Abstract
The evolution of optimal functioning and maintenance of the immune system is thought to be driven by the costs arising from the allocation of resources to immune functions rather than to growth and reproduction and by the benefits arising from higher defence if an infection occurs. In young animals there is a high premium for fast growth and competitiveness and a parasite-mediated trade-off is thus predicted between the allocation of resources to growth versus immune function. In a field study on nestling great tits (Parus major), we manipulated simultaneously the level of immune defence by a dietary supplementation of the immunostimulant methionine and ectoparasite (Ceratophyllus gallinae) abundance in the nest and thereby assessed both the costs and benefits of investing in immune defence. Nestlings supplemented with methionine grew slower during the experimental boost of their immune system compared to controls. Thereafter, however, nestlings with a boosted immune system grew at faster rates under parasite pressure compared to unstimulated birds. It experimentally shows the costs and benefits of investment in immunity and suggests that the evolution of optimum host defence is governed by a parasite-mediated allocation trade-off between growth and immune function.
Keywords: cost of immunity, ecological immunology, host–parasite interactions, life history, Parus major, trade-off
1. Introduction
Although the immune system of animals is a magnificent instrument for the defence against parasites, it is not perfect and considerable variation in immune system functioning is observed among individuals (Zuk & Stoehr 2002). It has been suggested that both the investment in an effective immune defence machinery and/or its use are costly (e.g. Martin et al. 2003; reviewed in Sheldon & Verhulst 1996; Norris & Evans 2000). Under natural conditions with limited access to energy and essential metabolites a higher investment in immune defence thus goes at the expense of investment in other traits such as growth (Soler et al. 2003; Brommer 2004), predator avoidance (Rigby & Jokela 2000) or sexual signalling and reproduction (e.g. Gustafsson et al. 1994; Nordling et al. 1998; Siva-Jothy et al. 1998; Veiga et al. 1998; Moreno et al. 1999; Ilmonen et al. 2000; Bonneaud et al. 2003; Jacot et al. 2004; Ahtiainen et al. 2005). Inherently the optimal solution for the resulting allocation trade-off (Sheldon & Verhulst 1996; Lochmiller & Deerenberg 2000; Norris & Evans 2000) is influenced by environmental factors, for example the risk of parasitism (Saino et al. 1998; Szép & Møller 1999; Merino et al. 2000).
Showing the costs and benefits of investment in immunity as well as the optimal levels of resource allocation into immune functions is problematic in a non-experimental set-up due to the condition-dependence of both fitness and immunocompetence (Sheldon & Verhulst 1996; Norris & Evans 2000). A direct, experimental manipulation of an individual's investment in immune defence is thus required. This might be either achieved by the use of lines artificially selected for high or low immunocompetence (e.g. Verhulst et al. 1999) or by supplementing individuals with immuno-enhancing drugs (Dietert et al. 1996).
Nestling passerines are a good model system to experimentally investigate costs and benefits of differential investment in immunity by the latter method. They grow at fast rates and body mass at fledging correlates with both survival during the post-fledging period and recruitment to the breeding population (Tinbergen & Boerlijst 1990; Lindèn et al. 1992; Both et al. 1999), implying high selective advantages of increased resource allocation to growth. However, early in life, young birds are also particularly vulnerable to parasites, due to both their limited mobility and their relatively naive immune system (Møller 1997). Investment in immunological defence that reduces the impact of parasites might thus pay when a considerable risk of infection is anticipated (Wakelin 1996; Wikel et al. 1996). Given the limited access to energy and nutrition under natural conditions, a trade-off between the investment into growth versus immune defence is thus predicted. This trade-off can be experimentally manipulated by supplementing nestling birds with methionine, a sulphur amino acid that enhances T-cell-functioning (Tsiagbe et al. 1987; Grimble & Grimble 1998). Methionine is routinely used in poultry research to increase general performance, immunocompetence and resistance against infection (Grimble & Grimble 1998), and has recently been used in two ecological field studies on magpies (Soler et al. 2003) and blue tits (Brommer 2004) to experimentally manipulate individual investment in immune defence. However, while Soler et al. (2003) and Brommer (2004) mainly concentrated on the experimental demonstration of the cost of immunity in terms of reduced growth or higher mortality, the aim of the present study was to assess both the costs and the benefits of an increased investment in immune defence in the presence and absence of parasites and thereby to explore the role of parasites in shaping the optimal solution of the investment trade-off between growth and immunity. We thus simultaneously manipulated the investment in immune function of great tit nestlings (Parus major) by an oral supplementation of an age-dependent dose of methionine and the load of their most common ectoparasite, the hen flea (Ceratophyllus gallinae) in a 2×2 design. We predict that an increased investment in immune defence is costly in terms of reduced growth rates, but that this investment pays if growing-up in the presence of parasites.
2. Material and methods
(a) Study species
The study was performed in 2004 in a population of great tits breeding in nest boxes in a mixed forest near Bern, Switzerland. The great tit is a small (16–20 g) hole-nesting passerine that produces one or two broods per year. It is one of the main hosts of the ectoparasitic hen flea (Tripet & Richner 1997). Hen fleas are nest-based parasites that suck blood from nestlings and adults and impair the fitness of their host by affecting growth, survival and future reproduction (Richner et al. 1993; Fitze et al. 2004).
(b) Experimental protocol
One day after hatching of the nestlings we heat-treated all nests in a microwave oven to kill naturally present parasites (Richner et al. 1993). Three days later, half the nests (n=38 broods) were experimentally infested by 40 female and 20 male hen fleas whereas the other half (n=37 broods) remained uninfested. The hen fleas used for the experimental infestation of the nests were obtained from old nest material collected at the beginning of the breeding season in the same forest.
Within each nest, nestlings were randomly assigned to the immune boosted (n=233 nestlings) or the control (n=254 nestlings) group. Immune boosted nestlings were supplemented with an age-dependent dose of methionine (dl-methionine, Sigma Chemicals, Germany) suspended in tap water (0.1 g ml−1). They received 100 μl of methionine suspension on day 4, 150 μl on day 5, 200 μl on day 6 and 7 and 250 μl on day 8 post-hatching, while the control nestlings received an equal quantity of tap water each day (Brommer 2004). Methionine is a sulphur amino acid that is required in considerable amounts during immune defence. Methionine demands are covered by both endogenous production and dietary ingestion. Insufficient sulphur amino acid supply compromises glutathione synthesis, resulting in impaired T-cell production and functioning (Grimble & Grimble 1998). Methionine is routinely provided in poultry farming to improve general performance and resistance against infection (Tsiagbe et al. 1987).
(c) Nestling growth
We measured the body mass gain of the nestlings (i) during the experimental boost of the immune system early in the nestlings' life (i.e. during the period of methionine supplementation) to assess the costs of an increased investment in immune defence and (ii) in the second half of the nestling period (i.e. after the nestlings had time to differentially invest in their immune defence in relation to their methionine treatment until shortly before fledging), to assess the benefits of their investment in immunity in relation to the manipulated parasite load in the nest. We therefore measured the body mass on day 4, before the start of the experimental boost of the immune system, on day 11, after the experimental manipulation of the nestlings' investment in immune defence had stopped and a third time on day 15 post-hatching, shortly before fledgling, using an electronic scale with a precision of 0.01 g. Growth early and late in the nestling period was calculated as the body mass gain from day 4 to day 11 and the body mass gain from day 11 to day 15, respectively. Nestling body mass was not significantly different between treatment groups at day 4 post-hatching, i.e. before the start of the methionine supplementation and parasite manipulation (nested ANOVA, brood: F73,410=0.819, p=0.851; parasite treatment: F1,73=1.302, p=0.258; methionine supplementation: F1,410=1.346, p=0.247; parasite treatment×methionine supplementation: F1,410=0.448, p=0.504). All nestlings were marked individually by clipping dorsal tufts on day 4 and were ringed with numbered aluminium rings on day 11.
(d) Immunocompetence
The T-cell-mediated immune response of the nestlings was assessed by a hypersensitivity assay using the lectin phytohaemagglutinin (PHA-P, Sigma Chemicals, Germany). The PHA assay provides a measure of the proliferative response of the circulating T lymphocytes to the injected mitogen (Goto et al. 1978; McCorkle et al. 1980; Cheng & Lamont 1988). Nestlings were injected subcutaneously with 0.1 mg of PHA-P dissolved in 0.02 ml of sterile phosphate-buffered saline in the centre of the left wing-web (patagium) 13 days post-hatching. The thickness of the patagium at the injection site was measured with a micrometer (Mitotuyo, Type 2046FB-60) to the nearest 0.01 mm prior to and 24 h (±1 h) after injection. The thickness of the wing-web 5 s after applying the micrometer was used as a standardized measure of swelling (Tschirren et al. 2005). The difference between the wing-web thickness before and 24 h after PHA injection was used as a measure of the cell-mediated immune response (Smits et al. 1999). Repeatability of the measurement of wing-web thickness was high (r>0.9, Tschirren et al. 2003). Measuring PHA response at the end of the nestling period and not e.g. after the methionine supplementation had stopped allowed demonstration that (i) methionine supplementation stimulates the T-cell mediated immune response, and probably also other components of the immune system that were not measured in this study, and that (ii) these immunostimulating effects lasted at least until the end of the nestling period, i.e. during the full second half of the nestling period when the beneficial effects of an increased investment in immune defence in relation to parasite abundance were assessed.
(e) Analyses
Nested ANOVAs were used to analyse the effects of the parasite and the methionine treatment on immune response and growth early and late in the nestling period. Parasite treatment, methionine treatment and the interaction between the two factors were included as fixed factors into the models, and the nest, nested within parasite treatment, was included as a random effect. For the analysis of differences between immune boosted and control nestlings within a given parasite-infestation group we used individual contrasts. All tests were two-tailed with a significance level set at p≤0.05. Residuals of the models were tested for normality and homoscedasticity. Statistical analyses were performed using the JMP statistical software (Sall & Lehmann 1996). Mean values±1 s.e. are shown.
3. Results
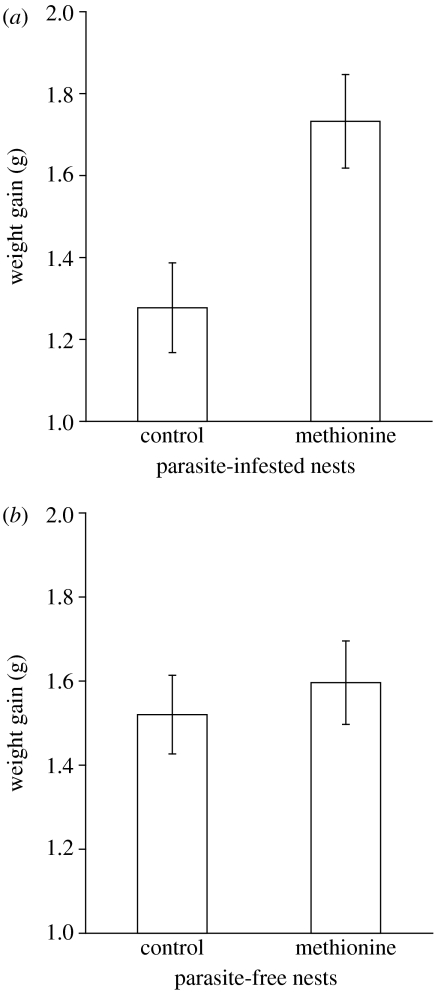
Methionine supplementation increased the nestlings' T-cell mediated immune response measured 14 days post-hatching (nested ANOVA, methionine supplementation: F1,410=4.932, p=0.027). Furthermore, during the supplementation experiment, immune boosted nestlings grew at significantly lower rates than controls independent of infection status (nested ANOVA, brood: F73,410=5.482, p<0.001; parasite treatment: F1,73=0.412, p=0.523; methionine supplementation: F1,410=61.969, p<0.001; parasite treatment×methionine supplementation: F1,410=0.008, p=0.931, figure 1a,b).
Figure 1.
Costs of increased investment in immune defence. Weight gain was lower in immune boosted nestlings compared to control nestlings in the first part of the nestling period, i.e. during the methionine supplementation, independent of the nestlings' state of infection; (a) parasite-infested nests, (b) parasite-free nests. Means±1 s.e. are shown.
After the nestlings had time to build up a functional immune defence, i.e. in the second half of the nestling period after the methionine supplementation had stopped, immune boosted nestlings grew at faster rates than controls in parasite-infested nests (figure 2a, individual contrast: F1,409=10.955, p<0.001) whereas in parasite-free nests the growth rate of immune-boosted and control nestlings did not differ significantly (figure 2b, individual contrast: F1,409=0.2685, p=0.555; nested ANOVA, brood: F73,410=4.812, p<0.001; parasite treatment: F1,73=0.072, p=0.789; methionine supplementation: F1,410=9.403, p=0.002; parasite treatment×methionine supplementation: F1,410=4.954, p=0.027).
Figure 2.
Benefits of increased investment in immune defence. In (a) parasite-infested nests immune boosted nestlings grew at significantly faster rates during the second part of the nestling period, i.e. after the methionine supplementation had stopped, compared to control nestlings. In (b) parasite-free nests, however, immune-boosted nestlings and control nestlings did not significantly differ in growth rates. Means±1 s.e. are shown.
4. Discussion
Methionine-supplemented nestlings showed a reduced body mass gain compared to control nestlings within the same nest during the experimental boost of the immune system. This result confirms previous studies on magpie and blue tit nestlings (Soler et al. 2003; Brommer 2004), which demonstrated the costs of immunity in terms of reduced growth and higher mortality by a direct manipulation of an individual's investment in immune defence. Beside the negative effect of the methionine supplementation on growth rates, methionine supplemented nestlings also showed higher responses against an injection of PHA, a measure of the T-cell mediated immune response routinely used in ecological field studies. This result makes it unlikely that the reduced growth rates of supplemented birds in our study, as well as in Soler et al. (2003) or Brommer (2004), are simply a result of detrimental effects of high methionine ingestion. The use of an experimental approach however highlights the trade-off between investment in immune defence and other life-history traits, i.e. the costs of an increased investment in immune defence in terms of reduced growth. These costs might reflect either the costs of building up powerful immune defence machinery, its use, or both. However, our experimental design does not allow for a direct separation and the discrimination between different costs will thus need further investigation. Furthermore, it is important to note that beside the T-cell mediated immune response assessed by the PHA assay, it is very likely that the supplementation of methionine also enhanced other components of the immune system, e.g. the humoral immune defence (e.g. Tsiagbe et al. 1987), which has not been assessed in our study.
To understand the evolution of optimum host defence one has to consider not only the costs but also the benefits of allocating resources into immune function (Sheldon & Verhulst 1996; Lochmiller & Deerenberg 2000; Norris & Evans 2000). In a second step, our study thus assessed the benefits of an increased investment in immune defence by manipulating the abundance of the nestlings' most common ectoparasite, the hen flea, thereby complementing previous studies by Soler et al. (2003) and Brommer (2004) that experimentally manipulated investment in immune defence to assess the costs of immunity. We found that after the experimental manipulation of the nestlings' investment in immune defence, i.e. after the methionine supplementation had stopped, immune-boosted nestlings grew at significantly faster rates in parasite-infested nests compared to controls, whereas in parasite-free nests, immune-boosted and control nestlings did not grow at significantly different rates. This result cannot be explained by compensatory growth alone because compensatory growth would lead to higher growth rates of methionine supplemented nestlings irrespective of their parasite treatment, (i.e. to a significant main effect of the methionine treatment, but a non-significant interaction effect between parasite treatment and methionine treatment). The significant interaction effect between parasite treatment and methionine supplementation thus emphasizes the beneficial effects of an increased investment in immune defence when exposed to parasites. This beneficial effect might be due to specific or unspecific immune reactions—against hen fleas and/or pathogens that are potentially transmitted by the fleas during the blood meal (Wakelin 1996; Wikel et al. 1996)—which were stimulated by the methionine supplementation (see above).
In conclusion, our study experimentally demonstrates the costs of immunity in terms of reduced growth rates, as well as the benefits of a higher investment in immune defence in the presence of parasites. This suggests that the evolution of optimum host defence is governed by a parasite-mediated allocation trade-off between growth and immune function and illustrates that the evolution of trade-offs and their optima can only be understood when both costs and benefits are measured within an organism's natural environment.
Acknowledgments
We are grateful to Verena Saladin and Linda Bischoff for field assistance, Jon Brommer for advice on the methionine supplementation, Erik Postma for support and discussion and two referees for valuable comments on the manuscript. The experiment was financially supported by the Swiss National Science Foundation (research grant no. 102017 to H.R. and a postdoctoral fellowship no. PBBEA-111206 to B.T.) and conducted under a licence provided by the Ethical Committee of the Office of Agriculture of the Canton of Bern, Switzerland.
References
- Ahtiainen J.J, Alatalo R.V, Kortet R, Rantala M.J. A trade-off between sexual signalling and immune function in a natural population of the drumming wolf spider Hygrolycosa rubrofasciata. J. Evol. Biol. 2005;18:985–991. doi: 10.1111/j.1420-9101.2005.00907.x. [DOI] [PubMed] [Google Scholar]
- Bonneaud C, Mazuc J, Gonzalez G, Haussy C, Chastel O, Faivre B, Sorci G. Assessing the cost of mounting an immune response. Am. Nat. 2003;161:367–379. doi: 10.1086/346134. doi:10.1086/346134 [DOI] [PubMed] [Google Scholar]
- Both C, Visser M.E, Verboven N. Density-dependent recruitment rates in great tits: the importance of being heavier. Proc. R. Soc. B. 1999;266:465–469. doi:10.1098/rspb.1999.0660 [Google Scholar]
- Brommer J.E. Immunocompetence and its costs during development: an experimental study in blue tit nestlings. Proc. R. Soc. B. 2004;271(Suppl. 3):S110–S113. doi: 10.1098/rsbl.2003.0103. doi:10.1098/rsbl.2003.0103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S, Lamont S.J. Genetic analysis of immunocompetence measures in a white leghorn chicken line. Poult. Sci. 1988;67:989–995. doi: 10.3382/ps.0670989. [DOI] [PubMed] [Google Scholar]
- Dietert R.R, Golemboski K.A, Kwak H, Ha R, Miller T.E. Environment-immunity interactions. In: Davison T.F, Morris T.R, Payne L.N, editors. Poultry immunology. Carfax Publishing; Oxford: 1996. pp. 343–356. [Google Scholar]
- Fitze P.S, Tschirren B, Richner H. Life history and fitness consequences of ectoparasites. J. Anim. Ecol. 2004;73:216–226. doi:10.1111/j.0021-8790.2004.00799.x [Google Scholar]
- Goto N, Kodama H, Okada K, Fujimoto Y. Suppression of phytohemagglutinin skin response in thymectomised chickens. Poult. Sci. 1978;57:246–250. doi: 10.3382/ps.0570246. [DOI] [PubMed] [Google Scholar]
- Grimble R.F, Grimble G.K. Immunonutrition: role of sulfur amino acids, related amino acids, and polyamines. Nutrition. 1998;14:605–610. doi: 10.1016/s0899-9007(98)80041-5. doi:10.1016/S0899-9007(98)80041-5 [DOI] [PubMed] [Google Scholar]
- Gustafsson L, Nordling D, Andersson M.S, Sheldon B.C, Qvarnström A. Infectious-diseases, reproductive effort and the cost of reproduction in birds. Phil. Trans. R. Soc. B. 1994;346:323–331. doi: 10.1098/rstb.1994.0149. [DOI] [PubMed] [Google Scholar]
- Ilmonen P, Taarna T, Hasselquist D. Experimentally activated immune defence in female pied flycatchers results in reduced breeding success. Proc. R. Soc. B. 2000;267:665–670. doi: 10.1098/rspb.2000.1053. doi:10.1098/rspb.2000.1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacot A, Scheuber H, Brinkhof M.W.G. Costs of an induced immune response on sexual display and longevity in field crickets. Evolution. 2004;58:2280–2286. doi: 10.1111/j.0014-3820.2004.tb01603.x. [DOI] [PubMed] [Google Scholar]
- Lindèn M, Gustafsson L, Pärt T. Selection on fledging mass in the collared flycatcher and the great tit. Ecology. 1992;73:336–343. [Google Scholar]
- Lochmiller R.L, Deerenberg C. Trade-offs in evolutionary immunology: just what is the cost of immunity? Oikos. 2000;88:87–98. doi:10.1034/j.1600-0706.2000.880110.x [Google Scholar]
- Martin L.B, Scheuerlein A, Wikelski M. Immune activity elevates energy expenditure of house sparrows: a link between direct and indirect costs? Proc. R. Soc. B. 2003;270:153–158. doi: 10.1098/rspb.2002.2185. doi:10.1098/rspb.2002.2185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCorkle F, Olah I, Glick B. The morphology of the phytohemagglutinin-induced cell response in the chicken's wattle. Poult. Sci. 1980;59:616–623. doi: 10.3382/ps.0592151. [DOI] [PubMed] [Google Scholar]
- Merino S, Møller A.P, de Lope F. Seasonal changes in cell-mediated immunocompetence and mass gain in nestling barn swallows: a parasite-mediated effect? Oikos. 2000;90:327–332. doi:10.1034/j.1600-0706.2000.900213.x [Google Scholar]
- Moreno J, Sanz J.J, Arriero E. Reproductive effort and T-lymphocyte cell-mediated immunocompetence in female pied flycatchers Ficedula hypoleuca. Proc. R. Soc. B. 1999;266:1105–1109. doi:10.1098/rspb.1999.0750 [Google Scholar]
- Møller A.P. Parasitism and the evolution of host life history. In: Clayton D.H, Moore J, editors. Host–parasite evolution: general principles and avian models. Oxford University Press; Oxford: 1997. pp. 105–127. [Google Scholar]
- Nordling D, Andersson M, Zohari S, Gustafsson L. Reproductive effort reduces specific immune response and parasite resistance. Proc. R. Soc. B. 1998;265:1291–1298. doi:10.1098/rspb.1998.0432 [Google Scholar]
- Norris K, Evans M.R. Ecological immunology: life history trade-offs and immune defence in birds. Behav. Ecol. 2000;11:19–26. doi:10.1093/beheco/11.1.19 [Google Scholar]
- Richner H, Oppliger A, Christe P. Effect of an ectoparasite on reproduction in great tits. J. Anim. Ecol. 1993;62:703–710. [Google Scholar]
- Rigby M.C, Jokela J. Predator avoidance and immune defence: costs and trade-offs in snails. Proc. R. Soc. B. 2000;267:171–176. doi: 10.1098/rspb.2000.0983. doi:10.1098/rspb.2000.0983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saino N, Calza S, Møller A.P. Effects of a dipteran ectoparasite on immune response and growth trade-offs in barn swallow, Hirundo rustica, nestlings. Oikos. 1998;81:217–228. [Google Scholar]
- Sall J, Lehmann A. Duxbury Press; New York: 1996. JMP start statistics. [Google Scholar]
- Sheldon B.C, Verhulst S. Ecological immunology: costly parasite defences and trade-offs in evolutionary ecology. Trends Ecol. Evol. 1996;11:317–321. doi: 10.1016/0169-5347(96)10039-2. doi:10.1016/0169-5347(96)10039-2 [DOI] [PubMed] [Google Scholar]
- Siva-Jothy M.T, Tsubaki Y, Hooper R.E. Decreased immune response as a proximate cost of copulation and oviposition in a damselfly. Physiol. Entomol. 1998;23:274–277. doi:10.1046/j.1365-3032.1998.233090.x [Google Scholar]
- Smits J.E, Bortolotti G.R, Tella J.L. Simplifying the phytohaemagglutinin skin-testing technique in studies of avian immunocompetence. Funct. Ecol. 1999;13:567–572. doi:10.1046/j.1365-2435.1999.00338.x [Google Scholar]
- Soler J.J, de Neve L, Perez-Contreras T, Soler M, Sorci G. Trade-off between immunocompetence and growth in magpies: an experimental study. Proc. R. Soc. B. 2003;270:241–248. doi: 10.1098/rspb.2002.2217. doi:10.1098/rspb.2002.2217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szép T, Møller A.P. Cost of parasitism and host immune defence in the sand martin Riparia riparia: a role for parent-offspring conflict? Oecologia. 1999;119:9–15. doi: 10.1007/s004420050755. doi:10.1007/s004420050755 [DOI] [PubMed] [Google Scholar]
- Tinbergen J.M, Boerlijst M.C. Nestling weight and survival in individual great tits (Parus major) J. Anim. Ecol. 1990;59:1113–1127. [Google Scholar]
- Tripet F, Richner H. The coevolutionary potential of a ‘generalist’ parasite, the hen flea Ceratophyllus gallinae. Parasitology. 1997;115:419–427. doi: 10.1017/s0031182097001467. doi:10.1017/S0031182097001467 [DOI] [PubMed] [Google Scholar]
- Tschirren B, Fitze P.S, Richner H. Sexual dimorphism in susceptibility to parasites and cell-mediated immunity in great tit nestlings. J. Anim. Ecol. 2003;72:839–845. doi:10.1046/j.1365-2656.2003.00755.x [Google Scholar]
- Tschirren B, Saladin V, Fitze P.S, Schwabl H, Richner H. Maternal yolk testosterone does not modulate parasite susceptibility or immune function in great tit nestlings. J. Anim. Ecol. 2005;74:675–682. doi:10.1111/j.1365-2656.2005.00963.x [Google Scholar]
- Tsiagbe V.K, Cook M.E, Harper A.E, Sunde M.L. Enhanced immune-responses in broiler chicks fed methionine-supplemented diets. Poult. Sci. 1987;66:1147–1154. doi: 10.3382/ps.0661147. [DOI] [PubMed] [Google Scholar]
- Veiga J.P, Salvador A, Merino S, Puerta M. Reproductive effort affects immune response and parasite infection in a lizard: a phenotypic manipulation using testosterone. Oikos. 1998;82:313–318. [Google Scholar]
- Verhulst S, Dieleman S.J, Parmentier H.K. A tradeoff between immunocompetence and sexual ornamentation in domestic fowl. Proc. Natl Acad. Sci. USA. 1999;96:4478–4481. doi: 10.1073/pnas.96.8.4478. doi:10.1073/pnas.96.8.4478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakelin D. Cambridge University Press; Cambridge, UK: 1996. Immunity to parasites. [Google Scholar]
- Wikel S.K, Bergmann D.K, Ramachandra R.N. Immunological-based control of blood-feeding arthropods. In: Wikel S.K, editor. The immunology of host–ectoparasitic arthropod relationships. CAB International; Oxon: 1996. pp. 290–315. [Google Scholar]
- Zuk M, Stoehr A.M. Immune defense and host life history. Am. Nat. 2002;160:S9–S22. doi: 10.1086/342131. doi:10.1086/342131 [DOI] [PubMed] [Google Scholar]