Abstract
The importance of behaviours as instigators or inhibitors of evolutionary change remains largely unresolved and this is in part because there are very few empirical examples of how behaviours affect evolutionary processes. By determining the environment of breeding, aggressive interactions over territories have the potential to strongly impact selection pressures experienced by individuals. Western bluebirds (Sialia mexicana) provide a unique opportunity to investigate the evolutionary importance of aggression, since their highly variable breeding habitat favours distinct foraging techniques and they also compete aggressively for nest boxes, a resource that is easy to manipulate. Here, I show experimentally that more aggressive males compete more effectively for territories with a high density of nest boxes and, as a consequence, aggressive and non-aggressive males are sorted into distinct breeding habitats that differ in the strength of selection on morphological traits. Specifically, males with longer tails and tarsi were favoured in open habitats where high agility is required to forage efficiently, whereas in forested habitats, where agility is less important, selection was weak. These results show that aggression can affect selection on a local scale by determining individual settlement patterns. More generally, because territorial interactions are important across a wide variety of taxa, these results suggest that aggressive behaviour has the potential to impact the evolutionary trajectory of many animal populations.
Keywords: aggression, territoriality, evolutionary change, Sialia mexicana
1. Introduction
Behaviours affect how organisms interact with their environment, and therefore can influence the evolutionary trajectory of a population (Mayr 1963; Wcislo 1989). On one hand, changes in behaviour can expose organisms to novel environments and, in turn, this sets the stage for subsequent evolution of the morphology, life history and physiology of an organism (Plotkin 1988; Wcislo 1989; West-Eberhard 2003). On the other hand, behavioural plasticity may buffer an organism from strong selection by allowing an individual to either avoid a stressful environment or to modify their interaction with the environment in order to maintain homeostasis (Wake et al. 1983; Huey et al. 2003; Badyaev 2005). Despite the extensive attention this topic has received, the relative importance of behaviour in driving or inhibiting evolutionary change remains largely unresolved. This is in part because there are very few empirical examples which link individual variation in ecologically important behaviours to evolutionary processes (Plotkin 1988; Huey et al. 2003; Losos et al. 2004; Sol et al. 2005).
Aggressive behaviour has great potential to affect selection pressures since it is often used to obtain a breeding territory (Stamps & Krishnan 1997), and therefore can affect individual fitness by determining the quality of environment in which offspring develop. The outcome of aggressive interactions will depend not only on an individual's own aggressive behaviour but will also depend on the aggressive phenotype of other individuals in the population. This makes aggressive interactions important for evolution because they can influence the local breeding environment for many individuals at once by determining territory spacing, population density and population dynamics (Moss et al. 1994; Watson et al. 1994). Therefore, even in the absence of behavioural plasticity, which is often assumed to be necessary for behaviours to affect evolutionary change (Wcislo 1989; West-Eberhard 2003), aggressive interactions can influence the evolutionary trajectory of populations if the frequency of aggressive and non-aggressive phenotypes changes over time (Mougeot et al. 2003). However, this perspective assumes that aggressive interactions will cause individuals to sort non-randomly with respect to environmental variation and that non-random settlement patterns will result in differential selection. The main objective of the current study is to experimentally test this and determine the relevance of aggressive interactions for influencing selection in a natural population.
Western bluebirds (Sialia mexicana) provide a unique opportunity to investigate the evolutionary consequences of aggression. I have previously shown that there is abundant variation in this behaviour among males and this variation is not due to plasticity but instead reflects consistent individual differences (Duckworth in press). These differences are likely to be ecologically important in western bluebirds because, as secondary cavity nesters, they depend on nest cavities to reproduce but cannot excavate their own and this leads to intense aggressive competition for this limited resource (Brawn & Balda 1988; Newton 1994). Bluebirds also aggressively defend large breeding territories in which they forage for themselves and their offspring, and habitat structure can vary widely among these territories (Guinan et al. 2000). Habitat variation is expected to have strong consequences for selection on morphology in this species because bluebirds use distinct foraging tactics depending on the amount of tree cover on their territory (Pinkowski 1979; Power 1980; Brawn 1991; R. A. Duckworth 2004, personal observation). In closed habitat, bluebirds mainly forage by perching on trees to scan the ground for prey, while on open territories with few trees, bluebirds must hover above or hop along the ground to search for prey (Pinkowski 1979; R. A. Duckworth 2004, personal observation) and these latter techniques require greater agility than the perch–forage strategy used in closed habitat (Osterhaus 1962; Pinkowski 1979; Balmford et al. 1993).
Here, I first show experimentally that more aggressive males acquire territories with a high density of nest boxes. Then, I show that aggressive interactions over nest boxes sort males into different breeding habitats where they experience differential selection on morphology that is concordant with the foraging tactics used in these habitats. Finally, in light of these results, I discuss the importance of aggressive interactions for influencing the evolutionary trajectory of animal populations.
2. Material and methods
(a) Nest-box manipulation experiments
Bluebirds prefer territories with multiple nest cavities and will guard more than one, particularly if they are within close proximity of one another (Meek & Robertson 1994; Plissner & Gowaty 1995). Therefore, to determine whether differences in aggression among males affect the outcome of competition for nest cavities, I conducted two experiments. First, I manipulated nest-box density after males had settled on territories. This experiment was designed to determine whether males modify their aggressive behaviour in response to changes in territory quality. Second, I manipulated nest-box density before males settled territories to determine whether aggressive behaviour affected male settlement patterns. These experiments were conducted on a population located in St Regis, Montana, USA on several miles of ranchland. The uniformly open habitat of this population makes it possible to isolate the effects of nest-box density while controlling for differences among territories in habitat structure and quality.
In 2004, I established 44 territories by placing single nest boxes ca 150 m apart along a linear transect (along a fencerow). These territories were established in early March before bluebirds arrived to the breeding grounds. Nest boxes were monitored weekly and once pairs chose a territory (as indicated by females initiating nest building), I designated territories as either control (single-box) or experimental (multi-box). On multi-box territories (n=18), I placed a second box 10–15 m from the first box and on single-box territories (n=13), I visited them but did not add a second nest box. Control and experimental territories were chosen so that they were spatially intermixed across the study area and so that the two groups did not differ in initiation date (t=0.85, p=0.40, n=30). I measured male aggressive behaviour on all territories within one week of the first laid egg. I predicted that if males modify the intensity of their territory defence in response to territory quality, then males breeding on multi-box territories should respond more aggressively compared to males breeding on single-box territories.
In 2005, I again conducted a nest-box manipulation experiment at this site with one key difference: I manipulated nest box density in early March before bluebirds arrived to the breeding grounds. I established 40 territories (different locations from 2004 were used to control for prior residency effects) by placing either two nest boxes 5–10 m apart or only one nest box in a linear transect along a fencerow; adjacent territories were ca 150 m apart. Once again, single-box and multi box territories were interspersed across the study area. Nest boxes were checked once a week to monitor breeding behaviour of pairs. I measured aggression of males during late nest building and early laying stages. For this experiment, I predicted that, if aggression determines the outcome of competition for nest cavities, more aggressive males should acquire preferred multi-box territories while less aggressive males should acquire single-box territories.
(b) Measurement of aggression
I measured male aggression by simulating a territorial intrusion of a common interspecific competitor of bluebirds, the tree swallow (Tachycinetas bicolor). I used a tree swallow for two reasons. First, a male's aggressive response to an interspecific competitor reliably indicates his aggressiveness toward conspecific males (Duckworth in press). Second, using conspecific males can lead to infanticide and/or divorce by the focal male while divorce and infanticide is never observed after presenting a heterospecific competitor (R. A. Duckworth 2004, personal observation).
To simulate territorial intrusions, I presented males with a live tree swallow in a wire cage placed on the nest box. The swallow was concealed until the focal male was within 100 m of the nest box. I observed a male's response from a blind approximately 15–30 m away. During the 2 min trial, I counted the number of times the male attacked the model, flew by it, or hovered near the model. I assigned each male an aggressiveness score of 1–6 with 1 indicating a non-aggressive response and 6 indicating the most aggressive response. Specifically, scores were assigned according to the following scale: 1=no aggressive behaviours, 2=hovering or flying by 1–5 times and 0 attacks, 3=hovering or flying by more than 5 times and 0 attacks, 4=1–5 attacks, 5=6–9 attacks and 6=10 or more attacks (see Duckworth in press for details). This assay of aggression is highly repeatable both within and across breeding stages and reproductive contexts (Duckworth in press).
(c) Naturally varying population
This component of the study was conducted on a separate population in part of Lolo National Forest in Montana USA which is located ca 110 km from the St Regis population. The core section of the study area is ca 60 ha and is characterized by open meadows interspersed with mixed stands of Douglas fir (Pseudotsuga menziesii) and ponderosa pine (Pinus ponderosa). In 2001, the study area was systematically searched for nest cavities suitable for bluebird nest sites. Most nest cavities were located in mature trees or snags, which are conspicuous and easy to locate in both open and forested habitat. Nest boxes were placed at or next to these natural cavities to mimic natural variation in the density of nest-cavities across the population while standardizing variation in nest-cavity quality. Therefore, unlike the experimental population, both the amount of forest cover as well as nest-cavity density varied naturally at this site (figure 1).
Figure 1.
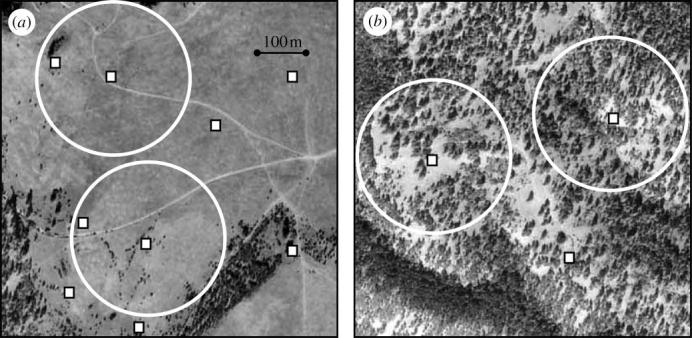
An example of typical (a) open and (b) closed territories. Circles indicate 150 m radius of area immediately surrounding the primary nest box and squares indicate the location of nest boxes. There is a higher density of nest boxes in open areas (e.g. nine versus three in (a) and (b), respectively). In (a), the two territories shown are multi-box while in (b), there is only one nest box in each male's territory.
The data for this study was collected during 2002–2004 breeding seasons. Each year, I trapped resident bluebirds, marked them with a unique colour band combination and measured wing chord and tail length using a ruler and tarsus length using Mitutoyo calipers. Morphological traits were measured twice for a subset of males to confirm repeatability of measurements (Wing: F35,38=35.65, r=0.97, p<0.0001, n=42; tail: F35,38=9.69, r=0.90, p<0.0001; tarsus: F35,38=19.56, r=0.95, p<0.0001). From March through July, I visited territories twice weekly to monitor nests and record observations of male territorial behaviour.
Aggressive behaviour was measured for 55 males using the methods described above. I recorded breeding success for each male as the number of offspring that survived to independence (two weeks post-fledge) and used this as a measure of annual breeding success in the analysis of selection on morphology. No other fitness measures were included in this study as there was no mortality of males during the breeding season.
(d) Measurement of territory characteristics
In the naturally varying population, territories were categorized as single-box or multi-box based on the number of nest boxes a male defended. Territories were considered to be multi-box if (i) males were observed defending more than one before the initiation of the first nest or (ii) a pair was observed nest building in multiple boxes before the initiation of the first nest. Most territorial interactions and foraging bouts of bluebirds occur within a 150 m radius of the nest box (Power 1980). Therefore, I recorded both the number of nest boxes and the distance to the next nearest nest box within this area. These measurements were used to determine whether acquiring single or multi-box territories was related to the local density of nest cavities. In addition, I used an aerial photo of the study site to assess the percentage of tree cover for each territory within a 150 m radius of the primary nest box (figure 1) using SigmaScan v. 5.0 image analysis software (Jandel Scientific). Each territory was measured three times to confirm the high repeatability of this measurement (F37,76=111.09, r=0.97, p<0.0001). For selection analyses, I categorized territories as open or closed based on the median per cent tree cover for all territories (median=18.7%, range=0.1–52.2%); open territories had less than or equal to 18.7% tree cover and closed territories had greater than 18.7% tree cover.
(e) Statistical analysis
I used general linear models (GLM) to analyse the relationship between aggression and the type of territory males acquired. This model included the number of boxes on a male's territory (multi versus single box), the amount of tree cover (open versus closed) and year as a covariate. To determine whether selection differed between habitats, I used a GLM with annual breeding success as the dependent variable and each morphological trait, habitat type and the interaction between morphology and habitat type as independent variables. Year was initially included as a covariate, but as its inclusion did not affect the results, it was omitted from the final model. Only significant interaction terms were included in the final model. A partial regression was used to analyse fecundity selection on male morphology in each habitat type and selection gradients were calculated as partial regression coefficients. Variables were standardized to a mean of zero and standard deviation of one before regression analyses and data were screened for outliers. I only used data for each male from their first year at the study site to ensure independence of data points and to control for any effect of prior residency on territory establishment. For selection analyses, I used a subset of males from the naturally varying population for which I obtained measurements of morphology (n=46). Males were captured opportunistically during the nest-box experiments (n=5 in 2004 and n=2 in 2005), and, therefore, there was not enough data on morphology in these populations for selection analyses. A post hoc power analysis was conducted using G*Power (Erdfelder et al. 1996) for a medium effect size of r=0.30 (Jennions & Møller 2003).
3. Results
(a) Aggression, body size and habitat
Reproductive success was not affected by habitat type (pooled: t=0.21, p=0.83, power=0.64) or whether the territory was multi-box (t=−0.69, p=0.49, power=0.60). Measurements of body size were unrelated to male aggressive behaviour (wing: F1,44=0.65, p=0.42; tail: F1,44=0.97, p=0.33; tarsus: F1,44=2.40, p=0.13, power=0.55), did not affect males' ability to acquire multi-box territories (wing: t=−1.12, p=0.27; tail: t=−1.41, p=0.17; tarsus: t=−0.14, p=0.87, power=0.53), and was not related to the type of habitat in which males settled (wing: t=0.04, p=0.97; tail: t=0.10, p=0.92; tarsus: t=−0.62, p=0.54, power=0.54).
(b) Aggression and settlement patterns
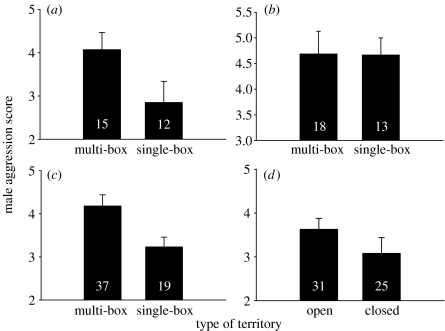
In both populations, more aggressive males acquired territories with multiple boxes (experimental population, 2005: one-tailed, t=1.95, p=0.03, n=27, figure 2a; naturally varying population: GLM: F1,51=9.71, p=0.003, figure 2c). However, when nest-box density was manipulated after males had already settled on territories, the aggressive response of males on single and multi-box territories did not differ (one-tailed: t=−0.05, p=0.45, power=0.52, figure 2b).
Figure 2.
Male aggression in relation to the type of territory males acquire. In the experimental population, more aggressive males acquired territories with multiple nest boxes when nest-box density was manipulated (a) before territory settlement and there was no difference in male aggressive behaviour when nest-box density was manipulated (b) after territory settlement. In the naturally varying population, (c) more aggressive males acquired territories with more nest boxes and (d) settled more often in open habitat. Bars indicate mean±s.e. and numbers on bars indicate sample sizes.
Unlike the experimental population, the distance between nest boxes in the naturally varying population was highly uneven among territories (mean±s.e. distance between boxes=164.40±8.18 m; range=26–319 m) and males guarded multiple nest boxes on territories that had a higher density of nest boxes (t=−3.74, p=0.0004, n=55) and shorter distances between the focal nest and the nearest nest box (t=4.59, p<0.0001, n=55). Territories with more open habitat had a higher density of nest boxes (t=−2.18, p=0.03) and a shorter distance between nest boxes (t=2.03, p<0.05), and thus more aggressive males settled more often on open territories while less aggressive males settled more frequently on closed territories (F1,51=4.80, p=0.03; figure 2d).
(c) Consequences of breeding habitat for selection on morphology
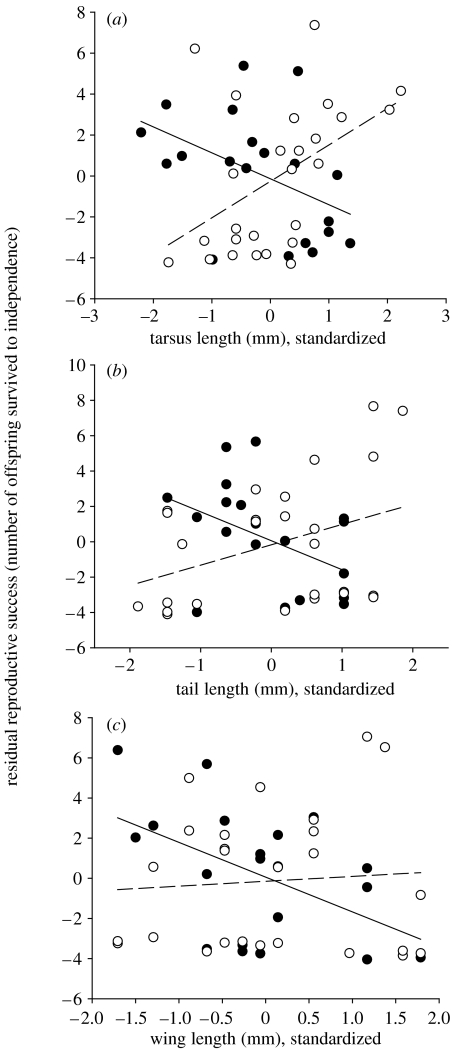
Breeding habitat influenced selection on morphology (tarsus length–habitat type interaction: F1,39=10.94, p=0.002; tail length–habitat type interaction: F1,39=7.20, p=0.01; figure 3a,b). In open habitat, males with longer tarsi (t=3.06, p=0.006, 95% confidence interval, CI=0.17–1.03) and tails (t=2.49, p=0.02, n=25, CI=0.06–1.12; table 1) were favoured. However, in closed habitat, selection on tail and tarsus was negative and not significant (tarsus: t=−1.32, p=0.20, CI=−0.70–0.16; tail: t=−0.19, p=0.85, CI=−0.76–0.69, n=21, power=0.37; table 1). There was no difference in the strength of selection on wing length among habitats (wing length–habitat type interaction: F1,38=0.05, p=0.83; table 1).
Figure 3.
Relationship between reproductive success (residual) and (a) tarsus, (b) tail and (c) wing length for males breeding in open (open circles, dashed line) or closed (closed circles, solid line) habitat.
Table 1.
Standardized selection gradients for fecundity selection on morphological traits of male western bluebirds breeding in closed or open habitats (*p<0.05; **p<0.01).
| habitat type | |||
|---|---|---|---|
| trait | open | closed | pooled across habitat |
| tail length | +0.63* | −0.06 | 0.23 |
| tarsus length | +0.54** | −0.30 | 0.09 |
| wing length | −0.32 | −0.44 | −0.27 |
4. Discussion
These results show that aggressive behaviour can strongly impact selection on morphological traits by determining the outcome of competitive interactions among males. I found that male aggressive behaviour was closely linked to male competitive performance—more aggressive males out-competed less aggressive males for preferred multi-box territories (figure 2a,c). In a population that varied naturally in the density of nest cavities and habitat characteristics, aggressive interactions over nest cavities sorted males into different habitats (figure 2d), and these habitat differences led to distinct selection on morphological traits (table 1; figure 3).
Competition for nest cavities seems to drive settlement patterns in this species—more aggressive males acquired territories with multiple boxes in both the experimental population where habitat was uniform and in the naturally varying population. As a secondary consequence of competition for nest cavities, some males settled on territories with habitat suboptimal for their particular morphology (figure 3). If individuals focused mainly on habitat type when choosing their territory, then it is expected that males with short tarsi and tails should avoid breeding in open habitat. However, the finding that some highly aggressive males—males that would be expected to have high resource holding capabilities—settled on territories with habitat that is suboptimal for their particular morphology suggests that habitat type is a secondary consideration to males in comparison with the number of nest cavities on a territory (figure 3). This idea is supported by many studies which show that competition for nest cavities is often fierce among secondary cavity nesters (Brawn 1990; Newton 1994; Merilä & Wiggins 1995). Thus, it appears that a highly aggressive phenotype increases the ability of males to secure nest cavities, yet a single-minded drive to obtain this limited resource may cause some males to breed in habitats that are suboptimal.
Most studies have focused on the flexibility of behaviours as the reason for their unique role in evolutionary change (Wcislo 1989; Price et al. 2003; Sol et al. 2005). However, in western bluebirds, the expression of aggression is highly consistent in males within and across breeding seasons (Duckworth in press). Moreover, I show here that male aggressive behaviour was not affected by changes in territory quality that occurred after males settled (figure 2b). This suggests that the association between male behaviour and the number of boxes acquired is not due to males modifying their behaviour in response to perceived territory quality, but instead, is the outcome of aggressive interactions among individuals highly consistent in their behaviour.
While competition for nest cavities undoubtedly plays an integral role in determining settlement patterns in this species (Brawn & Balda 1988; Plissner & Gowaty 1995; this study), this does not mean that aggressive competition over nest cavities is the only factor influencing territory settlement. There is growing evidence that consistent differences in behaviour among individuals may reflect personality types or different coping strategies (Koolhaas et al. 1999), and that consistent differences in aggression are often correlated with behaviours such as boldness and exploratory behaviour (Drent et al. 2003). In western bluebirds, more aggressive males disperse farther than less aggressive males (R. A. Duckworth unpublished data), suggesting that aggression is correlated with other behaviours which also play an important role in habitat selection. In the future, it will be important to measure different aspects of bluebird behaviour to determine whether these correlations affect settlement patterns.
The patterns of selection documented in this study should not only affect evolutionary change but may also provide a mechanism for the maintenance of variation in morphological traits. Breeding in open habitat favoured males with longer tails and tarsi which enables them to be more agile during aerial foraging (Balmford et al. 1993; Thomas 1997) and when foraging on the ground (Pinkowski 1979; Miles & Ricklefs 1984). In contrast, bluebirds foraging in closed habitats mainly forage on or from trees (Pinkowski 1979). Since habitat differences linked to functional variation in foraging ecology vary on an extremely fine scale in this system (e.g. opposite habitat types can be found on adjacent territories), it supports the idea that fine scale environmental heterogeneity can influence selection experienced across a population (Roff 1997). Thus, aggressive interactions over territories, by sorting males into different habitats, may facilitate the maintenance of variation in morphological traits in this species.
A consistent sorting of aggressive males into open habitat might favour the correlated evolution of aggression and male body size which could set the stage for ecological divergence within western bluebird populations (Schluter 1996). However, this divergence seems unlikely because in order for it to occur the aggressive phenotypes in the population and the distribution of nest cavities across the population would have to be stable over time. Studies on the role of aggression in producing population cycles suggest that this is unlikely (Watson et al. 1994; Matthiopoulos et al. 2000; Mougeot et al. 2003) and, moreover, the association between habitat type and nest cavity density varies both spatially and temporally depending on the ecology of a particular site (e.g. burned forests and open meadows with mature trees can both have high densities of nest cavities). In addition, conservation programmes often place high densities of nest boxes in areas where there are typically very low densities of natural cavities. These observations suggest that changes in both the distribution of nest cavities and in the frequency of aggressive phenotypes over time will produce a dynamic environment in which the effects of aggressive interactions on selection pressures are ever-changing (Chesson & Rosenzweig 1991; Mougeot et al. 2003). Thus, the results of this study suggest that the role of behaviour in evolutionary processes is complex and that plasticity of behaviour per se is not necessary for behaviours to affect the evolutionary trajectory of a population. Moreover, since aggressive interactions are common across a wide variety of taxa, the results of this study suggest that they have the potential to impact evolutionary processes in many animal species.
Acknowledgments
I thank David Pfennig, Trevor Price, Alex Badyaev, Steve Nowicki, Kevin Oh, Dana Seaman and Rebecca Young for comments and discussion which improved this paper. I thank the USFS and Hayes Creek residents for allowing me to work on their properties. Erv Davis generously donated nest boxes for this project and Nicki Clyde and Tina Dockter helped monitor the St Regis population. Funding for this project was provided by NSF (DDIG 0407952), Sigma Xi, Animal Behaviour Society, Duke University Biology Department and the American Museum of Natural History Frank M. Chapman Fund. This research was conducted in compliance with the Duke University Institutional Animal Care and Use committee (permit # A090-04-03).
References
- Badyaev A.V. Stress-induced variation in evolution: from behavioral plasticity to genetic assimilation. Proc. R. Soc. B. 2005;272:877–886. doi: 10.1098/rspb.2004.3045. doi:10.1098/rspb.2004.3045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balmford A, Thomas A.L.R, Jones I.L. Aerodynamics and the evolution of long tails in birds. Nature. 1993;361:628–631. doi:10.1038/361628a0 [Google Scholar]
- Brawn J.D. Interspecific competition and social behavior in violet–green swallows. Auk. 1990;107:606–608. [Google Scholar]
- Brawn J.D. Environmental effects on variation and covariation in reproductive traits of western bluebirds. Oecologia. 1991;86:193–201. doi: 10.1007/BF00317531. doi:10.1007/BF00317531 [DOI] [PubMed] [Google Scholar]
- Brawn J.D, Balda R.P. Population biology of cavity nesters in northern Arizona: do nest sites limit breeding densities? The Condor. 1988;90:61–71. [Google Scholar]
- Chesson P, Rosenzweig M. Behavior, heterogeneity, and the dynamics of interacting species. Ecology. 1991;72:1187–1195. [Google Scholar]
- Drent P.J, van Oers K, van Noordwijk A.J. Realized heritability of personalities in the great tit (Parus major) Proc. R. Soc. B. 2003;270:45–51. doi: 10.1098/rspb.2002.2168. doi:10.1098/rspb.2002.2168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckworth, R. A. In press. Behavioral correlations across reproductive contexts provide a mechanism for a cost of aggression. Behav. Ecol.
- Erdfelder E, Faul F, Buchner A. G*power: a general power analysis program. Behav. Res. Meth. Instrum. Comput. 1996;28:1–11. [Google Scholar]
- Guinan J.A, Gowaty P.A, Eltzroth E.K. The birds of North America. vol. 510. 2000. Western bluebird. pp. 1–31. [Google Scholar]
- Huey R.B, Hertz P.E, Sinervo B. Behavioral drive versus behavioral inertia in evolution: a null model approach. Am. Nat. 2003;161:357–366. doi: 10.1086/346135. doi:10.1086/346135 [DOI] [PubMed] [Google Scholar]
- Jennions M.D, Møller A.P. A survey of the statistical power of research in behavioral ecology and animal behavior. Behav. Ecol. 2003;45:438–445. doi:10.1093/beheco/14.3.438 [Google Scholar]
- Koolhaas J.M, Korte S.M, De Boer S.F, Van Der Vegt B.J, Van Reenen C.G, Hopster H, De Jong I.C, Ruis M.A.W, Blokhuis H.J. Coping styles in animals: current status in behavior and stress-physiology. Neurosci. Biobehav. Rev. 1999;23:925–935. doi: 10.1016/s0149-7634(99)00026-3. doi:10.1016/S0149-7634(99)00026-3 [DOI] [PubMed] [Google Scholar]
- Losos J.B, Schoener T.W, Spiller D.A. Predator-induced behaviour shifts and natural selection in field-experimental lizard populations. Nature. 2004;432:505–508. doi: 10.1038/nature03039. doi:10.1038/nature03039 [DOI] [PubMed] [Google Scholar]
- Matthiopoulos J, Moss R, Lambin X. The kin-facilitation hypothesis for red grouse population cycles: territory sharing between relatives. Ecol. Model. 2000;127:53–63. doi:10.1016/S0304-3800(99)00199-4 [Google Scholar]
- Mayr E. Harvard University Press; Cambridge, MA: 1963. Animal species and evolution. [Google Scholar]
- Meek S.B, Robertson R.J. Interspecific competition for nestboxes affects mate guarding in eastern bluebirds Sialia sialis. Anim. Behav. 1994;47:295–302. doi:10.1006/anbe.1994.1042 [Google Scholar]
- Merilä J, Wiggins D.A. Interspecific competition for nest holes causes adult mortality in the collared flycatcher. Condor. 1995;97:445–450. [Google Scholar]
- Miles D.B, Ricklefs R.E. The correlation between ecology and morphology in deciduous forest passerine birds. Ecology. 1984;65:1629–1640. [Google Scholar]
- Moss R, Parr R, Lambin X. Effects of testosterone on breeding density, breeding success and survival of red grouse. Proc. R. Soc. B. 1994;258:175–180. [Google Scholar]
- Mougeot F, Redpath S.M, Leckie F, Hudson P.J. The effect of aggressiveness on the population dynamics of a territorial bird. Nature. 2003;421:737–739. doi: 10.1038/nature01395. doi:10.1038/nature01395 [DOI] [PubMed] [Google Scholar]
- Newton I. The role of nest sites in limiting the numbers of hole-nesting birds—a review. Biol. Conserv. 1994;70:265–276. doi:10.1016/0006-3207(94)90172-4 [Google Scholar]
- Osterhaus M.B. Adaptive modifications in the leg structure of some North American warblers (Dendroica Parulidae) Am. Midl. Nat. 1962;68:474–486. [Google Scholar]
- Pinkowski B.C. Foraging ecology and habitat utilization in the genus Sialia. In: Dickson J.G, Conner R.N, Fleet R.R, Kroll J.C, Jackson J.A, editors. The role of insectivorous birds in forest ecosystems. Academic Press; New York, NY: 1979. [Google Scholar]
- Plissner J.H, Gowaty P.A. Eastern bluebirds are attracted to two-box nest sites. Wilson Bull. 1995;107:289–295. [Google Scholar]
- Plotkin H.C. Behavior and evolution. In: Plotkin H.C, editor. The role of behavior in evolution. The MIT Press; Cambridge, MA: 1988. [Google Scholar]
- Power H.W. The foraging behavior of mountain bluebirds with emphasis on sexual differences. Ornithol. Monogr. 1980;28:1–72. [Google Scholar]
- Price T.D, Qvarnström A, Irwin D. The role of phenotypic plasticity in driving genetic evolution. Proc. R. Soc. B. 2003;270:1433–1440. doi: 10.1098/rspb.2003.2372. doi:10.1098/rspb.2003.2372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roff R.A. Longman; New York, NY: 1997. Evolutionary quantitative genetics. [Google Scholar]
- Schluter D. Ecological causes of adaptive radiation. Am. Nat. 1996;148:S40–S64. doi:10.1086/285901 [Google Scholar]
- Sol D, Stirling G.D, Lefebvre L. Behavioral drive or behavioral inhibition in evolution: subspecific diversification in holarctic passerines. Evolution. 2005;59:2669–2677. [PubMed] [Google Scholar]
- Stamps J.A, Krishnan V.V. Functions of fights in territory establishment. Am. Nat. 1997;150:393–405. doi: 10.1086/286071. doi:10.1086/286071 [DOI] [PubMed] [Google Scholar]
- Thomas A.L.R. On the tail of birds. Bioscience. 1997;47:215–225. [Google Scholar]
- Wake D.B, Roth G, Wake M.H. On the problem of stasis in organismal evolution. J. Theor. Biol. 1983;101:211–224. doi:10.1016/0022-5193(83)90335-1 [Google Scholar]
- Watson A, Moss R, Parr R, Mountford M.D, Rothery P. Kin landownership, differential aggression between kin and non-kin, and population fluctuations in red grouse. J. Anim. Ecol. 1994;63:39–50. [Google Scholar]
- Wcislo W.T. Behavioral environments and evolutionary change. Annu. Rev. Ecol. Syst. 1989;20:137–169. doi:10.1146/annurev.es.20.110189.001033 [Google Scholar]
- West-Eberhard M. Oxford University Press; New York, NY: 2003. Developmental plasticity and evolution. [Google Scholar]