Abstract
In birds, poor rearing conditions usually have negative effects on T-cell-mediated immune response. However, earlier studies demonstrate that fitness-related traits such as body mass may show sex-specific patterns when subject to alteration of rearing conditions. Therefore, to investigate whether deterioration of rearing conditions influences the development of immune function differently in male and female nestlings, we performed brood size manipulation experiments on blue tit (Parus caeruleus) nestlings. To alter rearing conditions, some broods were increased by three nestlings soon after hatching, while other broods were left non-manipulated. Immune response was assessed as a hypersensitivity reaction to phytohaemagglutinin in 11-day-old nestlings. Additionally, we studied the consequences of brood size manipulation for fledgling body mass and tarsus length. The enlargement of brood size had different effects on the cellular immune responses of male and female nestlings, with males being more negatively affected than their female nest-mates. Sex-specific effects of poor rearing conditions were also recorded for tarsus length, such that tarsus growth was more retarded in female than in male nestlings. We discuss the effects of deterioration of rearing conditions on sex-specific development of cell-mediated immunity with respect to sexual dimorphism of size and developmental strategies in male and female nestlings.
Keywords: body size, brood size manipulation, immune function, Parus caeruleus, phytohaemagglutinin response
1. Introduction
Immune function seems to be one of the key physiological traits influencing fitness in animals (Møller & Saino 2004). Evidence is growing that variation in immune function may constitute an important determinant of survival in nestling birds. Nestlings that elicit higher immune response have been shown to survive better both during pre-fledging and post-fledging period (Nordling 1998; Gonzalez et al. 1999). As the maturation of immune function may take up to several weeks after hatching (Klasing & Leshchinsky 1999), rearing conditions experienced by nestlings at this time may substantially affect the development of immunity. Indeed, poor nutrition during growth has been demonstrated to slow the development of the immune system through impaired growth of lymphoid organs and suppressed humoral and cellular immune responses (Lochmiller et al. 1993; Birkhead et al. 1999; Hoi-Leitner et al. 2001). Recent studies have shown that nestlings from naturally large or experimentally enlarged broods, presumably thereby experiencing poor rearing conditions, have lower T-cell-mediated immune responses than nestlings from small or experimentally reduced broods (Saino et al. 1997; Hõrak et al. 1999).
However, previous studies have also demonstrated that alterations of rearing conditions may affect individual nestlings in the brood differently, e.g. some of them are more affected than the others. In general, the majority of studies showed that the larger sex suffers more pronounced negative consequences of deterioration of rearing conditions than the smaller sex (e.g. Nager et al. 2000; Velando 2002). Those studies, however, focused mainly on the consequences of different rearing circumstances for growth and final body size, paying little or no attention to other fitness-related traits, such as immune function. The immune system may show sex-specific development when subject to alteration of rearing conditions because it is highly dependent on nutrition (e.g. Gershwin et al. 1985), and sexes may differ in their competitive abilities as well as in their developmental strategies. To date, only very few studies have investigated how rearing conditions affect the development of the immune system in male and female nestlings in a natural population. In Eurasian kestrels (Falco tinnunculus), a species showing reversed sexual size dimorphism, cell-mediated immune response in male nestlings was enhanced when rearing conditions were improved by brood reduction as compared with control conditions, while the immune response of female nestlings was unaffected (Fargallo et al. 2002). In great tits (Parus major), adverse rearing conditions evoked by an experimental flea infestation had non-significant effect on nestling cell-mediated immunity and the effect did not differ between the sexes (Tschirren et al. 2003). However, as fleas do not only influence conditions experienced by the nestlings, but also activate immune function, it is not possible reach straightforward conclusions regarding sex-related development of immune function.
Here, we study the cell-mediated immune response to phytohaemagglutinin (PHA) in male and female blue tit (Parus caeruleus) nestlings under experimentally altered rearing conditions. Rearing conditions were manipulated by adding extra young to some nests, while the other nests were left non-manipulated. Blue tit is a sexually size dimorphic species: at fledging, females are 3.3% lighter and have 5.2% shorter tarsus than males (A. Dubiec, M. Cichoń & K. Deptuch 2002, personal observations). We predicted that either male or female nestlings might show more pronounced suppression of immune functions in response to brood size manipulation. Males, as a larger sex, may be more sensitive to poor nutrition and develop weaker immunity under poor rearing conditions, or they may alternatively develop stronger immunity under such conditions if they outcompete their sisters over limited food. In addition, male and female nestlings may show different priorities for developing potentially competing vital functions; if food is limited, male nestlings may prioritize growth over the development of immune function. This is very plausible because body size is an important determinant of male reproductive success in blue tit (Kempenaers et al. 1992). So, additionally we studied the consequences of brood size manipulation for development of sexual size dimorphism in body mass and tarsus length.
2. Material and methods
(a) General methods
The experiment was conducted on nest-box breeding blue tits in deciduous woodlands in southern Gotland (SE Sweden, 57°10′ N, 18°20′ E; for description of the study area see Pärt & Gustafsson 1989) in 2002. From the end of April onwards, nest-boxes were regularly visited to determine laying date, clutch size, hatching date (day 0) and the number of hatchlings. On days 11, 12 and 14 post-hatching, nestlings were weighed with a Pesola spring balance to the nearest 0.1 g and on day 14 their tarsus lengths were also measured with a digital calliper to the nearest 0.1 mm. The measurement of tarsus length in 14-days-old nestlings reflects the adult size, as the birds are already fully developed at that time (Merilä & Fry 1998). Since using body mass on day 11 or 12 post-hatching in the model did not change results qualitatively and in consequence did not change our conclusions, we do not present these analyses.
(b) Experimental procedures
An experimental brood size manipulation was employed to alter rearing conditions. In some nests, brood size was increased by three nestlings, while other nests were left non-manipulated constituting a control group. Broods were enlarged by transferring three randomly selected nestlings from a donor brood of the same hatching date on day 2 post-hatching. In most cases, donor broods provided nestlings to more than one other brood and hence they were not included in the analyses as a reduced group. In total, 25 pairs of control and enlarged broods, matched in terms of brood size (±1 nestling) and equal hatching date, were created. As three enlarged nests were deserted shortly after manipulation, 22 pairs were used in final analyses. Characteristics of control and enlarged broods prior to manipulation and on day 11 post-hatching are presented in table 1. Donor nestlings from enlarged broods were included in all analyses as (i) there was no evidence that they were disadvantaged by rearing in a non-natal environment (mixed model ANOVA with nest ID as a random factor and nestling origin as a fixed factor, donor versus natal nestlings (mean±s.e.); body mass on day 14 post-hatching: 10.84±0.16 versus 10.70±0.14, F1,266=2.22, p=0.138; tarsus length: 16.41±0.10 versus 16.22±0.09, F1,265=5.31, p=0.022; immune response: 0.844±0.033 versus 0.797±0.025, F1,261=2.91, p=0.089) and (ii) control and enlarged broods did not differ in the mean, within-brood, variance in immune response (control versus enlarged (mean±s.d.): 0.040±0.020 versus 0.037±0.016, t=0.60, d.f.=42, p=0.551); larger variance could have been expected among enlarged broods due to different origin of nestlings. The effect of brood size manipulation on male and female nestlings' traits may be associated with the brood sex composition. However, post-manipulation sex ratios (number of males/brood size) on day 2 post-hatching showed no difference between control and enlarged broods (control: 0.51±0.12, enlarged: 0.49±0.15; generalized linear model using a logit link function and binomial distribution: χ12=0.08, p=0.77).
Table 1.
Clutch size and the number of hatchlings before manipulation and on day 11 post-hatching in control and enlarged broods (mean±s.d.).
| control broods | enlarged broods | t | d.f. | p | |
|---|---|---|---|---|---|
| clutch size | 11.77±1.38 | 11.73±1.12 | 0.12 | 42 | 0.905 |
| hatching date | 51.00±2.76 | 51.18±2.74 | −0.22 | 42 | 0.827 |
| number of hatchlings | 10.91±1.54 | 11.22±1.15 | −0.78 | 42 | 0.442 |
| number of nestlings | 10.59±1.74 | 13.55±1.87 | −5.43 | 42 | <0.0001 |
(c) Assessment of cell-mediated immunity
T-cell-mediated immune function of nestlings was assessed as a response to phytohaemagglutinin (PHA, Sigma Chemicals) injection, which is a standard test used in avian studies (Lochmiller et al. 1993; Brinkhof et al. 1999; Tella et al. 2000). PHA is a bean extract that has a mitogenic effect on T lymphocytes, and its inoculation stimulates dense accumulation of lymphocytes (Goto et al. 1978). When nestlings were 11 days old, 0.2 mg of PHA suspended in 0.04 ml of physiological saline solution was inoculated in the middle of the right wing web (Smits et al. 1999). The thickness of the wing web was measured with a pressure-sensitive spessimeter (SM-12, Mitutoyo) prior to and 24 h after the PHA injection with an accuracy of 0.01 mm. Each measurement was taken three times and as it was highly repeatable (repeatability prior to injection: r=0.98, F540,1082=181.42, p<0.0001; post-injection: r=0.99, F540,1082=587.77, p<0.0001; Lessels & Boag 1987), the mean value was used in the further analyses. The level of immune response was calculated as a difference between mean wing web thickness prior to and after the injection.
(d) Molecular sex identification
Nestlings were sampled for blood usually 2 days after hatching. Blood (2–15 μl) was drawn from the leg vein into a capillary and transferred to an eppendorf tube filled with 96% ethanol. Samples were stored at room temperature until analysed. Nestling sex was assessed by amplification of two homologous genes located on sex chromosomes: CHD1W and CHD1Z (Griffiths et al. 1998; see Cichoń et al. 2003 for details).
(e) Statistical analysis
The effect of brood size manipulation on immune response and the parameters of body size in male and female nestlings were analysed in Proc Mixed in SAS v. 8 (SAS 2000) incorporating residual maximum likelihood methods. Brood size manipulation, offspring sex and their interaction were defined as fixed factors and pair of matched nests and nest ID nested in brood size manipulation and pair as random factors. By using nest ID as a random factor, the analyses take into account that the experimental units are the nests, not the individual nestlings. Non-significant interactions were removed from the full model at p>0.5. In case of significant interactions, brood size manipulation×sex post hoc contrasts were performed to specify how the traits under study were affected by experimental treatment within each of the sexes.
3. Results
(a) Effects of brood size manipulation and sex on cell-mediated immunity
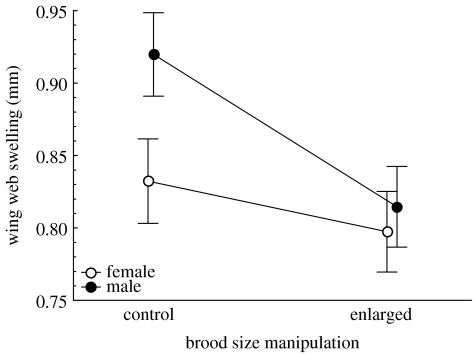
The experimental enlargement of brood size differentially affected cell-mediated immunity of male and female blue tit nestlings as indicated by a significant brood size manipulation×sex interaction (table 2). Male nestlings raised in enlarged broods had lower cellular response to PHA as compared with male nestlings from broods with non-manipulated numbers of nestlings (post hoc contrast, F1,42=8.23, p=0.006), while the immune response of females was not significantly affected by experimental treatment (post hoc contrast, F1,42=0.89, p=0.350, figure 1). Generally, nestlings reared in enlarged broods showed lower T-cell-mediated immune response than nestlings from control broods (least square mean±s.e., control broods: 0.876±0.03; enlarged broods: 0.806±0.02, table 2) and male nestlings developed stronger response than female nestlings (males: 0.867±0.02, females: 0.815±0.02, table 2).
Table 2.
The effects of brood size manipulation on T-cell-mediated immune response, body size and tarsus length in blue tit nestlings, analysed with mixed model nested ANOVA (Proc Mixed in SAS) with brood size manipulation (control versus enlarged broods), offspring sex and their interaction as fixed factors, and pair of matched nests and nest ID (nested in brood size manipulation and pair) as random factors. (Estimate (±s.e.) denotes variance components.)
| source | estimate | s.e. | d.f. | F | p |
|---|---|---|---|---|---|
| immune response | |||||
| brood size manipulation | 1, 42 | 4.68 | 0.036 | ||
| sex | 1, 21 | 5.86 | 0.025 | ||
| brood size manipulation×sex | 1, 443 | 4.06 | 0.045 | ||
| nest ID (brood size manipulation, pair) | 0.008 | 0.004 | |||
| pair | 0.001 | 0.003 | |||
| pair×sex | 0.002 | 0.002 | |||
| body mass | |||||
| brood size manipulation | 1, 42 | 4.22 | 0.046 | ||
| sex | 1, 21 | 106.73 | <0.0001 | ||
| brood size manipulation×sex | 1, 454 | 0.38 | 0.540 | ||
| nest ID (brood size manipulation, pair) | 0.353 | 0.084 | |||
| pair | 0 | ||||
| pair×sex | 0.002 | 0.009 | |||
| tarsus length | |||||
| brood size manipulation | 1, 42 | 1.77 | 0.191 | ||
| sex | 1, 21 | 194.42 | <0.0001 | ||
| brood size manipulation×sex | 1, 453 | 4.91 | 0.027 | ||
| nest ID (brood size manipulation, pair) | 0.085 | 0.031 | |||
| pair | 0.043 | 0.033 | |||
| pair×sex | 0.0002 | 0.005 |
Figure 1.
T-cell-mediated immune response (LS means±s.e. from the model in table 2) on day 12 post-hatching of male and female blue tit nestlings from control and enlarged broods.
(b) Effects of brood size manipulation and sex on body size
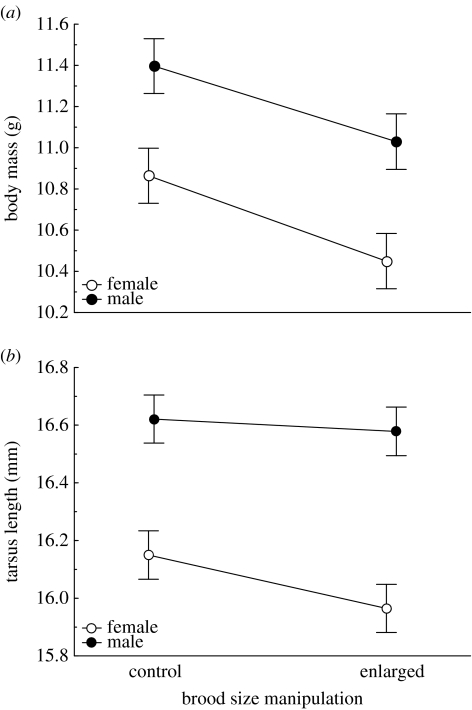
On day 14 post-hatching, nestlings reared in enlarged broods were significantly lighter than nestlings from control broods, but they did not differ in tarsus length (control versus enlarged broods, body mass: 11.12±0.13 versus 10.74±0.13; tarsus length: 16.40±0.08 versus 16.27±0.08; table 2). Male nestlings were heavier than female nestlings and had longer tarsus (body mass: males 11.20±0.10, females 10.66±0.10; tarsus length: males 16.60±0.07, females 16.07±0.07, table 2). Inter-sexual difference in body mass was similarly pronounced in both brood types; however, the expression of sexual dimorphism in tarsus length differed between control and enlarged broods as implied by a significant brood size manipulation×sex interaction (table 2, figure 2). Tarsus length of female nestlings was more affected by brood enlargement than male nestlings (post hoc contrasts; females: F1,42=4.22, p=0.046, males: F1,42=0.18, p=0.676).
Figure 2.
(a) Body mass and (b) tarsus length (LS means±s.e. from the models in table 2) on day 14 post-hatching of male and female blue tit nestlings from control and enlarged broods.
4. Discussion
Our study demonstrates that poor rearing conditions, simulated here by brood size enlargement, may differently affect the development of the cell-mediated component of immune function in male and female nestlings. We found that brood size enlargement negatively affected cellular immune response of male, but not female blue tit nestlings. Suppression of cell-mediated immunity in nestlings reared in enlarged broods has already been shown in a few species (Saino et al. 1997; Hõrak et al. 1999; Ilmonen et al. 2003; but see Bonneaud et al. 2003). However, only one more study, to our knowledge, has reported sex-specific effects of rearing environment on cell-mediated immunity. In sexually size dimorphic Eurasian kestrel, male nestlings from reduced broods had higher cellular immune response, while immune response of females, which are the larger sex, was not affected in comparison with control broods (Fargallo et al. 2002).
In blue tits larger body size probably places male nestlings at competitive advantage over the access to food, which in turn may improve development of the immune system. Indeed, we found that males had a higher cellular immune response than females in control broods. However, among nestlings subject to brood size enlargement, only males responded with suppression of the cellular component of immunity. Thus, competition does not seem to explain sexual differences in immune function. Similarly, immunosuppression in male Eurasian kestrel nestlings, observed under food restriction conditions, cannot be attributed to sexual differences in competitive abilities (Laaksonen et al. 2004). The suppression of immune response in males may indicate that male nestlings are more sensitive to poor rearing conditions than female nestlings, probably because they cannot meet energetic and nutritional requirements due to their larger body size. Alternatively, male and female nestlings may differ in their developmental strategies and the flexibility of adjusting these strategies to current rearing conditions. Males may show different priorities of resource allocation from females if the development of specific physiological and morphological components gives different fitness returns in different sexes. For example, body size may differently affect male and female fitness, in which case growth may be prioritized over the development of other functions (e.g. the development of an efficient immune system) in one of the sexes. In blue tits, male body size seems to be an important determinant of reproductive success, as males with longer tarsi have been reported to have higher chances of acquiring larger total fertilization success (Kempenaers et al. 1992). In this species there is no post-fledging skeletal growth, therefore body size attained at fledging corresponds to adult body size (Merilä & Fry 1998). In order to attain large structural body size, when rearing conditions deteriorate, male nestlings may reduce investments in some physiological functions, such as immune function, in favour of growth. Such a pattern of resource allocation may appear optimal if underdevelopment of the immune system at the nestling stage can be compensated later in life (Birkhead et al. 1999). Thus, when resources are limited it may be more important for male nestlings to sustain skeletal growth at the costs of the investment in immune function, while female nestlings may reduce the allocation of resources to growth in favour of immune defences. Our data seem to support such a possibility, as tarsus growth in male nestlings was affected by brood size enlargement to a much lesser extent than in female nestlings, and simultaneously the experimental treatment exerted stronger negative effects on cellular immune response in male nestlings. Råberg et al. (2005) also observed that in blue tits tarsus growth is more negatively affected by poor rearing conditions in female than in male nestlings.
Our results may also suggest that, under natural conditions, blue tit female nestlings secure only the very essential investment in immune function that is absolutely necessary to survive in a complex antigenic environment since, even when exposed to harsh rearing conditions, they raised their immune response to a level similar to the one found under non-manipulated conditions. Finally, the suppression of cellular immunity in males under poor growth conditions may be associated with the costs of immunopathology. Due to, for example, an increase in the level of heat-shock proteins (self-components subject to autoreactivity) in response to environmental stressors, the risk of autoimmune reactions in males from enlarged broods might be high in cases of upregulation of the immune system (Råberg et al. 1998).
Sexual dimorphism in nestling immune function has been investigated only recently, since molecular DNA-based techniques have enabled reliable sexing of nestlings (Griffiths et al. 1998). In the majority of studied species, including barn swallows (Hirundo rustica), American kestrels (Falco sparverius), Magellanic penguins (Spheniscus magellanicus), Alpine swifts (Apus melba) and white stork (Ciconia ciconia), male and female nestlings do not differ in cellular immune response (Tella et al. 2000, 2001; Saino et al. 2002; Jovani et al. 2004; Bize et al. 2005), in Eurasian kestrels and great tits females develop stronger cell-mediated immunity than males (Fargallo et al. 2002; Tschirren et al. 2003) and in blue tits males show stronger cellular response (this study). In some species, sex-related variation in immune function is more complex, e.g. in black-headed gull (Larus ridibundus) sex differences in immunity emerge only in the young hatching second in the hatching order, with females being more immunocompetent than males, while first- and third-hatched male and female nestlings do not differ in response against PHA (Müller et al. 2003). The mechanism behind the sex-specific variation in nestling immunity has not been identified yet, although lower immune responsiveness in male nestlings observed in some species has been associated with the elevated level of androgens (e.g. Mougeot et al. 2004).
In conclusion, this study shows that deterioration of rearing conditions as simulated by brood size enlargement differently affects the development of the immune system in male and female nestlings. Such sex-dependent phenomena may have important consequences for the optimization of clutch size and sex ratio. If large clutch size exerts more pronounced effects in one of the sexes, the adjustment of sex ratio to rearing conditions and clutch size may become a target of selection. Further studies are needed to investigate whether sex-specific differences in immune function in response to alteration of rearing environment exist among other bird species and whether these differences result from divergent strategies of resource allocation during growth and development of male and female nestlings.
Acknowledgments
We thank Natalia Pitala for help in the field. Barbara Tschirren, Wendt Müller, Toni Laaksonen and two anonymous referees provided valuable comments on previous versions of the manuscript. The research project was financially supported by the State Committee for Scientific Research, Republic of Poland, in years 2004–2007.
References
- Birkhead T.R, Fletcher F, Pellatt E.J. Nestling diet, secondary sexual traits and fitness in the zebra finch. Proc. R. Soc. B. 1999;266:385–390. doi:10.1098/rspb.1999.0649 [Google Scholar]
- Bize P, Roulin A, Tella J.L, Richner H. Female-biased mortality in experimentally parasitized Alpine swift Apus melba nestlings. Funct. Ecol. 2005;19:405–413. doi:10.1111/j.1365-2435.2005.00995.x [Google Scholar]
- Bonneaud C, Mazuc J, Gonzalez G, Haussy C, Chastel O, Faivre B, Sorci G. Assessing the cost of mounting an immune response. Am. Nat. 2003;161:367–379. doi: 10.1086/346134. doi:10.1086/346134 [DOI] [PubMed] [Google Scholar]
- Brinkhof M.W.G, Heeb P, Kölliker M, Richner H. Immunocompetence of nestling great tits in relation to rearing environment and parentage. Proc. R. Soc. B. 1999;266:2315–2322. doi:10.1098/rspb.1999.0925 [Google Scholar]
- Cichoń M, Dubiec A, Stoczko M. Laying order and offspring sex in blue tits Parus caeruleus. J. Avian Biol. 2003;34:355–359. doi:10.1111/j.0908-8857.2003.03201.x [Google Scholar]
- Fargallo J.A, Laaksonen T, Pöyri V, Korpimäki E. Inter-sexual differences in the immune response of Eurasian kestrel nestlings under food shortage. Ecol. Lett. 2002;5:95–101. doi:10.1046/j.1461-0248.2002.00290.x [Google Scholar]
- Gershwin M.E, Beach R.S, Hurley L.S. Academic Press; Orlando, FL: 1985. Nutrition and immunity. [Google Scholar]
- Gonzalez G, Sorci G, de Lope F. Seasonal variation in the relationship between cellular immune response and badge size in male house sparrows (Passer domesticus) Behav. Ecol. Sociobiol. 1999;46:117–122. doi:10.1007/s002650050600 [Google Scholar]
- Goto N, Kodama H, Okada K, Fujimoto Y. Suppression of phytohemagglutinin skin response in thymectomized chickens. Poult. Sci. 1978;52:246–250. doi: 10.3382/ps.0570246. [DOI] [PubMed] [Google Scholar]
- Griffiths R, Double M.C, Orr K, Dawson R.J.G. A DNA test to sex most birds. Mol. Ecol. 1998;7:1071–1075. doi: 10.1046/j.1365-294x.1998.00389.x. doi:10.1046/j.1365-294x.1998.00389.x [DOI] [PubMed] [Google Scholar]
- Hoi-Leitner M, Romero-Pujante M, Hoi H, Pavlova A. Food availability and immune capacity in serin (Serinus serinus) nestlings. Behav. Ecol. Sociobiol. 2001;49:333–339. doi:10.1007/s002650000310 [Google Scholar]
- Hõrak P, Tegelmann L, Ots I, Møller A.P. Immune function and survival of great tit nestlings in relation to growth conditions. Oecologia. 1999;121:316–322. doi: 10.1007/s004420050934. doi:10.1007/s004420050934 [DOI] [PubMed] [Google Scholar]
- Ilmonen P, Hasselquist D, Langefors Å, Wiehn J. Stress, immunocompetence and leukocyte profiles of pied flycatchers in relation to brood size manipulation. Oecologia. 2003;136:148–154. doi: 10.1007/s00442-003-1243-2. doi:10.1007/s00442-003-1243-2 [DOI] [PubMed] [Google Scholar]
- Jovani R, Tella J.L, Blanco G, Bertellotti M. Variable inter-annual relationships between T-cell mediated immunity and individual traits in White Storks. Ardeola. 2004;51:357–364. [Google Scholar]
- Kempenaers B, Verheyen G, van den Broeck M, Burke T, van Broeckhoven C, Dhondt A. Extra-pair paternity results from female preference for high quality males in the blue tit. Nature. 1992;357:494–496. doi:10.1038/357494a0 [Google Scholar]
- Klasing K.C, Leshchinsky T.V. Functions, costs, and benefits of the immune system during development. In: Adams N.J, Slotow R.H, editors. Proc. 22nd Int. Ornithological Congress, Durban, South Africa. Birdlife South Africa; Johannesburg, South Africa: 1999. pp. 2817–2835. [Google Scholar]
- Laaksonen T, Fargallo J.A, Korpimäki E, Lyytinen S, Valkama J, Pöyri V. Year- and sex-dependent effects of experimental brood sex ratio manipulation on fledging condition of Eurasian kestrels. J. Anim. Ecol. 2004;73:342–352. doi:10.1111/j.0021-8790.2004.00811.x [Google Scholar]
- Lessels C.M, Boag P.T. Unrepeatable repeatabilities: a common mistake. Auk. 1987;104:116–121. [Google Scholar]
- Lochmiller R.L, Vestey M.R, Boren J.C. Relationship between protein nutritional status and immunocompetence in northern bobwhite chicks. Auk. 1993;110:503–510. [Google Scholar]
- Merilä J, Fry J.D. Genetic variation and causes of genotype–environment interaction in the body size of blue tit (Parus caeruleus) Genetics. 1998;148:1233–1244. doi: 10.1093/genetics/148.3.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mougeot F, Irvine J.R, Seivwright L, Redpath S.M, Piertney S. Testosterone, immunocompetence, and honest sexual signaling in male red grouse. Behav. Ecol. 2004;15:930–937. doi:10.1093/beheco/arh087 [Google Scholar]
- Møller A.P, Saino N. Immune response and survival. Oikos. 2004;104:299–304. doi:10.1111/j.0030-1299.2004.12844.x [Google Scholar]
- Müller W, Dijkstra C, Groothuis T.G.G. Inter-sexual differences in T-cell-mediated immunity of black-headed gull chicks (Larus ridibundus) depend on the hatching order. Behav. Ecol. Sociobiol. 2003;55:80–86. [Google Scholar]
- Nager R.G, Monaghan P, Houston D.C, Genovart M. Parental condition, brood sex ratio and differential young survival: an experimental study in gulls (Larus fuscus) Behav. Ecol. Sociobiol. 2000;48:452–457. doi:10.1007/s002650000262 [Google Scholar]
- Nordling, D. 1998 Trade-offs between life-history traits and immune defence in the collared flycatcher Ficedula albicollis Ph.D. dissertation, Uppsala University, Sweden.
- Pärt T, Gustafsson L. Breeding dispersal in the collared flycatcher (Ficedula albicollis)—possible causes and reproductive consequences. J. Anim. Ecol. 1989;58:305–320. [Google Scholar]
- Råberg L, Grahn M, Hasselquist D, Svensson E. On the adaptive significance of stress-induced immunosuppression. Proc. R. Soc. B. 1998;265:1637–1641. doi: 10.1098/rspb.1998.0482. doi:10.1098/rspb.1998.0482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Råberg L, Stjernman M, Nilsson J.-Å. Sex and environmental sensitivity in blue tit nestlings. Oecologia. 2005;145:496–503. doi: 10.1007/s00442-005-0133-1. doi:10.1007/s00442-005-0133-1 [DOI] [PubMed] [Google Scholar]
- Saino N, Calza S, Møller A.P. Immunocompetence of nestling barn swallows in relation to brood size and parental effort. J. Anim. Ecol. 1997;66:827–836. [Google Scholar]
- Saino N, Ambrosini R, Martinelli R, Calza S, Møller A.P, Pilastro A. Offspring sexual dimorphism and sex-allocation in relation to parental age and paternal ornamentation in the barn swallow. Mol. Ecol. 2002;11:1533–1544. doi: 10.1046/j.1365-294x.2002.01542.x. doi:10.1046/j.1365-294X.2002.01542.x [DOI] [PubMed] [Google Scholar]
- SAS 2000 SAS/STAT User's Guide. Version 8.2. Cary, NC: SAS Institute, Inc.
- Smits J.E, Bortolotti G.R, Tella J.L. Simplifying the phytohaemagglutinin skin-testing technique in studies of avian immunocompetence. Funct. Ecol. 1999;13:567–572. doi:10.1046/j.1365-2435.1999.00338.x [Google Scholar]
- Tella J.L, Bortolotti G.R, Dawson R.D, Forero M.G. The T-cell-mediated immune response and return rate of fledgling American kestrels are positively correlated with parental clutch size. Proc. R. Soc. B. 2000;267:891–895. doi: 10.1098/rspb.2000.1086. doi:10.1098/rspb.2000.1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tella J.L, Forero M.G, Bertellotti M, Donázar J.A, Blanco G, Ceballos O. Offspring body condition and immunocompetence are negatively affected by high breeding densities in a colonial seabird: a multiscale approach. Proc. R. Soc. B. 2001;268:1455–1461. doi: 10.1098/rspb.2001.1688. doi:10.1098/rspb.2001.1688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschirren B, Fitze P.S, Richner H. Sexual dimorphism in susceptibility to parasites and cell-mediated immunity in great tit nestlings. J. Anim. Ecol. 2003;72:839–845. doi:10.1046/j.1365-2656.2003.00755.x [Google Scholar]
- Velando A. Experimental manipulation of maternal effort produces differential effects in sons and daughters: implications for adaptive sex ratios in the blue-footed booby. Behav. Ecol. 2002;13:443–449. doi:10.1093/beheco/13.4.443 [Google Scholar]