Abstract
The advent of highly active anti-retroviral therapy (HAART) has dramatically decreased the rate of AIDS-related mortality and significantly extended the life span of patients with AIDS. A variety of metabolic side effects are associated with these therapies, one of which is metabolic bone disease. A higher prevalence of osteopenia and osteoporosis in HIV-infected patients receiving anti-retroviral therapy than in patients not on therapy has now been reported in several studies. Several factors have been demonstrated to influence HIV-associated decreases in bone mineral density (BMD), including administration of nucleoside reverse transcriptase inhibitors (NRTIs). In this article, discussion will focus on the molecular pathogenesis and treatment of HAART-associated osteopenia and osteoporosis.
Keywords: HIV, osteopenia/osteoporosis, HAART, NRTI, osteoclastogenesis, T cell cytokines, mitochondrial toxicity, RANK/RANKL/OPG
INTRODUCTION
Bone remodeling depends on the tightly integrated activity of two major cell types, osteoblasts, which make new bone (bone formation), and osteoclasts, which destroy old bone (bone resorption). Therefore, the balance between the number and activity of osteoclasts and osteoblasts is crucial in determining bone mass, which is directly related to bone fragility and fracture risk. Two important molecules: macrophage colony-stimulating factor (M-CSF) and receptor for activation of nuclear factor-kappa B ligand (RANKL) produced from osteoblasts/stromal cells regulate the differentiation, function, and survival of osteoclasts.1 Two transcription factors, Runx2 and Osterix, have been reported to regulate osteoblast differentiation.
HIV Infection and Bone Disease
Bone metabolism in HIV-infected individuals has been studied since the late 1980s. Before the widespread use of highly active anti-retroviral therapy (HAART), studies indicated that bone mineral metabolism was only minimally affected in HIV-infected patients. Serrano et al. assessed histomorphometry in HIV-positive patients and found that many parameters of histomorphometry were significantly lower in patients than in controls.2 Paton et al. reported that 45 HIV-infected patients had marginally lower bone mineral density (BMD) at the lumbar spine. None of the patients had reduced BMD to levels associated with a diagnosis of osteoporosis.3 Recently, Amiel et al. analyzed BMD in 48 HIV-infected treatment-naïve patients, 49 HIV-infected patients on protein inhibitors, 51 HIV-infected patients on no-protein inhibitors, and 81 HIV-uninfected control subjects. The results showed a significant decrease of BMD in all HIV-infected patients compared to the control subjects.4
The increasing frequency of osteopenia and osteoporosis being reported in HIV-infected patients receiving anti-retroviral therapy suggests an independent role of anti-retroviral drugs in altering bone metabolism.
HAART and Bone Diseases
HAART is a complex therapeutic regimen consisting of up to 15 anti-retroviral agents. In general, HAART includes two major therapeutic regimens: a protease inhibitor (PI)-based regimen and a nucleoside reverse transcriptase inhibitor (NRTI)-based regimen. The PI-based regimen uses one or two PIs combined with two NRTIs, whereas the NRTI-based regimen uses two NRTIs combined with one non-nucleoside reverse transcriptase inhibitor (NNRTI). With more effective therapies as a result of HAART, the prevalence of HAART-associated bone diseases has increased.
A high incidence of osteopenia/osteoporosis has been associated with both PI and NRTI use. Tebas et al. measured BMD in AIDS patients receiving a PI and found that 50% of the patients had osteopenia and 21% had osteoporosis.5 This incidence is significantly increased compared to patients without therapy or normal controls. Moore et al. confirmed that 71% of HIV-infected patients on PI therapy have reduced BMD.6 Jain et al. compared the effect of various PIs on bone resorption and found that some PIs, but not all, increase bone resorption.7 Similarly, Carr et al. reported that 3% of 44 HIV-infected patients receiving NRTIs developed osteoporosis and 22% developed osteopenia.8 Tsekes et al. determined BMD and whole body fat by dual-energy X ray absorbance (DEXA) of HIV-infected patients on Zidovudine (AZT) and other NRTIs and found significant decreases in both body fat and BMD.9
It has also been noted that HIV-infected patients have an increased risk for osteonecrosis of the hip.10 Keruly et al. reported 15 cases of avascular hip necrosis in HIV-infected patients and suggested that the incidence of osteonecrosis in HIV-infected patients was higher than the general HIV-negative population.11 Allison et al. summarized the epidemiology, etiology, and clinical management of osteonecrosis.10 It is not known whether osteonecrosis of the hip is attributable to HIV infection itself, HAART, or other HIV-associated complications. Belmonte et al. suggested a possible association between the presence of anti-phospholipid antibodies and osteonecrosis.12
MOLECULAR PATHOGENESIS OF HAART-ASSOCIATED BONE LOSS
At the cellular level, the regulation of osteoclast differentiation by PIs has been investigated in vitro by measuring the expression of RANK/RANKL/OPG. The results showed that some PIs inhibit osteogenesis and expression of osteoprotegerin (OPG) leading to increased osteoclastogenesis and bone resorption.7 Wang et al. reported that the PI, Indinavir, attenuates the function and recruitment of osteoblasts increasing bone loss, whereas another PI, Ritonavir, blocks osteoclastogenesis decreasing bone loss.13 To study the pathogenesis of NRTI-associated bone mineral loss, we examined the contributions of NRTIs on osteoclastogenesis, T cell cytokines, and mitochondrial toxicity.
Increased Osteoclastogenesis
We reported the osteoclastogenic effects of AZT in vitro and in vivo.14 AZT enhances osteoclastogenesis in RAW264.7 cells and primary mouse osteoclast precursors in the presence of RANKL. This osteoclastogenic effect of AZT is associated with increased activities of the tartrate-resistant acid phosphatase (TRAP) promotor and the NF-κB transcription factor.14 The osteoclastogenic effect of AZT was concentration dependent (Fig. 1A), and the expression of TRAP and calcitonin receptor (CTR), but not RANK, were markedly increased by AZT plus RANKL (Fig. 1B). Note that in the experiment shown in Figure 1A, the incubation time is only 3 days, which explains the low value in the presence of RANKL alone. Maximum stimulation of osteoclastogenesis by RANKL alone requires 5–6 days.14
FIGURE 1.
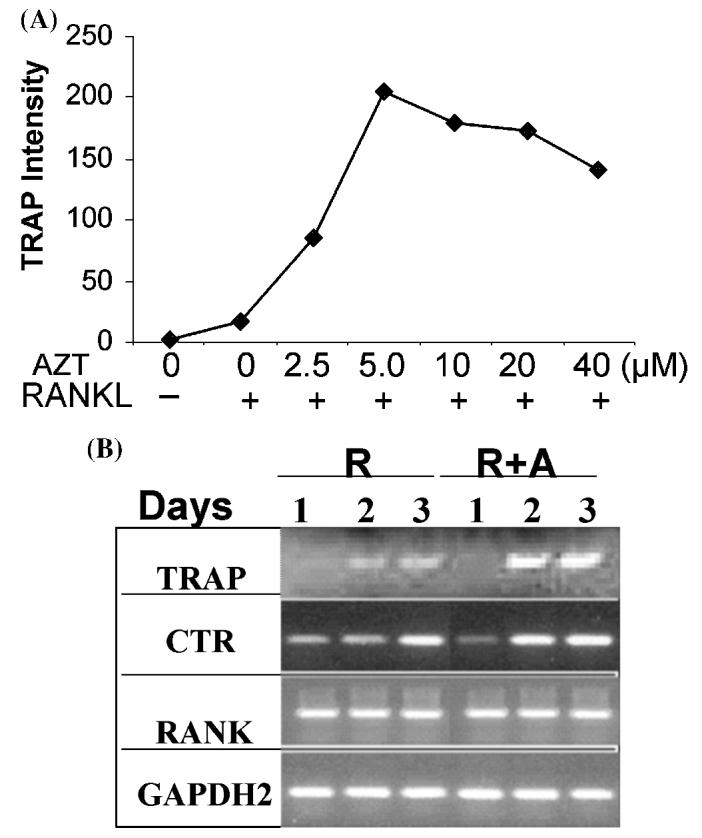
The osteoclastogenic effect of AZT in RAW264.7 cells (A) RAW264.7 cells were treated with AZT at indicated concentrations in the presence of RANKL (50 ng/mL) for 3 days. Osteoclasts were measured by TRAP staining assay. The Y-axis represents the TRAP intensity per well. (B) RAW264.7 cells were treated with RANKL (50 ng/mL, R) or RANKL plus AZT (25 μM, R + A) for 1–3 days as indicated and total mRNAs were isolated. RT-PCR for tartrate-resistant acid phosphatase (TRAP), calcitonin receptor (CTR), RANK, and GAPDH2 genes was performed.
We recently extended our studies to include two other NRTI compounds, didanosine (ddI) and lamivudine (3TC), alone and in combination. In the absence of RANKL, these NRTIs failed to stimulate the differentiation of RAW264.7 cells into TRAP-positive osteoclasts, whereas in the presence of RANKL treatment both ddI and 3TC, like AZT, significantly increased TRAP-positive osteoclasts. Furthermore, there were neither additive nor synergistic effects on osteoclastogenesis when cells are treated with various combinations of NRTIs.15
Although bone marrow is usually used as a source of osteoclast precursors, functional osteoclasts can be differentiated in vitro from human peripheral blood mononuclear cells (PBMCs). Susa et al. described an in vitro protocol for the production of functional osteoclasts in the presence of RANKL and M-CSF using both fresh and frozen PBMCs.16 We therefore determined the osteoclastogenic effect of AZT in PBMCs.15 Here we compared the effect of HIV infection on osteoclastogenesis efficacy of AZT using PBMCs from HIV-negative and HIV-positive HAART-naïve patients. The results show that AZT plus RANKL profoundly increased osteoclastogenesis in HIV-positive HAART-naïve PBMCs and a similar osteoclastogenic efficacy of AZT in HIV-negative PBMCs (Fig. 2). These results support the conclusion that HIV infection does not itself accelerate osteoclastogenesis or the effect of AZT in vitro.
FIGURE 2.
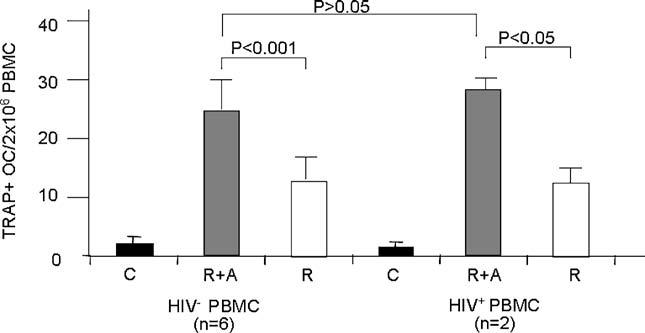
The osteoclastogenic effect of AZT in PBMCs. PBMCs (2 × 106 PBMCs per well) from two HIV-positive, HAART-naïve patients and six age-, sex-matched HIV-negative individuals were cultured with RANKL alone (R), or RANKL plus AZT (R+A) in the presence of M-CSF for 2 weeks. Treatment with M-CSF alone was a control (C). The number of multinucleated TRAP positive osteoclasts were counted. The results of the Student's t-test are indicated.
The effects of AZT on bone remodeling were also demonstrated in vivo.14 Compared to control mice, BMD in mice treated with AZT was significantly reduced by 6.15 ± 2.02% (P < 0.05, Fig. 3A). Histomorphometric analysis was performed on spine sections. Compared to control mice, both the number (OcN, P < 0.05) and surface (OcS, P < 0.001) of osteoclasts in AZT-treated mice were markedly increased (Fig. 3B). The number (ObN) and surface (ObS) of osteoblasts were not significantly changed in the NRTI-treated animals. These results indicate that decreased BMD in AZT-treated mice is mediated by increased osteoclastogenesis resulting in increased bone resorption.
FIGURE 3.
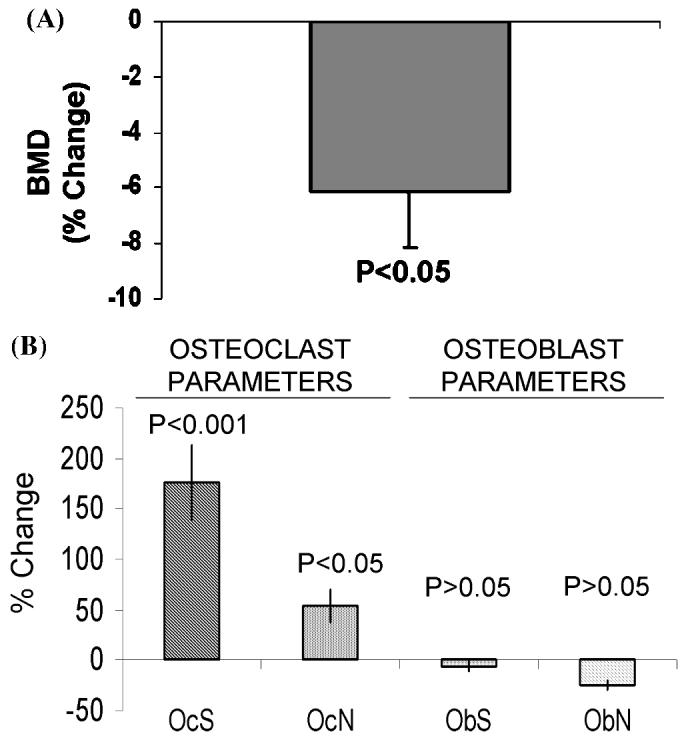
Effects of AZT on BMD and bone histomorphometry in mice. Mice, 5 months old, were orally fed PBS (n = 6, control) or AZT (n 5, AZT 100 mg/kg/day) for 30 days. (A) Bone mineral density (BMD) was measured by=DEXA. (B) Spines were fixed, decalcificated, and stained with trichrome. Histomorphometry was analyzed in three vertebrae of each animal. Osteoclast parameters included number (OcN) and surface (OcS) of osteoclasts. Osteoblast parameters included number (ObN) and surface (ObS) of osteoblasts. All parameters were compared to the control values, and reported as percentage change induced by AZT. The results were mean ± SE. P values are compared to controls.
T Cell Cytokines
There is a close relationship between the function of T cells and bone remodeling.17,18 The immune system affects normal bone growth and restructuring. In many inflammatory conditions, including autoimmune diseases, allergy, infection, and injury, bone metabolism is disrupted. Various inflammatory cytokines released from T cells or macrophages, such as interferon-α (IFN-α), IFN-β, and tumor necrosis factor-α (TNF-α), various interleukins, CD40L, granulocyte-macrophage colony-stimulating factor (GM-CSF), and macrophage inflammation protein-1 (MIP-1), have been reported to modulate osteoclast formation and function.17,18 Moreover, activated T cells release RANKL that can trigger osteoclastogenesis and bone loss.17,19,20 In addition, a crucial counter-regulatory mechanism has been discovered to inhibit the RANKL-induced maturation and activation of osteoclasts. Activated T cells secrete IFN-γ that blocks osteoclastogenesis in vitro.17,21,22 In HIV infection, an overexpression of RANKL has been documented.23,24 Elevated RANKL alone or in combination with TNF-α in HIV infection augments HIV replication forming a positive feedback loop.24 In addition, various cytokines triggering osteoclastogenesis have been reported to be changed in HIV infection and in HAART-treated AIDS patients.25,26 However, our in vitro studies using RAW264.7 cells indicate that TNF-α neither promoted osteoclastogenesis when used alone nor enhanced RANKL-induced osteoclastogenesis.15
Mitochondrial Toxicity
Mitochondria perform numerous important cell functions, including energy production, redox signaling, calcium storage, and the regulation of cell growth and death. In this regard, it has been shown that mitochondrial damage and dysfunction is involved in the pathogenesis of numerous metabolic degenerative disorders, such as diabetes, lipodystrophy, and heart diseases. Lactic acidosis is a common feature among certain forms of mitochondrial disease, which has also been linked to the observed decrease in BMD associated with NRTI therapy. Carr et al. suggested that these effects are caused by nucleoside analogue effects on osteoblast mitochondria.8 However, other reports failed to show a significant correlation between NRTI-associated mitochondrial toxicity and osteopenia/osteoporosis.27
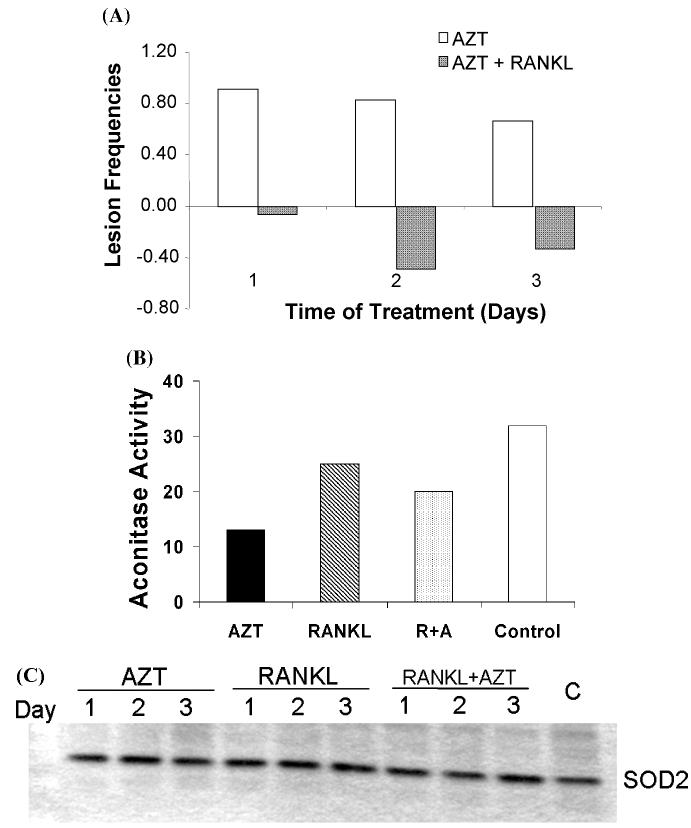
Mitochondria have their own DNA (mtDNA), which is distinct from the nuclear DNA (nDNA). The mtDNA is replicated by a γ-polymerase, which has a relatively high error rate compared to the α-DNA polymerase which is important for nDNA replication. Whereas the success of NRTIs is based upon their ability to interfere with HIV reverse transcriptase and thus inhibit viral replication,28 NRTIs also inhibit γ-polymerase and mtDNA synthesis. The mtDNA depletion in mitochondria results in mitochondrial damage and dysfunction. Interestingly, RANKL appears to prevent mtDNA damage associated with NRTI treatment. Figure 4A shows that AZT induces mtDNA damage while RANKL treatment completely reverses mtDNA damage associated with AZT treatment.
FIGURE 4.
Mitochondrial toxicity of AZT. (A) RAW264.7 cells (2 × 106) were plated in 100-mm plates and treated with AZT (25 μM), RANKL (100 ng/mL), and AZT plus RANKL for 1–3 days. Cells were washed with cold PBS twice and then cell pellets were collected and stored in −80°C. Mitochondrial DNA damage was determined by qPCR. The frequency of mtDNA damage induced by AZT and AZT+RANKL was expressed relative to the frequency of mtDNA damage in RANKL-treated cells. (B) RAW264.7 cells (2 × 106) were treated as indicated for 3 days. Aconitase activity of cell lysates from RAW264.7 cells treated as indicated [AZT, RANKL, RANKL + AZT (R+A)] for 3 days, and (C) Western blot for mitochondrial SOD in RAW264.7 cells treated as indicated [AZT, RANKL, RANKL + AZT no treatment (c)] for 1–3 days.
Mitochondrial superoxide dismutase (SOD) is an important cellular antioxidant that converts the superoxide to H2O2.29 Mitochondrial aconitase is widely recognized as a citric acid cycle enzyme that is highly sensitive to oxidative inactivation by superoxide radicals, and consequently, its activity has been used as a measure of oxidant load associated with superoxide levels. Because of their importance in cellular energy and oxidant regulation, mitochondrial SOD and aconitase are important markers of mitochondrial function and oxidant stress. Consequently, we quantified mitochondrial SOD protein levels and aconitase activity in RAW264.7 cells treated with AZT for 3 days with AZT, RANKL, or AZT + RANKL via Western blot and spectrophotometric assays, respectively. Aconitase activity in RAW264.7 cells treated with AZT for 3 days was decreased (Fig. 4B) to 41% of the activity in the control suggesting that AZT treatment resulted in inactivation of aconitase. In contrast, aconitase activity was ameliorated by addition of RANKL, to approximately 78% of the control. Western blot analysis of mitochondrial SOD showed that protein levels were not significantly altered (Fig. 4C). Overall, these data suggest that AZT induces mtDNA damage and increased oxidant stress (aconitase inactivation) that is prevented in the presence of RANKL. Because it has been reported that RANKL expression is elevated by HIV infection,23,24 it is conceivable that NRTI therapy on a background of increased RANKL levels minimizes the deleterious effects of NRTIs on osteoclast mitochondria, thereby promoting osteopenia/osteoporosis.
CLINICAL TREATMENT OF HAART-ASSOCIATED BONE LOSS
Currently, the management of osteopenia/osteoporosis in HIV-infected patients is focused on reducing the risks for bone loss and includes intake of calcium and vitamin D, weight-bearing exercise, recombinant growth hormone, or other anabolic agents.30,31 Bisphosphonates are also widely used as a principal drug.32,33
It has been reported that anabolic steroids increase spinal bone density after treatment but no effect on the rate of fractures has been documented.34 However, a study of the steroid, oxandrolone, had no effect on BMD among a small group of HIV-infected individuals.35 On the other hand, bisphosphonates, such as alendronate (ALN) have been used to treat or prevent osteopenia/osteoporosis in AIDS patients on HAART.32,33 Inhibition of osteoclast activity and increase of apoptosis of osteoclasts by bisphosphonates are considered the key mechanisms responsible for their efficacy in treating osteopenia and osteoporosis.36 Their efficacy in treating HIV-1-infected patients is consistent with the increased osteoclastogenesis induced by AZT in our studies.
New therapeutic agents for treating bone disease include OPG and RANK-Fc fusion proteins. The OPG fusion protein is being evaluated for treatment of various metabolic bone and joint disorders caused by elevation of activated osteoclasts and for diminishing advanced bone pain induced by cancer metastasis.37 RANK-Fc is a recombinant soluble form of RANK, in which the extra-cellular domain is expressed as a fusion protein with human IgG-Fc. Studies in vitro have shown that RANK-Fc functions as an antagonist to RANK-mediated signaling and acts to sequester endogenously produced RANKL.38 The potent antiresorptive effects of RANK-Fc in vivo highlight the potential utility of disrupting RANK signaling as a novel therapeutic approach in humoral hyper-calcemia of malignancy and possibly multiple myeloma and skeletal metastases associated with osteolysis.39 An anti-osteoclastogenic effect of RANK-Fc in HIV-infected cells in vitro has been reported implying that RANK-Fc may provide a therapeutic option for HIV-associated osteopenia and osteoporosis.23 However, no clinical trial treating HIV-associated osteopenia/osteoporosis has been reported.
CONCLUSION
Although AZT and other NRTIs have been beneficial in slowing disease progression of AIDS, osteopenia and osteoporosis are important complications affecting these patients. With the number of AIDS patients receiving HAART increasing, the incidence of osteopenia/osteoporosis is expected to increase. However, there are many controversial issues regarding the pathogenesis and mechanisms responsible for bone loss in HIV-infected patients on HAART. Increased osteoclastogenesis induced by AZT is likely to play a critical role in the pathogenesis of osteopenia and osteoporosis in these patients. Because the osteoclastogenic effect of AZT requires the presence of RANKL, interrupting RANKL by OPG, RANK-Fc, or other compounds may be potent therapeutic options, possibly better than bisphosphonates alone. Carefully controlled clinical prospective trails and additional basic science studies are still required to determine the incidence, etiology, risk factors, and most appropriate treatment for the bone complications in HIV-infected patients.
REFERENCES
- 1.Teitelbaum SL. Bone resorption by osteoclasts. Science. 2000;289:1504–1508. doi: 10.1126/science.289.5484.1504. [DOI] [PubMed] [Google Scholar]
- 2.Serrano S, et al. Bone remodelling in human immunodeficiency virus-1-infected patients. A histomorphometric study. Bone. 1995;16:185–191. doi: 10.1016/8756-3282(94)00028-x. [DOI] [PubMed] [Google Scholar]
- 3.Paton NI, et al. Bone mineral density in patients with human immunodeficiency virus infection. Calcif. Tissue Int. 1997;61:30–32. doi: 10.1007/s002239900288. [DOI] [PubMed] [Google Scholar]
- 4.Amiel C, et al. BMD is reduced in HIV-infected men irrespective of treatment. J. Bone Miner. Res. 2004;19:402–409. doi: 10.1359/JBMR.0301246. [DOI] [PubMed] [Google Scholar]
- 5.Tebas P, et al. Accelerated bone mineral loss in HIV-infected patients receiving potent anti-retroviral therapy. AIDS. 2000;14:F63–F67. doi: 10.1097/00002030-200003100-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moore AL, et al. Reduced bone mineral density in HIV-positive individuals. AIDS. 2001;15:1731–1733. doi: 10.1097/00002030-200109070-00019. [DOI] [PubMed] [Google Scholar]
- 7.Jain RG, Lenhard JM. Select HIV protease inhibitors alter bone and fat metabolism ex vivo. J. Biol. Chem. 2002;277:19247–19250. doi: 10.1074/jbc.C200069200. [DOI] [PubMed] [Google Scholar]
- 8.Carr A, et al. Osteopenia in HIV-infected men: association with asymptomatic lactic acidemia and lower weight pre-anti-retroviral therapy. AIDS. 2001;15:703–709. doi: 10.1097/00002030-200104130-00005. [DOI] [PubMed] [Google Scholar]
- 9.Tsekes G, et al. Body composition changes in protease inhibitor-naive HIV-infected patients treated with two nucleoside reverse transcriptase inhibitors. HIV Med. 2002;3:85–90. doi: 10.1046/j.1468-1293.2002.00105.x. [DOI] [PubMed] [Google Scholar]
- 10.Allison GT, et al. Osteonecrosis in HIV disease: epidemiology, etiologies, and clinical management. AIDS. 2003;17:1–9. doi: 10.1097/01.aids.0000042940.55529.93. [DOI] [PubMed] [Google Scholar]
- 11.Keruly JC, et al. Increasing incidence of avascular necrosis of the hip in HIV-infected patients. J. Acquir. Immune. Defic. Syndr. 2001;28:101–102. doi: 10.1097/00042560-200109010-00017. [DOI] [PubMed] [Google Scholar]
- 12.Belmonte MA, et al. Avascular necrosis of bone in human immunodeficiency virus infection and antiphospholipid antibodies. J. Rheumatol. 1993;20:1425–1428. [PubMed] [Google Scholar]
- 13.Wang MW, et al. The HIV protease inhibitor ritonavir blocks osteoclastogenesis and function by impairing RANKL-induced signaling. J. Clin. Invest. 2004;114:206–213. doi: 10.1172/JCI15797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pan G, et al. AZT enhances osteoclastogenesis and bone loss. AIDS Res. Hum. Retroviruses. 2004;20:608–620. doi: 10.1089/0889222041217482. [DOI] [PubMed] [Google Scholar]
- 15.Pan G, et al. Modulation of osteoclastogenesis induced by nucleoside reverse transcriptase inhibitors. AIDS Res. Hum. Retroviruses (Submitted) 2006;2(6):1–13. doi: 10.1089/aid.2006.22.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Susa M, et al. Human primary osteoclasts: in vitro generation and applications as pharmacological and clinical assay. J. Transl. Med. 2004;2(6):1–13. doi: 10.1186/1479-5876-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takayanagi H, et al. T-cell-mediated regulation of osteoclastogenesis by signalling cross-talk between RANKL and IFN-gamma. Nature. 2000;408:600–605. doi: 10.1038/35046102. [DOI] [PubMed] [Google Scholar]
- 18.Wei S, et al. Interleukin-4 reversibly inhibits osteoclastogenesis via inhibition of NF-kappa B and mitogen-activated protein kinase signaling. J. Biol. Chem. 2002;277:6622–6630. doi: 10.1074/jbc.M104957200. [DOI] [PubMed] [Google Scholar]
- 19.Wei S, et al. IL-1 mediates TNF-induced osteoclastogenesis. J. Clin. Invest. 2005;115:282–290. doi: 10.1172/JCI23394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wong TY, So MK. Primary human immunodeficiency virus infection: heightened awareness needed. Hong Kong Med. J. 2001;7:205–208. [PubMed] [Google Scholar]
- 21.Manoury-Schwartz B, et al. High susceptibility to collagen-induced arthritis in mice lacking IFN-gamma receptors. J. Immunol. 1997;158:5501–5506. [PubMed] [Google Scholar]
- 22.Vermeire K, et al. Accelerated collagen-induced arthritis in IFN-gamma receptor-deficient mice. J. Immunol. 1997;158:5507–5513. [PubMed] [Google Scholar]
- 23.Fakruddin JM, Laurence J. HIV envelope gp120-mediated regulation of osteoclastogenesis via receptor activator of nuclear factor kappa B ligand (RANKL) secretion and its modulation by certain HIV protease inhibitors through interferon-gamma/RANKL cross-talk. J. Biol. Chem. 2003;278:48251–48258. doi: 10.1074/jbc.M304676200. [DOI] [PubMed] [Google Scholar]
- 24.Fakruddin JM, Laurence J. Interactions among human immunodeficiency virus (HIV)-1, interferon-gamma and receptor of activated NF-kappa B ligand (RANKL): implications for HIV pathogenesis. Clin. Exp. Immunol. 2004;137:538–545. doi: 10.1111/j.1365-2249.2004.02568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aukrust P, et al. Decreased bone formative and enhanced resorptive markers in human immunodeficiency virus infection: indication of normalization of the bone-remodeling process during highly active anti-retroviral therapy. J. Clin. Endocrinol. Metab. 1999;84:145–150. doi: 10.1210/jcem.84.1.5417. [DOI] [PubMed] [Google Scholar]
- 26.Biswas DK, et al. Inhibition of HIV-1 replication by combination of a novel inhibitor of TNF-alpha with AZT. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 1998;18:426–434. doi: 10.1097/00042560-199808150-00003. [DOI] [PubMed] [Google Scholar]
- 27.Cossarizza A, Moyle G. Anti-retroviral nucleoside and nucleotide analogues and mitochondria. AIDS. 2004;18:137–151. doi: 10.1097/00002030-200401230-00002. [DOI] [PubMed] [Google Scholar]
- 28.Papadopulos-Eleopulos E, et al. A critical analysis of the pharmacology of AZT and its use in AIDS. Curr. Med. Res. Opin. 1999;15:S1–S45. doi: 10.1185/03007999909114096. [DOI] [PubMed] [Google Scholar]
- 29.Benov L, Fridovich I. Growth in iron-enriched medium partially compensates Escherichia coli for the lack of manganese and iron superoxide dismutase. J. Biol. Chem. 1998;273:10313–10316. doi: 10.1074/jbc.273.17.10313. [DOI] [PubMed] [Google Scholar]
- 30.Tai VW, et al. Effects of recombinant human growth hormone on fat distribution in patients with human immunodeficiency virus-associated wasting. Clin. Infect. Dis. 2002;35:1258–1262. doi: 10.1086/343051. [DOI] [PubMed] [Google Scholar]
- 31.Grinspoon S, Mulligan K. Weight loss and wasting in patients infected with human immunodeficiency virus. Clin. Infect. Dis. 2003;36:S69–S78. doi: 10.1086/367561. [DOI] [PubMed] [Google Scholar]
- 32.Schambelan M, et al. Management of metabolic complications associated with anti-retroviral therapy for HIV-1 infection: recommendations of an International AIDS Society-USA panel. J. Acquir. Immune. Defic. Syndr. 2002;31:257–275. doi: 10.1097/00126334-200211010-00001. [DOI] [PubMed] [Google Scholar]
- 33.Mondy K, Tebas P. Emerging bone problems in patients infected with human immunodeficiency virus. Clin. Infect. Dis. 2003;36:S101–S105. doi: 10.1086/367566. [DOI] [PubMed] [Google Scholar]
- 34.Eiken P, et al. Effects on bone mass after eight years of hormonal replacement therapy. Br. J. Obstet. Gynaecol. 1997;104:702–707. doi: 10.1111/j.1471-0528.1997.tb11981.x. [DOI] [PubMed] [Google Scholar]
- 35.Lawal A, et al. Effect of oxandrolone upon bone mineral density content in malnourished HIV+ patients; Eighth Conference on Retroviruses and Opportunistic Infections; Chicago, IL. Feb. 4–8, 2001; 2001. no.636. [Google Scholar]
- 36.Evio S, et al. Effects of alendronate and hormone replacement therapy, alone and in combination, on bone mass and markers of bone turnover in elderly women with osteoporosis. J. Clin. Endocrinol. Metab. 2004;89:626–631. doi: 10.1210/jc.2003-030198. [DOI] [PubMed] [Google Scholar]
- 37.Luger NM, et al. Osteoprotegerin diminishes advanced bone cancer pain. Cancer Res. 2001;61:4038–4047. [PubMed] [Google Scholar]
- 38.Anderson DM, et al. A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature. 1997;390:175–179. doi: 10.1038/36593. [DOI] [PubMed] [Google Scholar]
- 39.Oyajobi BO, et al. Therapeutic efficacy of a soluble receptor activator of nuclear factor kappaB-IgG Fc fusion protein in suppressing bone resorption and hypercalcemia in a model of humoral hypercalcemia of malignancy. Cancer Res. 2001;61:2572–2578. [PubMed] [Google Scholar]