Abstract
Scale-free foraging patterns are widespread among animals. These may be the outcome of an optimal searching strategy to find scarce, randomly distributed resources, but a less explored alternative is that this behaviour may result from the interaction of foraging animals with a particular distribution of resources. We introduce a simple foraging model where individual primates follow mental maps and choose their displacements according to a maximum efficiency criterion, in a spatially disordered environment containing many trees with a heterogeneous size distribution. We show that a particular tree-size frequency distribution induces non-Gaussian movement patterns with multiple spatial scales (Lévy walks). These results are consistent with field observations of tree-size variation and spider monkey (Ateles geoffroyi) foraging patterns. We discuss the consequences that our results may have for the patterns of seed dispersal by foraging primates.
Keywords: foraging, Lévy walks, spider monkeys, scale invariance, seed dispersal
1. Introduction
Many animal species, including albatrosses (Diomedea exulans; Viswanathan et al. 1996), jackals (Canis adustus; Atkinson et al. 2002) and spider monkeys (Ateles geoffroyi) (Ramos-Fernández et al. 2004) move in their environment along apparently erratic trajectories which can be accurately described as Lévy walks. These are random walks composed of a sum of independent steps (or sojourns), but with markedly non-Gaussian statistics due to a diverging mean square of the step length. That is, the probability distribution P(l) for the length l of each sojourn is broadly distributed and self-similar over a wide range of scales. Normal (Brownian) random walks are characterized by steps with finite mean square length, which contribute by roughly the same amount to the overall displacement, giving rise to Gaussian diffusion. In contrast, Lévy walks generate anomalous (namely, faster) diffusion as they are dominated by very large, though infrequent, steps (Bouchaud & Georges 1990; Shlesinger et al. 1993; Klafter et al. 1996). Recently, we have determined the distribution of the sojourns made by fruit-eating spider monkeys foraging in a semi-evergreen tropical forest in Yucatan, Mexico (Ramos-Fernández et al. 2004). The results show power-law behaviour , with an exponent , which suggests that these foraging movements may indeed be described by Lévy walks (for Lévy processes, 1<α≤3). Similar scaling laws, with α also close to 2, have been reported for other animals (Viswanathan et al. 1999), and even, more recently, for human travels (Brockmann et al. 2006).
Lévy foraging behaviour may be the outcome of an optimal searching strategy to find scarce, randomly distributed resources (Viswanathan et al. 1999; Bartumeus et al. 2005). A less explored alternative is that this behaviour may be the outcome of the distribution of resources themselves. It is well known that many animals (bees, rodents, primates) do not forage randomly but rely instead on cognitive maps to navigate their environment (Collett et al. 1986; Garber 1989; Dyer 1994). These maps may contain information on the location of different targets and the geometric relationships between them (Kamil & Jones 1997). Animals are also able to evaluate the amount of food present at different locations within their environment (Shettleworth et al. 1988; Janson 1998) and in some cases integrate this information with their spatial knowledge (Janson 1998). In the field, we have observed that spider monkeys follow regular routes to travel between feeding sites within a limited area of approximately 2 km2 (Ramos-Fernández & Ayala-Orozco 2003; Valero 2004).
Here, we introduce a novel foraging model, in which the territory is composed of many targets (food patches) with varying sizes and in which the spatial structure can be varied; foragers know the location and size of the targets and adhere to a simple foraging strategy (i.e. maximizing food intake in a minimum travelled distance). We use the model to explore the conditions that lead to Lévy foraging patterns. Our numerical simulations show that a particular target size frequency distribution, similar to the tree-size frequency distribution measured in the field, induces the most non-Gaussian trajectories. The corresponding step length distribution is a Lévy law with α=2, in agreement with the collected foraging data (Ramos-Fernández et al. 2004). The results are also consistent with the observed distribution of waiting times, i.e. the time spent feeding in trees between two moves (Ramos-Fernández et al. 2004). We discuss the relevance of our findings for understanding seed dispersal patterns by frugivorous primates.
2. The base model
We model the foraging environment as a two-dimensional square domain of area, set to unity for convenience, containing N point-like targets (N≫1). In a first approximation, targets are randomly and independently distributed in space (Poisson process). The size of the system can be thought of as the size of the territory of a group of monkeys. Targets represent the trees with fruits that monkeys eat; we assign to each target i a random integer ki≥1 representing the target's size, or fruit content. Recent work (Enquist & Niklas 2001; Niklas et al. 2003) has found that in many tropical and temperate forests, the probability p(k) of observing a tree of size k (estimated as the diameter at breast height (DBH); see more discussion on this in §3) falls as an inverse power law. Thus, we assume that ki is distributed according to a inverse power-law probability distribution
| 2.1 |
where C is the normalization factor and is a fixed exponent characterizing the environment, being the only parameter of the model. If the exponent β is close to 1, p(k) decays slowly with k, implying that the range of target size is very broad, with essentially all sizes present. In contrast, when β≫1, practically all targets have the same size and the probability of finding larger ones (ki=2, 3, …) is negligible. Many types of forests have been found to be characterized by values in the range 1.5≤β≤4 (Enquist & Niklas 2001; Niklas et al. 2003).
We now consider a forager located at a starting point near the centre of the domain. The forager knows the location and size of all targets in the system. The following rules of motion are recursively implemented:
the forager located at the target number i will move in a straight line to a target j such that the quantity is minimal among all available targets in the system, where is the distance separating the two targets and is the size of target j;
the forager does not choose an already visited target, as it is assumed that visited targets no longer contain fruits.
According to rule (a), valuable targets (large k) may be chosen even if they are not the nearest to the monkey's position. The quantity roughly represents a cost/gain ratio for a move. Our assumption that foragers know the position and size of all targets could be relaxed by assuming that they only know a random subset of the total. Those known targets would still vary in size according to the same overall distribution (equation (2.1)), and the results reported here would not change. We emphasize that, once the random environment is set and an initial position chosen, the trajectory, although complicated, is not random but deterministic. In appendix A, we develop two modifications of our model designed to test the robustness of the results. Further improvements, not considered here, could relax rule (b) and allow revisiting after a period of time that is sufficient for a patch to replenish.
3. Results of the base model
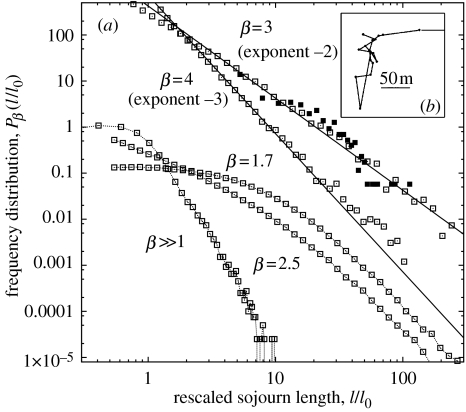
We first analyse the average properties of the trajectories composed of many steps, and their variations with the type of environment, namely, the resource exponent β. The numerical results can be summarized from figure 1a.
For 1≤β≤2, the sojourn length frequency distribution, denoted as , is very broad and is only limited by a characteristic length-scale of order of the system size (L=1), which is much larger than the mean separation distance l0 between two nearby targets (l0∼N−1/2).
For 2≤β≤4, is broad but does not significantly depend on the size of the system: in the sub-range 3≤β≤4, it is very well fitted by a power-law distribution for large l, , with a measured exponent α≈β−1, while important deviations from power-law behaviour appear in the sub-range 2≤β<3.
For , decays faster than , meaning that sojourns between nearby targets entirely dominate the statistics.
Figure 1.
(a) Normalized step length distribution for various resource exponents β, obtained from simulations with targets in a square domain, and averaged over 10 independent environment realizations (in each run, the number of visited sites is small compared with N). The length is the average distance between two nearby targets. The curves and 4 are translated upward for clarity. Solid lines are inverse power laws l−2 and l−3. The filled squares correspond to the monkeys' foraging patterns collected in the field. (b) Spatial map of a trajectory performed by a spider monkey in the field (detail; see Ramos-Fernández et al. (2004) for details on the field study).
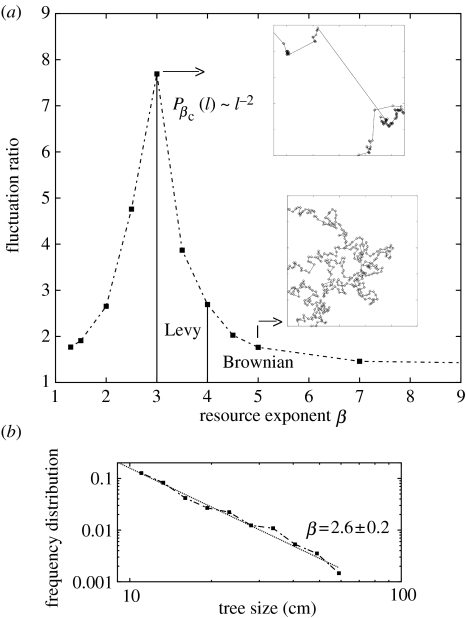
These results can be understood qualitatively as follows. At large values of the resource exponent β, all targets are practically identical in size and the forager travels to the nearest unvisited one (Lima et al. 2001), performing trajectories resembling those already observed in some herbivores (Gross et al. 1995). In contrast, for small β, the largest targets available in the system are much larger than average and also relatively numerous. As the distance from any point of origin increases, there is a higher probability of finding increasingly larger targets, so that sometimes the forager will decide to visit them in spite of the distance it has to travel. In that lower exponent range, the length of a long sojourn can be of the order of the system size, with the resulting trajectories resembling those of a random walker trapped in a finite domain. Lévy-like trajectories occur at intermediate values of β, for which large targets are indeed present, but are still scarce and far apart from each other. In this regime, the trajectories are not strongly affected by the finite size of the system except after very long times. In figure 2a, we show numerical results for the relative step length fluctuations as a function of β, along with typical trajectories. The resource size distribution with is special because it induces the largest relative fluctuations on the length of the sojourn l. These trajectories contain the largest number of length-scales, characterized by P(l)∼l−α, with α≈2 (see figure 1a).
Figure 2.
(a) Fluctuation ratio (mean square length to square mean length) associated with the step length distributions as a function of the resource exponent β. The vertical lines are guides to the eye. Insets: spatial maps of typical trajectories for β=3 (top) and 5 (bottom). (b) Tree-size frequency distribution in semi-evergreen medium forest in La Pantera, in the southeastern Yucatan Peninsula, Mexico. This is the same forest type with the same species composition as the spider monkey study site. Data conform to a power law with exponent (±0.2 standard error). Adjustment was performed using a least-squares regression. Data consist of the diameter at breast height of a total of 250 trees ranging from 10 to 63.4 cm. See Cairns et al. (2003) for more details on the study site and procedures. Data were kindly provided by the Centro de Investigación Científica de Yucatán.
It is worth noting that the special state at most resembles the trajectories described by spider monkeys in the field (Ramos-Fernández et al. 2004). Additionally, this resource exponent value is close to that which characterizes the real variation in tree size, as measured in the field in an area close to the study site. The tree-size distribution at this site can be fitted as a power law with exponent value 2.6±0.2 (figure 2b). These results are based on measurements of the DBH, which is commonly regarded as one of the most accurate methods for estimating fruit abundance of tropical tree species (Chapman et al. 1992). It is observed in some examples that the mass of reproductive structures is roughly a linear function of DBH (McDiarmid et al. 1977; Snook et al. 2005), as well as of tree size (Crawley 1997; Fenner & Thompson 2005).
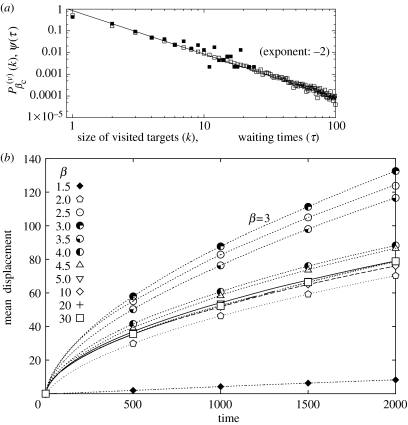
Our model accounts for another feature that illustrates the interaction between spider monkeys and their environment. In the field, once a monkey has stopped at a given tree, it tends to stay there for a random time-interval, τ, that is also distributed as a power law (Ramos-Fernández et al. 2004). In spite of the fact that most waiting times are short (5–10 min), a significant number of them are longer than 2 h. As reported in figure 3a, the measured waiting time distribution of spider monkeys can be fitted by the law with (Ramos-Fernández et al. 2004). We then assume that the time spent on a tree is proportional to the amount of food available at that tree, which is also proportional to the tree size. In figure 3a, we also show the frequency distribution of the size of the targets visited by the model forager, noted as , for the special value of the model. Such distribution is not straightforward: the walker visits a subset of targets that have relatively larger sizes compared with the overall distribution p(k) of equation (2.1). This is due to the attractiveness of larger targets in the choice process. We observe , with . This numerical value is in quantitative agreement with the waiting time exponent measured in the field, as would be expected under the assumption that the time spent feeding on a tree is proportional to its size or its fruit content.
Figure 3.
(a) Waiting time distribution. Once a monkey has stopped at a tree, it stays there for a time τ before moving to another site. The measured waiting time distribution of spider monkeys in the field is plotted here as filled squares, and is fitted by an inverse power law, with (one time unit represents a 5 min interval; see Ramos-Fernández et al. 2004). Also plotted—as open squares—is the distribution of the size of the targets visited by the walker in the model, at the particular exponent value . We observe , with the same value . One iteration of the model is equivalent to 5 min in the field observations of spider monkeys. (b) Mean displacement of the model walker, , as a function of the duration of the walk t. A walker arriving at a target of size k stays there for a time t=k before moving towards the next target at a constant speed (l0 per unit time).
Fruit-eating monkeys swallow the seeds of many tree species and deposit them away from the parent tree after an average transit time of 4.4 h in the case of spider monkeys (Lambert 1998). During this time, a spider monkey may travel distances of tens to hundreds of meters. Indeed, in previous work (Ramos-Fernández et al. 2004), we observed that during the early morning period, when monkeys forage away from their sleeping trees, their mean displacement grows algebraically with time, as t0.85. This growth rate is faster than ordinary diffusion, but slower than straight-line motion. In the model, one can similarly define the mean displacement of the walker as the average quantity , where t is the total duration of the walk, R is the walker's position vector and t0 an arbitrary origin time. A walker arriving at a target of size k stays there for a time equal to k (see above and figure 3a) before moving towards the next target at a constant speed, e.g. l0 per unit time. In figure 3b, we have plotted the resulting displacement as a function of time t for various resource exponents β. We observe that the mean displacement of the forager away from its starting point after any given time is maximal when the environment has the target size frequency distribution exponent . This property is independent of the forager's speed: we have verified that, for any fixed value of the speed of motion between two targets, the displacement is always maximal when β=3. This result still holds if the distribution (2.1) is truncated at a particular size kmax (if p(k>100)=0, for instance).
Qualitatively, this result implies that at low β values (less than 3) the large quantity of large targets slows down the forager's progression through the system. At large β values (greater than 3), on the other hand, most targets are small and very large ones are so scarce that they have little impact on the trajectories. In this case, waiting times are short, but so are the lengths of the steps. An intermediate situation (shorter waiting times, long step lengths) is achieved for , the value at which the mean displacement, and therefore dispersal, are maximal.
4. Discussion
Despite its simplicity, the model shows a rich variety of behaviour. By varying its main parameter, which describes the decay of the tree-size frequency distribution, the trajectories of a forager following a simple optimization rule can differ widely. The agreement found between the field exponents (for step length, tree size and waiting time distributions) and their theoretical values at the special parameter βc=3 suggests that the model correctly captures the interactions between spider monkeys and their environment. At these values, forager trajectories contain the largest possible fluctuations regarding the length of constituent sojourns and thus can be correctly characterized as Lévy walks.
Viswanathan et al. (1999) suggested that Lévy walks may be part of an optimal searching strategy to find scarce, randomly distributed resources. Their argument is based on the fact that, compared to Brownian foragers (who perform walks with a constant length of constituent sojourns), Lévy foragers would reach new, unvisited areas in shorter times as well as having a smaller probability of reaching areas already visited. However, many animal species, from insects to primates, have been shown to possess a sophisticated knowledge of resource location (e.g. Garber 1989; Dyer 1994). Our results suggest that Lévy walks arise as a consequence of food intake maximization in a spatially disordered, heterogeneous environment where the location of resources is at least partially known.
A crucial assumption of our model is that spider monkeys rely on mental maps in their foraging as they move from one fruiting tree to another. Recent work (Valero 2004) carried out in the same study site as the one where the movement patterns were first studied (Ramos-Fernández et al. 2004) found that spider monkeys can orient their straight-line movements toward existing fruiting trees at distances of up to 1500 m. These distances appear too great to have been the result of detection by sight alone and instead suggest that spider monkeys use some kind of mental representation of the location of current feeding sources (Valero 2004). A variant of our model (see appendix A) introduces a degree of error of 65% in the foraging rule employed by foragers, effectively eliminating, in many cases, the best option available. Still, the statistics of the foraging patterns remain unaffected.
The distribution of resources on which spider monkeys actually rely on is close to an inverse power law with exponent β=3. Similarly, tree-size distribution measurements, reported elsewhere (Enquist & Niklas 2001; Niklas et al. 2003) in several forest types, have shown that typical exponent values are in the range 1.5≤β≤4. Our results highlight some of the consequences that power-law size distributions in tree communities can have on the foraging patterns of animals that utilise them as a resource.
The phenology of trees deserves special attention, as the diet of foragers largely depends on the timing of fruiting (Rathcke & Lacey 1985). So far, we have not included different species in our model, and it is clear that there are times when more abundant or larger tree species would provide larger amounts of fruit (Newstrom et al. 1994). This could imply that the total number of feeding trees, as well as their size frequency distribution (e.g. the exponent β) at any one time, might not remain constant across seasons. The model can provide testable predictions of the way in which foraging trajectories would vary. For instance, the results above still hold if fruiting trees are modelled as a random subset of the overall set of targets. Additionally, the spatial structure of feeding trees could change with time, a feature that can be incorporated into the model with the help of the Markov point processes mentioned in appendix A.
Our results may have important consequences for the ecology of the trees that spider monkeys use, particularly on the seed dispersal patterns. Long-distance seed dispersal has been identified as a key determinant of the spatial and genetic structure, as well as the species composition, of tree communities (Janzen 1970; Cain et al. 2000; Nathan & Muller-Landau 2000; Pacheco & Simonetti 2000; Chave et al. 2002). Frugivorous primate species, in particular, are important seed dispersers as they have been shown to disperse the seeds of twice as many species as birds (Clark & Poulsen 2001), affecting the spatial patterns of seed abundance at various spatial scales (Julliot 1997; Wehncke et al. 2003). These primate species are known to disperse seeds at modal distances of 200–500 m, although the longest observed dispersion events can go up to 840 m for capuchin monkeys (Wehncke et al. 2003) or 1 km for woolly monkeys (Stevenson 2000). Such a ‘fat tail’ in the probability of seed dispersion as a function of distance from the source has been suggested by Clark et al. (1999) to be a more appropriate representation of the dispersion probability function. In our model, we find that the movement patterns described by foragers could disperse seeds precisely in this way. By moving in a series of short sojourns followed by rare but very long ones, seeds would be deposited frequently near the source but also, infrequently, at very long distances from it.
The results presented in appendix A, on the other hand, show that dispersal is also sensitive to the existing spatial structure of trees at short-to-intermediate length-scales. Under the same foraging rules and size distribution of resources, seeds are likely to be spread further in a community that is spatially random/uniform (with the Poisson forest as a limiting case) than in a spatially structured/heterogeneous community (e.g. clumped). In order to tell whether foragers would be contributing to the evolution of the tree population toward a particular size or spatial structure, a description of forest dynamics should be further coupled to the present model. For instance, many factors would influence the growth success of a seed that has arrived at a given site: the distance from parent tree (Janzen 1970; Harms et al. 2000), nutrient availability, competition with other seeds or predation (Jordano 1992; Nathan & Muller-Landau 2000; Adler & Muller-Landau 2005). Our model does not specifically address the issue of species diversity. However, it gives some insights on a mechanism (among many) which can have an impact on diversity at intermediate scales in communities composed of individuals with known size and spatial distributions.
In summary, we have identified a novel mechanism by which a realistic, scale-invariant distribution of tree size generates Lévy-walk foraging movements as an emergent pattern. These findings have been supported by various field measurements. Our results should be considered as an alternative explanation of the prevalence of Lévy walks in animal behaviour, especially of species with sophisticated knowledge about their environment. On the other hand, these foraging patterns may have important consequences for the dynamics of tree communities.
Acknowledgements
We are indebted to Robert M. May, Annette Ostling, Tim Coulson, Michael P. Hassell, François Leyvraz, Eliane Ceccon, Gregory S. Gilbert, Barbara Ayala, Michael F. Shlesinger and Joseph Klafter for fruitful discussions, and to the late Ingrid Olmsted from the Centro de Investigación Científica de Yucatán for the data on tree size in a tropical forest in the Yucatan Peninsula. This work was supported by CONACYT grants 40867-F and G32723-E, SEMARNAT–CONACYT grant 0536, DGAPA grants IN-111000, IN-100803 and IN-118306 and the Instituto Politécnico Nacional.
Appendix A
Appendix A. Robustness of the model
The model above relies on a few basic assumptions. To further investigate the robustness and the generality of its predictions, we also present two variants including more specific hypotheses.
(a) Variant I: tree correlations
Trees in tropical forests are usually not randomly distributed in space. They tend to be aggregated in many cases and clumping is particularly strong among conspecifics (Crawley 1997; Condit et al. 2000). Spatial correlations still exist across different species, although they are much lower (Pélissier 1998). The diet of spider monkeys is composed of hundreds of different species (van Roosmalen & Klein 1987; Ramos-Fernández & Ayala-Orozco 2003). Even in a single month, spider monkeys may have access to more than 20 different species (Ramos-Fernández 2001). Even though the random (Poisson) assumption made above may be not too far from reality as far as the overall set of available trees is concerned, it is instructive to further take into account an a priori existing stand structure to study its impact on forager trajectories. In this first variant of the model, the target size distribution in equation (2.1) and the foraging rules are unchanged, but the targets composing the system are now correlated in space.
Markov point processes are phenomenological methods that are widely used in forestry statistics to generate correlated spatial patterns (Ripley 1977; Stoyan & Penttinen 2000). A pairwise interaction ϕ(lmn) is introduced, depending on the distance separating two trees m and n, for instance, ϕ being related to the likelihood that two trees are found at a given distance. Further, the forest is modelled by N points (N=7.2×104 here) such that the probability density of finding them at positions {x1, …, xN} is set as proportional to , the sum running on all possible pairs of points. Representative patterns are obtained after many computer iterations by using a depletion–replacement algorithm usual in Monte-Carlo calculations (Binder & Heermann 2002). We choose a standard shape for ϕ(l): the pair interaction is supposed to be infinitely repulsive (hard-core) at short distances, attractive at intermediate distances, whereas targets do not interact directly at larger distances. More specifically, ϕ(l)=∞ for 0<l<σ; ϕ(l)=−γ for σ<l<R and ϕ(l)=0 for l>R, where σ=0.5d, R=3d, with d=4.47×10−3. The hard-core repulsion produces a fairly regular pattern at short length-scales, while the attractive potential (interaction strength γ≥0) generates positive correlations further away (clumps). Correlations become negligible for sufficiently large separation distances.
We then consider two cases: In Case (1), any pair of targets {m, n} interact through a unique potential, with γ=0.1 as described earlier. In Case (2), a pair of targets interact with the potential of Case (1) only if the ratio of their sizes km/kn is not larger than 2 (with the convention km≥kn); if not, the value γ=0 is set in their interaction.
In Case (1), the interaction between targets does not depend on their sizes: the method produces point patterns with spatial correlations, the sizes of neighbouring targets remaining uncorrelated, as in the base model. Case (2), on the other hand, favours configurations where targets of similar sizes are more likely to be clumped, producing an effective repulsion between targets of very different sizes. Inhibition is commonly observed in evergreen tropical forests between adult and young trees, in part due to competition for light (Pélissier 1998).
In Case (2), the overall target–target pair correlation function g(l), related to Ripley's K function (Stoyan & Penttinen 2000), decays slowly towards the value unity with the separation distance l, and indicates larger clumps than in Case (1): g(l=σ)≈1.84 and g(l=5σ)≈0.99 for Case (1), whereas g(l=σ)≈1.43 and g(l=5σ)≈1.14 for Case (2) with a size distribution exponent β=3. (A Poisson process gives g(l)=1 for any l.) The values above are of the same order of magnitude as the ones measured in tropical forest stands that include all species (Pélissier 1998).
(b) Variant II: imperfect foraging efficiency
In order to relax the assumption of perfect knowledge by the foragers, the second variant of the base model modifies the foraging rule (a), while the targets remain Poisson-distributed in space and with independent weights. In this variant, we assume that the forager is inexact in evaluating the distance to a given target, as well as its fruit content. The forager located at target i will evaluate the cost of a move to target j as , where and are subjective distances and fruit contents deviating from the real ones: and , with e a constant lower than 1 and {ηj, λj} two random numbers uniformly distributed in the interval [−1,1]. Before each move, a pair of random numbers {ηj, λj} are attributed independently of each unvisited target j, making its attractiveness over or underestimated. The forager moves to the target of lowest among the unvisited js. The results presented here have been obtained with e=0.30, corresponding to an error of typical amplitude of 65%.
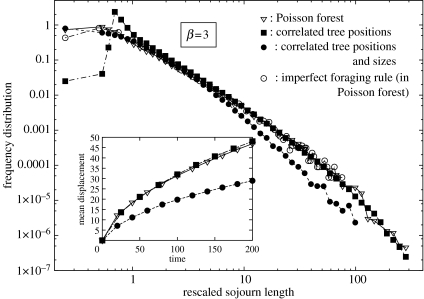
We now discuss the results given by the two variants of the model which address its robustness. Figure 4 displays the frequency distribution of the lengths of the sojourns, the tree-size distribution exponent being fixed to the value β=3. Spatial correlations between targets modify the shape of P(l) at short distances. However, the scaling law P(l)∼l−2 at large l found for uncorrelated targets is not modified with the introduction of spatial correlations without size affinity (variant I—Case (1)). The average mean displacement of the forager is also unchanged, as shown in the inset of figure 4.
Figure 4.
Frequency distribution of the sojourn length given by the different variants of the model as described in appendix A, for β=3. Inset: corresponding mean displacement as a function of time (same legends).
Interestingly, deviations from the base behaviour arise when targets are correlated in size (variant I—Case (2)), the frequency of long sojourns being noticeably lower. This feature can be explained by the fact that large targets (those that can be reached after a long sojourn) tend to be clumped in this case, which reduces the distance between them compared to the Poisson situation. When reaching a clump of large targets, the forager may not need to give long sojourns for a while. As a consequence, the mean displacement (and therefore dispersion) is significantly reduced compared to the Poisson case, as shown in the inset of figure 4.
When the foraging rule is imperfect and with a typical error of 65% (variant II), no qualitative differences in the distributions are found with respect to the perfect, deterministic rule (figure 4).
References
- Adler F.R, Muller-Landau H.C. When do localized natural enemies increase species richness? Ecol. Lett. 2005;8:438–447. doi:10.1111/j.1461-0248.2005.00741.x [Google Scholar]
- Atkinson R.P.D, Rhodes C.J, Macdonald D.W, Anderson R.M. Scale-free dynamics in the movement patterns of jackals. Oikos. 2002;98:134–140. doi:10.1034/j.1600-0706.2002.980114.x [Google Scholar]
- Bartumeus F, da Luz M.G.E, Viswanathan G.M, Catalan J. Animal search strategies: a quantitative random-walk analysis. Ecology. 2005;86:3078–3087. [Google Scholar]
- Binder K, Heermann D.W. Springer; Berlin, Germany: 2002. Monte Carlo simulation in statistical physics. [Google Scholar]
- Bouchaud J.-P, Georges A. Anomalous diffusion in disordered media: statistical mechanisms, models and physical applications. Phys. Rep. 1990;195:127–293. doi:10.1016/0370-1573(90)90099-N [Google Scholar]
- Brockmann D, Hufnagel L, Geisel T. The scaling laws of human travel. Nature. 2006;439:462–465. doi: 10.1038/nature04292. doi:10.1038/nature04292 [DOI] [PubMed] [Google Scholar]
- Cain M.L, Milligan B.G, Strand A.E. Long-distance seed dispersal in plant populations. Am. J. Bot. 2000;87:1217–1227. [PubMed] [Google Scholar]
- Cairns M.A, Olmsted I, Granados J, Argaez J. Composition and aboveground tree biomass of a dry semi-evergreen forest on Mexico's Yucatan Peninsula. Forest Ecol. Manag. 2003;186:125–132. doi:10.1016/S0378-1127(03)00229-9 [Google Scholar]
- Chapman C.A, Chapman L.J, Wrangham R, Hunt K, Gebo D, Gardner L. Estimators of fruit abundance of tropical trees. Biotropica. 1992;24:527–531. [Google Scholar]
- Chave J, Muller-Landau H.C, Levin S.A. Comparing classical community models: theoretical consequences for patterns of diversity. Am. Nat. 2002;159:1–23. doi: 10.1086/324112. doi:10.1086/324112 [DOI] [PubMed] [Google Scholar]
- Clark C.J, Poulsen J.R. The role of arboreal seed dispersal groups on the seed rain of a lowland tropical forest. Biotropica. 2001;33:606–620. [Google Scholar]
- Clark J.S, Silman M, Kern R, Macklin E, HilleRisLambers J. Seed dispersal near and far: patterns across temperate and tropical forests. Ecology. 1999;80:1475–1494. [Google Scholar]
- Collett T.S, Cartwright B.A, Smith B.A. Landmark learning and visuo-spatial memories in gerbils. J. Comp. Physiol. A. 1986;158:835–851. doi: 10.1007/BF01324825. doi:10.1007/BF01324825 [DOI] [PubMed] [Google Scholar]
- Condit R, et al. Spatial patterns in the distribution of tropical tree species. Science. 2000;288:1414–1418. doi: 10.1126/science.288.5470.1414. doi:10.1126/science.288.5470.1414 [DOI] [PubMed] [Google Scholar]
- Crawley M.J. Blackwell Science; Oxford, UK: 1997. Plant ecology. [Google Scholar]
- Dyer F.C. Spatial cognition and navigation in insects. In: Real L.A, editor. Behavioral mechanisms in evolutionary ecology. University of Chicago Press; Chicago, IL: 1994. pp. 66–98. [Google Scholar]
- Enquist B.J, Niklas K.J. Invariant scaling relations across tree-dominated communities. Nature. 2001;410:655–660. doi: 10.1038/35070500. doi:10.1038/35070500 [DOI] [PubMed] [Google Scholar]
- Fenner M, Thompson K. Cambridge University Press; Cambridge, UK: 2005. The ecology of seeds. [Google Scholar]
- Garber P.A. The role of spatial memory in primate foraging patterns: Saguinus mystax and Saguinus fuscicollis. Am. J. Primatol. 1989;19:203–216. doi: 10.1002/ajp.1350190403. doi:10.1002/ajp.1350190403 [DOI] [PubMed] [Google Scholar]
- Gross J.E, Zank C, Thompson Hobbs N, Spalinger D.E. Movement rules for herbivore in spatially heterogeneous environments: responses to small scale patterns. Landscape Ecol. 1995;10:209–217. doi:10.1007/BF00129255 [Google Scholar]
- Harms K.E, Wright S.J, Calderon O, Hernandez A, Herre E.A. Pervasive density-dependent recruitment enhances seedling diversity in a tropical forest. Nature. 2000;404:493–495. doi: 10.1038/35006630. doi:10.1038/35006630 [DOI] [PubMed] [Google Scholar]
- Janson C.H. Experimental evidence for spatial memory in foraging wild capuchin monkeys, Cebus apella. Anim. Behav. 1998;55:1229–1243. doi: 10.1006/anbe.1997.0688. doi:10.1006/anbe.1997.0688 [DOI] [PubMed] [Google Scholar]
- Janzen D.H. Herbivores and the number of tree species in tropical forests. Am. Nat. 1970;104:501–528. doi:10.1086/282687 [Google Scholar]
- Jordano P. Fruits and frugivory. In: Fenner M, editor. The ecology of regeneration in plant communities. CAB International; New York, NY: 1992. pp. 105–156. [Google Scholar]
- Julliot C. Impact of seed dispersal of red howler monkeys Alouatta seniculus on the seedling population in the understorey of tropical rain forest. J. Ecol. 1997;85:431–440. [Google Scholar]
- Kamil A.C, Jones J.E. The seed-storing corvid Clark's nutcracker learns geometric relationships among landmarks. Nature. 1997;390:276–279. doi:10.1038/36840 [Google Scholar]
- Klafter J, Shlesinger M.F, Zumofen G. Beyond Brownian motion. Phys. Today. 1996;49:33–39. [Google Scholar]
- Lambert J.E. Primate digestion: interactions among anatomy, physiology, and feeding ecology. Evol. Anthropol. 1998;9:8–20. doi:10.1002/(SICI)1520-6505(1998)7:1<8::AID-EVAN3>3.0.CO;2-C [Google Scholar]
- Lima G.F, Martinez A.S, Kinouchi O. Deterministic walks in random media. Phys. Rev. Lett. 2001;87:010603. doi: 10.1103/PhysRevLett.87.010603. doi:10.1103/PhysRevLett.87.010603 [DOI] [PubMed] [Google Scholar]
- McDiarmid R.W, Ricklefs R.E, Foster M.S. Dispersal of Stemmadenia donnell-smithii (Apocynaceae) by birds. Biotropica. 1977;9:9–25. [Google Scholar]
- Nathan R, Muller-Landau H.C. Spatial patterns of seed dispersal, their determinants and consequences for recruitment. Trends Ecol. Evol. 2000;15:278–285. doi: 10.1016/s0169-5347(00)01874-7. doi:10.1016/S0169-5347(00)01874-7 [DOI] [PubMed] [Google Scholar]
- Newstrom L.E, Frankie G.W, Baker H.G, Colwell R.K. Diversity of long-term flowering patterns. In: McDade L.A, Bawa K.S, Hespenheide H.A, Hartshorn G.S, editors. La Selva: ecology and natural history of a neotropical rain forest. University of Chicago Press; Chicago, IL: 1994. pp. 142–160. [Google Scholar]
- Niklas K.J, Midgley J.J, Rand R.H. Tree size frequency distributions, plant density, age and community disturbance. Ecol. Lett. 2003;6:405–411. doi:10.1046/j.1461-0248.2003.00440.x [Google Scholar]
- Pacheco L.F, Simonetti J.A. Genetic structure of a Mimosoid tree deprived of its seed disperser, the spider monkey. Conserv. Biol. 2000;14:1766–1775. doi: 10.1111/j.1523-1739.2000.99182.x. [DOI] [PubMed] [Google Scholar]
- Pélissier R. Tree spatial patterns in three contrasting plots of a southern Indian tropical moist evergreen forest. J. Trop. Ecol. 1998;14:1–16. doi:10.1017/S0266467498000017 [Google Scholar]
- Ramos-Fernández, G. 2001 Patterns of association, feeding competition and vocal communication in spider monkeys, Ateles geoffroyi Ph.D. dissertation, University of Pennsylvania, PA, USA. (see http://repository.upenn.edu/dissertations/AAI3003685/)
- Ramos-Fernández G, Ayala-Orozco B. Population size and habitat use in spider monkeys at Punta Laguna, Mexico. In: Marsh L.K, editor. Primates in fragments: ecology and conservation. Kluwer Academic Publishers; New York, NY: 2003. pp. 191–210. [Google Scholar]
- Ramos-Fernández G, Mateos J.L, Miramontes O, Larralde H, Cocho G, Ayala-Orozco B. Lévy walk patterns in the foraging movements of spider monkeys (Ateles geoffroyi) Behav. Ecol. Sociobiol. 2004;55:223–230. doi:10.1007/s00265-003-0700-6 [Google Scholar]
- Rathcke B, Lacey E.P. Phenological patterns of terrestrial plants. Annu. Rev. Ecol. Syst. 1985;16:179–214. doi:10.1146/annurev.es.16.110185.001143 [Google Scholar]
- Ripley B.D. Modelling spatial patterns (with discussion) J. R. Stat. Soc. Ser. B. 1977;39:172–212. [Google Scholar]
- Shettleworth S.J, Krebs J.R, Stephens D.W, Gibbon J. Tracking a fluctuating environment: a study of sampling. Anim. Behav. 1988;36:87–105. [Google Scholar]
- Shlesinger M.F, Zaslavsky G.M, Klafter J. Strange kinetics. Nature. 1993;363:31–37. doi:10.1038/363031a0 [Google Scholar]
- Snook L.K, Cámara-Cabrales L, Kelty M.J. Six years of fruit production by mahogany trees (Swietenia macrophylla King): patterns of variation and implications for sustainability. Forest Ecol. Manag. 2005;206:221–235. doi:10.1016/j.foreco.2004.11.003 [Google Scholar]
- Stevenson P.R. Seed dispersal by woolly monkeys (Lagothrix lagothricha) at Tinigua National Park, Columbia: dispersal distance, germination rates, and dispersal quantity. Am. J. Primatol. 2000;50:275–289. doi: 10.1002/(SICI)1098-2345(200004)50:4<275::AID-AJP4>3.0.CO;2-K. doi:10.1002/(SICI)1098-2345(200004)50:4<275::AID-AJP4>3.0.CO;2-K [DOI] [PubMed] [Google Scholar]
- Stoyan D, Penttinen A. Recent applications of point process methods in forestry statistics. Stat. Sci. 2000;15:61–78. doi:10.1214/ss/1009212674 [Google Scholar]
- Valero, A. 2004 Spider monkey (Ateles geoffroyi) travel patterns in a subtropical forest of Yucatan, Mexico. Ph.D. thesis, School of Psychology, University of St Andrews, St Andrews, UK, p. 155.
- van Roosmalen M.G.M, Klein L.L. The spider monkeys, Genus Ateles. In: Mittermeier R.A, Rylands A.B, editors. Ecology and behavior of neotropical primates. World Wide Fund; Washington, DC: 1987. pp. 455–537. [Google Scholar]
- Viswanathan G.M, Afanasyev V, Buldyrev S.V, Murphy E.J, Prince P.A, Stanley H.E. Lévy flight search patterns of wandering albatrosses. Nature. 1996;381:413–415. doi: 10.1038/nature06199. doi:10.1038/381413a0 [DOI] [PubMed] [Google Scholar]
- Viswanathan G.M, Buldyrev S.V, Havlin S, da Luz M.G.E, Raposo E.P, Stanley H.E. Optimizing the success of random searches. Nature. 1999;401:911–914. doi: 10.1038/44831. doi:10.1038/44831 [DOI] [PubMed] [Google Scholar]
- Wehncke E.V, Hubbell S.P, Foster R.B, Dalling J.W. Seed dispersal patterns produced by white-faced monkeys: implications for the dispersal limitation of neotropical tree species. J. Ecol. 2003;91:677–685. doi:10.1046/j.1365-2745.2003.00798.x [Google Scholar]