Abstract
Females of many species can gain benefits from being choosy about their mates and even exhibit context-dependent investment in reproduction in response to the quality of their breeding situation. Here, we show that if a male house wren is provided with surplus nest boxes in his territory, his mate lays a larger clutch with a significantly higher proportion of sons. This response to a territory characteristic directly associated with male competitive ability, and ultimately to male reproductive success, suggests that male competition over access to high-quality territories with surplus nest boxes (i.e. those able to support polygyny) may influence female reproductive investment decisions. The results of this study have interesting implications, particularly considering the important role that studies of cavity nesting birds utilizing nest boxes have played in advancing our understanding of behaviour, ecology and evolution.
Keywords: clutch size, differential allocation, offspring sex ratio, parental investment, sexual selection, Troglodytes aedon
1. Introduction
The great statistician and population geneticist R. A. Fisher famously took up a challenge from Darwin and used informal game theoretic ideas to develop the logic for why a population's sex ratio should be near 50 : 50 (Fisher 1930). Fisher's principle of equal allocation postulated that, when the costs of producing males and females were equal, frequency-dependent selection would provide greater fitness benefits to individuals producing the minority sex, thus favouring equal allocation to the production of sons and daughters (Fisher 1930). In modern game-theoretic terms, Fisher's principle predicts a population level evolutionarily stable strategy of a 50 : 50 sex ratio. A universal strategy of equal investment in the sexes by all parents is one way to achieve this equilibrium. Fisher's principle of equal allocation at the population level did not explicitly consider the possibility of a mixed strategy, with adaptive differences in investment in the sexes by different parents within a population. Modern evolutionary theory predicts adaptive deviations from equal parental investment in the two sexes of offspring, both within and between populations, when the fitness returns of sons and daughters differ (Hamilton 1967; Trivers & Willard 1973; Williams 1979; Bull & Charnov 1988; Wade et al. 2003). There is a growing empirical literature documenting adaptive adjustments to the offspring sex ratio by individuals within populations in various taxa, especially birds (Appleby et al. 1997; Komdeur et al. 1997; Badyaev et al. 2002), but also mammals (Sheldon & West 2004; Krüger et al. 2005), possibly including humans (Catalano 2003; Grant & Yang 2003).
The most influential theory for adaptive offspring sex ratios within a population (e.g. brood sex ratios) is the Trivers–Willard hypothesis that natural selection can favour systematic individual deviations from equal parental investment in the two sexes under certain well-defined conditions that should cancel out, on average, over the local breeding population (Trivers & Willard 1973). Trivers & Willard argued that selection would favour a conditional strategy of sex ratio allocation when the opportunity costs of investing in sons and daughters varied with parental condition. They framed their hypothesis in terms of polygynous species, where males have higher variance in reproductive success than females, but the reverse pattern should and apparently does hold when the marginal benefits of investing in daughters are higher (Andersson et al. 2003).
In terms of an individual's offspring sex ratio, deviations from parity should be adaptive in any situation in which parents can increase inclusive fitness by biasing offspring sex ratios (reviewed in Cockburn et al. 2002), for instance when more attractive fathers tend to produce sexier or healthier sons. Fisher focused on differential costs of offspring production (the cost of reproduction hypothesis: Myers 1978) and Trivers–Willard focused on differential benefits. Alternative adaptive hypotheses exist, e.g. in cases of sex-specific natal philopatry, females may adjust sex ratio in response to future costs of competition or benefits of helpers (local resource competition, Clark 1978; local resource enhancement, Emlen et al. 1986). Among birds, females appear to have remarkable control over characteristics of their offspring, adapting the sex ratio as well as clutch size to their own condition (Pietiainen & Kolunen 1993; Nager et al. 1999), to the quality or attractiveness of their mate (Petrie & Williams 1993; Ellegren et al. 1996; Svensson & Nillson 1996; Sheldon et al. 1999), his courtship displays (Polo et al. 2004) or territory (Komdeur et al. 1997) and to differences in environmental conditions (Badyaev et al. 2002; Suorsa et al. 2003).
House wrens (Troglodytes aedon) are small (10–12 g), migratory, secondary cavity nesters (Johnson 1998). Male house wrens arrive at our study site (Barry County, Michigan, USA) in late April. Males begin singing and placing sticks in available cavities shortly after arrival. The first females arrive several days later. After pairing, males may continue singing to advertise for secondary mates (Johnson & Kermott 1991) and supplying males with surplus nest sites may increase the frequency of polygyny (Johnson & Kermott 1991). Acquiring additional social mates, either through polygyny or sequential monogamy, increases male reproductive success (Poirier et al. 2004; Whittingham & Dunn 2005). As a result, competition among males for access to territories containing multiple nest sites should lead to a correlation between cavity availability in a male's territory and male quality or attractiveness. Thus, cavity availability might be a signal used by females to assess mate quality. Females are expected to bias offspring sex ratios towards the sex with the higher inclusive fitness returns. Should components of male quality be heritable and affect the fitness of sons more than daughters, then females paired to higher quality mates would be expected to bias offspring sex ratios towards males.
2. Material and methods
(a) Experimental manipulation
We conducted this study at the Lux Arbor Reserve, Kellogg Biological Station (42°29′ N, 85°28′ W). Lux Arbor Reserve is a 529 ha site consisting of fragmented habitats including hardwood and softwood forests, open fields, wetlands and agricultural areas. In early April of each year, before house wrens had returned to the breeding grounds, we established 53 sites. Each site contained three nest boxes (5–10 m apart), placed along forested edges wherever possible, and sites were separated by approximately 100 m. The spacing of sites allowed individual males to control access to only a single cluster of three boxes. We manipulated the availability of nest-boxes within these sites by providing males with access to either one or three nest boxes.
Prior to male arrival we left only a single nest box open at each site, plugging the entrance holes of the two adjacent nest boxes with rubber stoppers. We paired territories by male settlement date and randomly assigned territories to a single or surplus cavity treatment within each pair by removing rubber stoppers from the nest boxes in surplus cavity territories immediately after male settlement, but before females settled on male territories. Pairing treatments by settlement date and randomly assigning treatments within those pairs decoupled any potential correlations between treatment and male quality or habitat quality (other than the number of nest boxes). Therefore, we could examine the effect of cavity availability on female reproductive investment independent of differences in male characteristics or habitat characteristics not related to cavity availability. The experimental treatments were maintained through the pairing and egg-laying periods.
Although polygyny can be fairly common in populations of house wrens, particularly in populations nesting in boxes (Johnson & Kermott 1991; Poirier et al. 2004), the frequency of polygyny was relatively low at our study site (less than 5%). Since our focus was on whether the potential for polygyny affected female investment in reproductive success, we intentionally spaced nest boxes close together within territories in order to allow males to easily defend the extra nest boxes in surplus cavity territories. Mated males with surplus nest boxes in their territories appear to advertise for additional mates (N. S. Dubois, personal observation), but the close spacing of surplus boxes within territories may have allowed primary females to exclude secondary females from settling (Slagsvold & Lifjeld 1994). Two cases of polygyny occurred on territory sites included in our dataset, but in both cases, males acquired secondary females after the primary females had already completed investment in eggs. Therefore, no females are included after their status shifted from monogamous to primary female.
We manipulated ‘early season’ nests of monogamous and first-mated females initiated prior to mid-June in 2001 and 2002 (2001, n=29; 2002, n=45). Early season nests included the first nest attempts of double-brooded females and single-brooded females initiated before the start of any second brood nests. Ten females were known to have initiated first brood nests in both years. For these females, we randomly selected one nest to include in our analyses. Therefore, the sample sizes indicated include each female only once.
We identified the day that the first pieces of lining material appeared in the nest cup as a female's nest initiation date and the day that the first egg of a clutch was laid as a female's laying date. We recorded clutch size only for completed clutches. We considered a clutch complete once incubation had started and the number of eggs in the nest remained unchanged for two consecutive days. Since some nests failed between nest lining and clutch completion (e.g. due to suspected abandonment, depredation, or egg-removal by house wrens), sample sizes differ for analyses of laying date (single cavity, n=38; surplus cavity, n=34) and clutch size (single cavity, n=32; surplus cavity, n=32).
Adult females were captured opportunistically at different stages in the breeding cycle and individually marked with unique combinations of colour bands. We collected standard morphological measurements: length of exposed culmen, tarsus length (measured from behind the intertarsal joint) and unflattened wing chord. We found that mass measurements varied considerably with timing within the breeding cycle (see also Freed 1981) and did not consider them to be reliable indicators for use in estimates of female condition. Ageing by moult limits is not reliable during the breeding season (Pyle 1997); therefore, we were only able to separate returning females (at least 2 years old) from females breeding on the study site for the first time based on banding data.
(b) Genetic sexing
We collected blood samples (5–40 μl) from 12 day old nestlings for a subset of nests (single cavity, n=20; surplus cavity, n=17) included in the experimental manipulation. Samples were stored in Queen's Lysis Buffer (Seutin et al. 1991) at 4 °C. DNA was extracted using the QIAamp DNA Mini Kit (Qiagen Inc., Valencia, CA). We used a polymerase chain reaction technique to determine the sex of nestlings (Fridolfsson & Ellegren 1999), with the following PCR conditions: initial denaturation (94 °C, 2 min), followed by 35 cycles of strand denaturation (94 °C, 30 s), primer annealing (46–47 °C, 30 s) and DNA extension (72 °C, 90 s) and 5 min final extension at 72 °C. We sexed 208 nestlings from 37 nests. We validated the sexing procedure in two ways, first by correctly identifying 12 adults of known sex (six males and six females) using this protocol. Second, 13 individuals that had been sexed as nestlings returned to breed in subsequent years (a 14th adult that had been sexed as a nestling could not be positively identified due to a transcription error in record-keeping). In all 13 cases, the sex assigned through molecular analysis as a juvenile matched the sex assigned to breeding adults based on behaviour and morphological characteristics in the field.
(c) Analyses
We used standard non-parametric tests for statistical analyses of clutch size and laying date (Sokal & Rohlf 1995). For analyses of laying date, the first egg of each year was considered lay date 0. MANOVA was used to analyse female size data (PROC GLM with MANOVA statement in SAS v. 8.1). Logistic regression analysis of sex ratios was conducted using generalized linear models (PROC GENMOD, type 3 test statistics) with a logit link function and binomial errors (Wilson & Hardy 2002). We used number of male nestlings as the dependent variable and the total number of sexed nestlings in the brood as the binomial denominator. We assessed model fit by looking at the residual deviance, which is distributed asymptotically as χ2 and checked standardized residuals for normality. The data were slightly underdispersed (residual mean deviance=0.73), which leads to conservative tests (Wilson & Hardy 2002). Therefore, we scaled the variance by s (Pearson's χ2 divided by the residual d.f.). We report offspring sex ratios as proportion of males. These means are back transformed from the parameters fitted during statistical analysis.
We assessed deviations of the population brood sex ratio from parity using a replicated goodness-of-fit test (Sokal & Rohlf 1995). In order to calculate the G statistic for this test, we had to exclude one single-box territory nest that contained no males and three females. The excluded nest was an incompletely sexed brood (only three nestlings out of seven eggs survived to day 12) and its removal had little effect on the average offspring sex ratio for single-box territories.
When there were no significant year or year×treatment effects, we combined data from both years and present models including only the treatment effect. Since there was a significant year effect on female size, we present the full model including main effects and interactions for that analysis. For all data, means±standard error are reported.
(d) Use of animals in research
Use of animals in research was approved by Michigan State University's All-University Committee on Animal Use and Care and was conducted under the appropriate federal and state permits.
3. Results
(a) Clutch size and offspring sex ratio
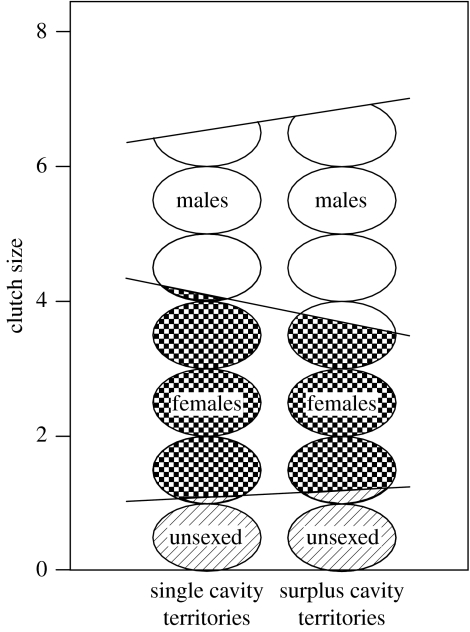
Females mated to males with surplus cavities in their territories produced larger clutches (single versus surplus: 6.5±0.10, n=32 versus 6.9±0.17, n=32; Wilcoxon Rank Sum: ts=2.3, p<0.02) and produced a higher proportion of sons (single versus surplus: 0.44±0.039, n=20 versus 0.56±0.041, n=17; GENMOD with binomial errors: F1,35=4.8, p<0.04), than females mated to males with single cavities in their territories (figure 1). Similar patterns in offspring sex ratio were observed when the analysis was limited to nests for which all hatchlings were sexed (single versus surplus: 0.46±0.046, n=16 versus 0.57±0.054, n=10; GENMOD with binomial errors: F1,24=2.5, p<0.14). At the population level (combining treatments), brood sex ratios did not differ from parity (replicated goodness-of-fit-test: GT=28.9, d.f.=37, p>0.8). Only a subset of the completed clutches was sexed, but differences in clutch sizes for the nests from which young were sexed were similar to those of the entire population (single versus surplus: 6.6±0.11, n=20 versus 7.1±0.21, n=17; Wilcoxon Rank Sum: Us=226, p<0.09).
Figure 1.
Clutch size and offspring sex ratio for females mated to males with single cavities and surplus cavities in their territories during the pairing and egg-laying stages (clutch size: single; n=32, surplus; n=32, p<0.04; offspring sex ratio as proportion of sons: single; n=20, surplus; n=17, p<0.04). Offspring sex ratios were determined for a subset of the nests used in the analysis of clutch size. The mean proportion of sons for each treatment was derived from model parameters fitted during statistical analysis of this smaller dataset. Proportions of sons (open symbols), daughters (stippled symbols) and unsexed offspring (striped symbols) are represented.
(b) Maternal effects
Returning females were not more likely to settle in surplus cavity territories than females breeding on the study site for the first time (χ12=0.12, p>0.7, single n=33, surplus n=32, nine unidentified females that lined nests were excluded from this analysis). Nor did females mated to males with surplus cavities in their territories advance laying dates (Wilcoxon Rank Sum: ts=0.57, p>0.5, single n=38, surplus n=34). There were no differences in the size of females settling in surplus versus single cavity territories (table 1, MANOVA: treatment effect, Pillai's trace value=0.09, F3,51=1.7, p>0.17; year effect, Pillai's trace value=0.27, F3,51=6.3, p<0.001; year×treatment, Pillai's trace value=0.07, F3,51=1.2, p>0.3; 17 females that lined nests were excluded from this analysis due to missing or incomplete data).
Table 1.
Morphological measurements (mean±standard error) for females settling in territories containing single and surplus cavities at the beginning of the breeding season. (MANOVA: treatment effect, p>0.17.)
| morphological variable | single cavity territories (n=29) | surplus cavity territories (n=28) |
|---|---|---|
| tarsus length (mm) | 19.7±0.11 | 19.6±0.12 |
| unflattened wing chord (mm) | 49.2±0.31 | 48.9±0.32 |
| exposed culmen length (mm) | 12.1±0.12 | 12.4±0.13 |
(c) Unsexed offspring
On average 1.1±0.26 (n=20) and 1.2±0.29 (n=17) offspring (including unhatched eggs) were unsexed in single and surplus cavity territories, respectively, but neither the occurrence of nests with at least one unhatched egg nor at least one unsexed hatchling differed between treatments (eggs: χ12<0.01, p>0.9; hatchlings: χ12=2.0, p>0.16; table 2).
Table 2.
Number of nests (and percentage within treatments) used in analysis of brood sex ratios in which one or more unhatched eggs and/or unsexed nestlings occurred. (Treatments were applied during the pairing and egg-laying stages.)
| all eggs hatched | at least one egg did not hatch | ||||
|---|---|---|---|---|---|
| treatment | all nestlings sexed | nestlings incompletely sexed | all nestlings sexed | nestlings incompletely sexed | totals |
| single cavity | 7 (35%) | 1 (5%) | 9 (45%) | 3 (15%) | 20 |
| surplus cavity | 6 (35%) | 1 (6%) | 4 (24%) | 6 (35%) | 17 |
4. Discussion
Burley (1986) proposed that females that acquire more attractive mates in a particular mating attempt might increase investment in reproduction (the differential allocation hypothesis), thereby maximizing lifetime fitness. Attractive mates might provide genetic benefits (Norris 1993; Sheldon et al. 1997; Møller & Alatalo 1999) and/or direct benefits such as parental care (Hill 1991; Voltura et al. 2002; Siefferman & Hill 2003), thus increasing the reproductive value and possibly decreasing the production cost of offspring produced from that breeding attempt. Females mated to attractive males have been shown to adjust reproductive allocation to produce more eggs (Petrie & Williams 1993), lay larger eggs (Cunningham & Russell 2000), deposit higher levels of testosterone in eggs (Gil et al. 1999), participate in fewer extra-pair copulations (Burley et al. 1994), as well as facultatively adjust the sex ratio of their offspring (Svensson & Nillson 1996; Sheldon et al. 1999). The observed patterns of clutch size and sex ratio adjustment in nests of female house wrens mated to males with surplus cavities in their territories suggests that females perceive these territories and/or associated males as high-quality breeding situations and invest in reproduction accordingly. The fact that similar patterns were observed in both years of the study suggests that they are not simply an artefact of sampling (Griffith et al. 2003).
Male house wrens challenge one another through chases and physical fights over the defence of territories and the nest sites within them (Kendeigh 1941). Since opportunities for polygyny are limited by the availability of suitable nest sites in a territory, intense competition over access to nest sites should lead to a correlation between male competitive ability and cavity availability within a territory under non-manipulated conditions. As a result, cavity availability could signal mate attractiveness or viability to females. Should these traits be heritable and affect the fitness of sons more than daughters, then we would expect females mated to attractive or high-quality males to produce male-biased clutches (e.g. Ellegren et al. 1996; Svensson & Nillson 1996; Sheldon et al. 1999). One possible pathway might be if sons of males with multiple cavities in their territories are more likely to have an intra-specific competitive advantage in acquiring high-quality territories themselves. In great tits (Parus major), where male size is related to resource holding potential, females bias sex ratios towards sons when mated to larger males (Kölliker et al. 1999).
Both the observed increase in clutch size and bias in offspring sex ratio towards males are consistent with differential allocation by females mated to males with surplus cavities in their territories, but an alternative possibility is that clutch size and offspring sex ratio differed between treatments because older females or females in better condition had preferential access to territories containing surplus cavities (the differential access hypothesis, Burley 1986). These alternatives may be confounded, even in experimental manipulations, if treatments are not randomly applied across females as well as males (Sheldon 2000). Studies on house wrens have documented adjustment of offspring sex ratios in response to maternal condition (Whittingham et al. 2002), hatch order (Albrecht 2000, but see Johnson et al. 2005), and mating status (Albrecht & Johnson 2002), with females biasing offspring sex ratios towards daughters when fewer resources are available to allocate to offspring. While we cannot rule out the possibility that females mated to males with surplus cavity territories were in better condition or of higher quality, our data do not seem to be consistent with this hypothesis. Differential access (sorting) would lead us to expect returning females (which were at least 2 years old) to settle disproportionately on territories with surplus nest boxes. We have no evidence for this. Nor did females mated to males with surplus cavities in their territories have earlier laying dates, which would have been expected if they were in better condition. In house wrens, female condition effects on offspring sex ratio may be more strongly manifested in second broods (Whittingham et al. 2002), which were not examined in this study.
Whether differences in reproductive investment occur before pairing (differential access) or after pairing (differential allocation), our findings suggest that the availability of surplus cavities in male house wren territories affects maternal investment. Females responded to the presence of surplus cavities, either as a signal of male quality, or possibly to the resource of additional nest sites itself. Plissner & Gowaty (1995) found that primarily monogamous Eastern bluebirds (Sialia sialis) were attracted to multi-box territories before the onset of breeding activity, but there was no effect of surplus boxes on reproductive success. We cannot exclude the possibility that females might directly benefit from surplus nest boxes if surplus boxes provide opportunities to re-nest more quickly in the event of nest failure, but we suggest that such a benefit would be more likely to act directly on female reproductive success in a given breeding season rather than the inclusive fitness of sons and daughters produced in that territory.
In polygynous species, additional nest sites in a territory increase the potential for polygyny. Many studies have demonstrated costs of polygyny for females, such as reduced paternal assistance and lower reproductive success (e.g. Lifjeld & Slagsvold 1989; Pinxten & Eens 1994; Kempenaers 1995; Sandell et al. 1996). Even prior to the acquisition of a secondary female, a first-mated female may face reduced paternal care from males attempting to attract additional mates (Smith 1995). Male parental care during the nestling period can affect female reproductive success in house wrens (Johnson et al. 1992), but costs of polygyny appear to affect the reproductive success of secondary females (Johnson et al. 1993) much more strongly than primary females (Czapka & Johnson 2000). Therefore, for first-mated females, benefits of mating with males with surplus nest boxes in their territories may outweigh any potential costs.
In the cooperatively breeding Seychelles warbler (Acrocephalus sechellensis), females produce the helping sex (females) in high resource territories where helping increases parental reproductive success, but produce the dispersing sex (males) in low resource territories where philopatric daughters would compete with parents for resources (Komdeur et al. 1997). One might imagine that female wrens residing in surplus cavity territories perceived reduced nest site competition and therefore increased investment in the philopatric sex. However, it seems unlikely that local resource competition (Clark 1978; Gowaty 1993) accounts for the observed biases in wren offspring sex ratio. In house wrens, natal return rates are low (generally less than 5% in an Illinois population, Drilling & Thompson 1988; similar rates on our study site, N. S. Dubois unpublished work) with small differences between male and female offspring (approx. 60% of natal returns were male, N. S. Dubois unpublished work). Moreover, the empty nest boxes in three-box territories were defended, and therefore would not be available for additional males (but they would be available for females).
The question remains as to whether the offspring sex ratios we observed on brood day twelve reflect female investment in sex ratios at laying and/or hatching. Overall, mortality of hatched young was low (less than 5%), and limiting our analyses to nests from which all hatchlings were sexed produced the same patterns in offspring sex ratio between treatments. On average, size differences between male and female offspring within a brood are small (less than 3.1%, N. S. Dubois unpublished work). Therefore, it seems unlikely that the observed differences in offspring sex ratios were due to sex-biased nestling mortality. However, the sex bias could have occurred at laying or might have resulted from differential hatch success. Occurrences of hatch failure were similar across treatments, suggesting that the bias in offspring sex ratios may reflect differences at laying.
We suggest that the observed differences in offspring sex ratio are due to facultative adjustment of offspring sex ratios by females in response to differences in the reproductive value of sons and daughters under different breeding conditions. These differences in offspring sex ratio among females are apparent at the individual level, but are masked by the population offspring sex ratio. The results of this study demonstrate that territory characteristics such as surplus cavity availability can influence reproductive investment in house wrens, both in terms of the number and sex of offspring produced. The results of this study have interesting implications for nest-box studies, particularly those utilizing high densities of nest-boxes for polygynous species. Close spacing of nest boxes probably increases the occurrence of multi-cavity territories compared to natural cavities and might have consequences on female reproductive investment, including the number and sex ratio of offspring produced.
Acknowledgments
The authors thank T. Price and L. Whittingham for comments on an earlier draft of the manuscript, as well as three anonymous reviewers. J. Glazer, T. Grattan, C. Noud, T. Polosky and E. Zontek provided help in the field. N.S.D. gratefully acknowledges support from a NSF Graduate Research Fellowship, MSU Distinguished Fellowship, KBS Research Training Grant funded by NSF (DBI-9602252), Sigma Xi Grants-In-Aid-of-Research, Lauff Research Award, Wallace Award and the Departments of Zoology and EEBB at MSU. This is Kellogg Biological Station contribution number 1212.
References
- Albrecht D.J. Sex ratio manipulation within broods of house wrens, Troglodytes aedon. Anim. Behav. 2000;59:1227–1234. doi: 10.1006/anbe.1999.1420. doi:10.1006/anbe.1999.1420 [DOI] [PubMed] [Google Scholar]
- Albrecht D.J, Johnson L.S. Manipulation of offspring sex ratio by second-mated female house wrens. Proc. R. Soc. B. 2002;269:461–465. doi: 10.1098/rspb.2001.1914. doi:10.1098/rspb.2001.1914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson M, Wallander J, Oring L, Akst E, Reed J.M, Fleischer R.C. Adaptive seasonal trend in brood sex ratio: test in two sister species with contrasting breeding systems. J. Evol. Biol. 2003;16:510–515. doi: 10.1046/j.1420-9101.2003.00533.x. doi:10.1046/j.1420-9101.2003.00533.x [DOI] [PubMed] [Google Scholar]
- Appleby B.M, Petty S.J, Blakey J.K, Rainey P, MacDonald D.W. Does variation of sex ratio enhance reproductive success of offspring in tawny owls (Strix aluco)? Proc. R. Soc. B. 1997;264:1111–1116. doi:10.1098/rspb.1997.0153 [Google Scholar]
- Badyaev A.V, Hill G.E, Whittingham L.A. Population consequences of maternal effects: sex-bias in egg-laying order facilitates divergence in sexual dimorphism between bird populations. J. Evol. Biol. 2002;15:997–1003. doi:10.1046/j.1420-9101.2002.00462.x [Google Scholar]
- Bull J.J, Charnov E.L. How fundamental are Fisherian sex ratios? Oxford Surv. Evol. Biol. 1988;5:96–135. [Google Scholar]
- Burley N. Sexual selection for aesthetic traits in species with biparental care. Am. Nat. 1986;127:415–445. doi:10.1086/284493 [Google Scholar]
- Burley N.T, Enstrom D.A, Chitwood L. Extra-pair relations in zebra finches: differential male success results from female tactics. Anim. Behav. 1994;48:1031–1041. doi:10.1006/anbe.1994.1336 [Google Scholar]
- Catalano R.A. Sex ratios in the two Germanies: a test of the economic stress hypothesis. Hum. Reprod. 2003;18:1972–1975. doi: 10.1093/humrep/deg370. doi:10.1093/humrep/deg370 [DOI] [PubMed] [Google Scholar]
- Clark A.B. Sex ratio and local resource competition in a prosimian primate. Science. 1978;201:163–165. doi: 10.1126/science.201.4351.163. [DOI] [PubMed] [Google Scholar]
- Cockburn A, Legge S, Double M.C. Sex ratios in birds and mammals: can the hypotheses be disentangled? In: Hardy I.C.W, editor. Sex ratios: concepts and research methods. Cambridge University Press; Cambridge, UK: 2002. pp. 266–286. [Google Scholar]
- Cunningham E.J.A, Russell A.F. Egg investment is influenced by male attractiveness in the mallard. Nature. 2000;404:74–77. doi: 10.1038/35003565. doi:10.1038/35003565 [DOI] [PubMed] [Google Scholar]
- Czapka S.J, Johnson L.S. Consequences of mate sharing for first-mated females in a polygynous songbird, the house wren. Wilson Bull. 2000;112:72–81. [Google Scholar]
- Drilling N.E, Thompson C.F. Natal and breeding dispersal in house wrens (Troglodytes aedon) Auk. 1988;105:480–491. [Google Scholar]
- Ellegren H, Gustafsson L, Sheldon B.C. Sex ratio adjustment in relation to paternal attractiveness in a wild bird population. Proc. Natl Acad. Sci. USA. 1996;93:11 723–11 728. doi: 10.1073/pnas.93.21.11723. doi:10.1073/pnas.93.21.11723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emlen S.T, Emlen J.M, Levin S.A. Sex-ratio selection in species with helpers-at-the-nest. Am. Nat. 1986;127:1–8. doi: 10.1086/303148. doi:10.1086/284463 [DOI] [PubMed] [Google Scholar]
- Fisher R.A. Clarendon; Oxford: 1930. The genetical theory of natural selection. [Google Scholar]
- Freed L.A. Loss of mass in breeding wrens: stress or adaptation? Ecology. 1981;62:1179–1186. [Google Scholar]
- Fridolfsson A.-K, Ellegren H. A simple and universal method for molecular sexing of non-ratite birds. J. Avian Biol. 1999;30:116–121. [Google Scholar]
- Gil D, Graves J, Hazon N, Wells A. Male attractiveness and differential testosterone investment in zebra finch eggs. Science. 1999;286:126–128. doi: 10.1126/science.286.5437.126. doi:10.1126/science.286.5437.126 [DOI] [PubMed] [Google Scholar]
- Gowaty P.A. Differential dispersal, local resource competition, and sex ratio variation in birds. Am. Nat. 1993;141:263–280. doi: 10.1086/285472. doi:10.1086/285472 [DOI] [PubMed] [Google Scholar]
- Grant V.J, Yang S. Achieving women and declining sex ratios. Hum. Biol. 2003;75:917–927. doi: 10.1353/hub.2004.0005. [DOI] [PubMed] [Google Scholar]
- Griffith S.C, Örnborg J, Russell A.F, Andersson S, Sheldon B.C. Correlations between ultraviolet coloration, overwinter survival and offspring sex ratio in the blue tit. J. Evol. Biol. 2003;16:1045–1054. doi: 10.1046/j.1420-9101.2003.00550.x. doi:10.1046/j.1420-9101.2003.00550.x [DOI] [PubMed] [Google Scholar]
- Hamilton W.D. Extraordinary sex ratios. Science. 1967;156:477–488. doi: 10.1126/science.156.3774.477. [DOI] [PubMed] [Google Scholar]
- Hill G.E. Plumage coloration is a sexually selected indicator of male quality. Nature. 1991;350:337–339. doi:10.1038/350337a0 [Google Scholar]
- Johnson L.S. House wren (Troglodytes aedon) In: Poole A, Gill F, editors. The birds of North America. Vol. 380. The Birds of North America, Inc; Philadelphia, PA: 1998. [Google Scholar]
- Johnson L.S, Kermott L.H. Effect of nest-site supplementation on polygynous behavior in the house wren (Troglodytes aedon) Condor. 1991;93:784–787. [Google Scholar]
- Johnson L.S, Merkle M.S, Kermott L.H. Experimental evidence for importance of male parental care in monogamous house wrens. Auk. 1992;109:662–664. [Google Scholar]
- Johnson L.S, Kermott L.H, Lein M.R. The cost of polygyny in the house wren Troglodytes aedon. J. Anim. Ecol. 1993;62:669–682. [Google Scholar]
- Johnson L.S, Wimmers L.E, Johnson B.G, Milkie R.C, Molinaro R.L, Gallagher B.S, Masters B.S. Sex manipulation within broods of house wrens? A second look. Anim. Behav. 2005;70:1323–1329. doi:10.1016/j.anbehav.2005.03.021 [Google Scholar]
- Kempenaers B. Polygyny in the blue tit: intra- and inter-sexual conflicts. Anim. Behav. 1995;49:1047–1064. doi:10.1006/anbe.1995.0134 [Google Scholar]
- Kendeigh S.C. Territorial and mating behavior of the house wren. Illinois Biol. Monogr. 1941;18:1–120. [Google Scholar]
- Kölliker M, Heeb P, Werner I, Mateman A.C, Lessells C.M, Richner H. Offspring sex ratio is related to male body size in the great tit (Parus major) Behav. Ecol. 1999;10:68–72. doi:10.1093/beheco/10.1.68 [Google Scholar]
- Komdeur J, Daan S, Tinbergen J, Mateman C. Extreme adaptive modification in sex ratio of the Seychelles warbler's eggs. Nature. 1997;385:522–525. doi:10.1038/385522a0 [Google Scholar]
- Krüger O, Radford A.N, Anderson C, Liversidge R. Successful sons or superior daughters: sex-ratio variation in springbok. Proc. R. Soc. B. 2005;272:375–381. doi: 10.1098/rspb.2004.2943. doi:10.1098/rspb.2004.2943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lifjeld J.T, Slagsvold T. Allocation of parental investment by polygynous pied flycatcher males. Ornis Fennica. 1989;66:3–14. [Google Scholar]
- Møller A.P, Alatalo R.V. Good-genes effects in sexual selection. Proc. R. Soc. B. 1999;266:85–91. doi:10.1098/rspb.1999.0607 [Google Scholar]
- Myers J.H. Sex ratio adjustment under food stress: maximization of quality or numbers of offspring? Am. Nat. 1978;112:381–388. doi:10.1086/283280 [Google Scholar]
- Nager R.G, Monaghan P, Griffiths R, Houston D.C, Dawson R. Experimental demonstration that offspring sex ratio varies with maternal condition. Proc. Natl Acad. Sci. USA. 1999;96:570–573. doi: 10.1073/pnas.96.2.570. doi:10.1073/pnas.96.2.570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris K. Heritable variation in a plumage indicator of viability in male great tits Parus major. Nature. 1993;362:537–539. doi:10.1038/362537a0 [Google Scholar]
- Petrie M, Williams A. Peahens lay more eggs for peacocks with larger trains. Proc. R. Soc. B. 1993;251:127–131. [Google Scholar]
- Pietiainen H, Kolunen H. Female body condition and breeding of the Ural owl Strix uralensis. Funct. Ecol. 1993;7:726–735. [Google Scholar]
- Pinxten R, Eens M. Male feeding of nestlings in the facultatively polygynous European starling: allocation patterns and effect on female reproductive success. Behaviour. 1994;129:113–140. [Google Scholar]
- Plissner J.H, Gowaty P.A. Eastern bluebirds are attracted to two-box sites. Wilson Bull. 1995;107:289–295. [Google Scholar]
- Poirier N.E, Whittingham L.A, Dunn P.O. Males achieve greater reproductive success through multiple broods than through extrapair mating in house wrens. Anim. Behav. 2004;67:1109–1116. doi:10.1016/j.anbehav.2003.06.020 [Google Scholar]
- Polo V, Viega J.P, Cordero P.J, Viñuela J, Monaghan P. Female starlings adjust primary sex ratio in response to aromatic plants in the nest. Proc. R. Soc. B. 2004;271:1929–1933. doi: 10.1098/rspb.2004.2801. doi:10.1098/rspb.2004.2801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyle P. Slate Creek Press; Bolinas, CA: 1997. Identification guide to North American birds: part I Columbidae to Ploceidae. [Google Scholar]
- Sandell M.I, Smith H.G, Bruun M. Paternal care in the European starling, Sturnus vulgaris: nestling provisioning. Behav. Ecol. Sociobiol. 1996;39:301–309. doi:10.1007/s002650050293 [Google Scholar]
- Seutin G, White B.N, Boag P.T. Preservation of avian blood and tissue samples for DNA analyses. Can. J. Zool. 1991;69:82–90. [Google Scholar]
- Sheldon B.C. Differential allocation: tests, mechanisms and implications. Trends Ecol. Evol. 2000;15:397–402. doi: 10.1016/s0169-5347(00)01953-4. doi:10.1016/S0169-5347(00)01953-4 [DOI] [PubMed] [Google Scholar]
- Sheldon B.C, West S.A. Maternal dominance, maternal condition, and offspring sex ratio in ungulate mammals. Am. Nat. 2004;163:40–54. doi: 10.1086/381003. doi:10.1086/381003 [DOI] [PubMed] [Google Scholar]
- Sheldon B.C, Merilä J, Qvarnström A, Gustafsson L, Ellegren H. Paternal genetic contribution to offspring condition predicted by size of male secondary sexual character. Proc. R. Soc. B. 1997;264:297–302. doi:10.1098/rspb.1997.0042 [Google Scholar]
- Sheldon B.C, Andersson S, Griffith S.C, Örnborg J, Sendecka J. Ultraviolet colour variation influences blue tit sex ratios. Nature. 1999;402:874–877. doi:10.1038/47239 [Google Scholar]
- Siefferman L, Hill G.E. Structural and melanin coloration indicate parental effort and reproductive success in male eastern bluebirds. Behav. Ecol. 2003;14:885–861. doi:10.1093/beheco/arg063 [Google Scholar]
- Slagsvold T, Lifjeld J.T. Polygyny in birds: the role of competition between females for male parental care. Am. Nat. 1994;143:59–94. doi:10.1086/285596 [Google Scholar]
- Smith H.G. Experimental demonstration of a trade-off between mate attraction and paternal care. Proc. R. Soc. B. 1995;260:45–51. [Google Scholar]
- Sokal R.R, Rohlf F.J. 3rd edn. W.H. Freeman and Co; New York: 1995. Biometry: the principles and practice of statistics in biological research. [Google Scholar]
- Suorsa P, Helle H, Huhta E, Jäntti A, Nikula A, Hakkarainen H. Forest fragmentation is associated with primary brood sex ratio in the treecreeper (Certhia familiaris) Proc. R. Soc. B. 2003;270:2215–2222. doi: 10.1098/rspb.2003.2490. doi:10.1098/rspb.2003.2490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson E, Nillson J.-Å. Mate quality affects offspring sex ratio in blue tits. Proc. R. Soc. B. 1996;263:357–361. [Google Scholar]
- Trivers R.L, Willard D.E. Natural selection of parental ability to vary the sex ratio of offspring. Science. 1973;179:90–92. doi: 10.1126/science.179.4068.90. [DOI] [PubMed] [Google Scholar]
- Voltura K.M, Schwagmeyer P.L, Mock D.W. Parental feeding rates in the house sparrow, Passer domesticus: are larger-badged males better fathers? Ethology. 2002;108:1011–1022. doi:10.1046/j.1439-0310.2002.00831.x [Google Scholar]
- Wade M.J, Shuster S.M, Demuth J.P. Sexual selection favors female-biased sex ratios: the balance between the opposing forces of sex-ratio selection and sexual selection. Am. Nat. 2003;162:403–414. doi: 10.1086/378211. doi:10.1086/378211 [DOI] [PubMed] [Google Scholar]
- Whittingham L.A, Dunn P.O. Effects of extra-pair and within-pair reproductive success on the opportunity for selection in birds. Behav. Ecol. 2005;16:138–144. doi:10.1093/beheco/arh140 [Google Scholar]
- Whittingham L.A, Valkenaar S.M, Poirier N.E, Dunn P.O. Maternal condition and nestling sex ratio in house wrens. Auk. 2002;119:125–131. [Google Scholar]
- Williams G.C. The question of adaptive sex ratio in outcrossed vertebrates. Proc. R. Soc. B. 1979;205:567–580. doi: 10.1098/rspb.1979.0085. [DOI] [PubMed] [Google Scholar]
- Wilson K, Hardy I.C.W. Statistical analysis of sex ratios: an introduction. In: Hardy I.C.W, editor. Sex ratios: concept and research methods. Cambridge University Press; Cambridge, UK: 2002. pp. 48–92. [Google Scholar]