Abstract
Male Drosophila melanogaster (Canton-S strain) exhibit aggression in competition for resources, to defend territory, and for access to mates. In the study reported here, we asked: (i) how long flies fight; (ii) whether flies adopt distinct winning and losing strategies as hierarchical relationships are established; (iii) whether flies exhibit experience-dependent changes in fighting strategies in later fights; and (iv) whether flies fight differently in second fights against familiar or unfamiliar opponents. The results showed that flies fought for up to 5 h. As hierarchical relationships were established, behavioral strategies changed: winners progressively lunged more and retreated less, whereas losers progressively lunged less and retreated more. Encounters between flies were frequent during the first 10 min of pairing and then dropped significantly. To ask whether flies remembered previous fights, they were re-paired with familiar or unfamiliar opponents after 30 min of separation. In familiar pairings, there were fewer encounters during the first 10 min of fighting than in unfamiliar pairings, and former losers fought differently against familiar winners than unfamiliar winners. Former losers lost or no decision was reached in all second fights in pairings with familiar or unfamiliar winners or with naive flies. Winner/winner, loser/loser, and naive/naive pairings revealed that losers used low-intensity strategies in later fights and were unlikely to form new hierarchical relationships, compared with winners or socially naive flies. These results strongly support the idea that learning and memory accompany the changes in social status that result from fruit fly fights.
Keywords: behavior, dominance, fruit fly, social status, fighting
Most investigations of learning and memory in Drosophila have used controlled classical or operant conditioning paradigms to elicit these phenomena (1–7). Learning and memory have been demonstrated in various social contexts in fruit flies as well (6, 8–11). We have been studying aggression as a behavior indicative of individual fitness in fruit flies and other organisms. Here, we asked whether learning and memory accompany aggression and the changes in social status resulting from dyadic interactions between pairs of male flies.
Recent studies of aggression in Drosophila melanogaster have simplified an experimental setup used in earlier studies (12–14), to demonstrate fighting and decision-making behavior during interactions between pairs of socially naive male flies (15). The resultant ethogram of the behavior listed 13 identifiable offensive and defensive behavioral patterns: 9 were offensive in nature, whereas 4 were defensive. During fights, various combinations of patterns were observed during brief meetings between the flies, called encounters. Over the course of several encounters, a stable hierarchical relationship was established, in which one fly could be designated the “winner,” the other the “loser” (14, 15). Flies that initiated fights at high-intensity levels were more likely to win, suggesting early consequences of high levels of aggression (15). In addition, behavioral strategies seemed to change over the course of a fight (14, 15). Notably, losers took longer to reengage winners after encounters that progressed to high-intensity levels (15). These and related observations raised the possibility that learning and memory might accompany the changes in social status resulting from agonistic interactions between male fruit flies. Fighting behavior between females in competition for a nutrient resource alone (fresh yeast paste) was examined in a subsequent study, and the behavioral patterns observed were compared with male patterns (16). A striking difference between male and female fights was that no stable hierarchical relationships were formed during the female fights.
In a variety of classical or operant conditioning paradigms, fruit flies generate learned behavioral responses that they associate with visual, tactile, or olfactory cues. Through the use of differing training protocols, mutant fly strains and pharmacological manipulations, the resultant memories can be subdivided into distinct categories showing both interdependent and independent properties. These have been most extensively characterized by using an olfactory classical conditioning protocol (for reviews, see refs. 7 and 17), but similarities have been reported in the classification of categories by using operant learning protocols as well (18, 19). In the classical mode, four distinct categories have been identified: (i) short-term memory (decays over ≈1 h, cAMP-dependent); (ii) mid-term memory (decays over 4–5 h, also cAMP-dependent); (iii) anesthesia-resistant memory (lasting up to 4 days, seen after massed or spaced trials but also seen for shorter periods of time after single trials, not dependent on protein synthesis and likely to involve the protein kinase C pathway); and (iv) long-term memory [lasting a week or longer, dependent on spaced trials and protein synthesis, cAMP response element-binding protein (CREB)-dependent] (for reviews, see refs. 4, 7, 17, 20, and 21). Anesthesia-resistant and long-term memory seem to lie along separable and possibly mutually exclusive pathways (22). Learning in a social context is more complex and likely to involve multiple sensory modalities and elements of both classical and operant conditioning. In one example of socially induced learning and memory, D. melanogaster females in groups become conditioned to low levels of activity and avoid aggressive encounters with other flies (11). This experience leads to a long-lasting behavioral change that can be observed after only 15 min of social exposure. A particularly well studied example of the social modulation of behavior is the courtship suppression seen toward virgin females when males have been preexposed to mated, unreceptive females (called courtship conditioning; refs. 6, 8–10, and 23–26). Like classical conditioning, courtship conditioning involves short- and long-term components. Effects can last as long as 9 days after training depending on the training protocol used (24).
Here, we demonstrate that learning and memory, in the form of fight strategy adaptation by winners and losers and status-dependent behavioral changes, accompany aggression between pairs of socially naive male fruit flies. The studies focus on explorations of the following: (i) the nature of the dominance relationship established in extended fight trials between pairs of male Drosophila, (ii) the persistence of the dominance relationship and related changes in behavioral strategy after a period of separation, and (iii) the transfer of status-related behaviors to pairings with new opponents.
Results
Indicators of Dominant and Subordinate Status.
Despite their relative genetic homogeneity, enormous variability is seen in fruit fly fights in terms of the latency to begin fighting, the number of encounters and the duration of the fights. Accordingly, large numbers of trials had to be carried out to gather statistically valid data sets. The number of valid trials obtained for each experimental series is indicated in the text describing that series. In the first study, 63 pairs of male flies were introduced into fight chambers (as described in ref. 15) under normal laboratory conditions in competition for a headless female resource positioned on a small food cup. In 18 of these pairings, interactions were observed on the food surface and recorded for 5 h after introducing flies into the chamber. Status was assigned by using the following definition: after three lunges by one fly (with no intervening retreats) and three retreats by the opponent (with no intervening lunges), the fly that lunged was identified as the winner and the fly that retreated as the loser. We saw essentially no reversals in status assignment once this criterion was satisfied. Fights not satisfying this criterion were considered “draws.” In 12/18 pairings in the first series of studies, status could be assigned to opponents based on the three-lunge/three-retreat rule. Within 30 min, winners identified by this method spent significantly more time on the food surface than losers (two-factor repeated measures ANOVA, main effect of Status, P < 0.01, n = 12 trials) (Fig. 1A).
Of the behavioral patterns scored, we focused on lunge and retreat because of their overall high frequencies and the systematic status-dependent changes in their usage by animals over the course of a fight. After transferring the experimental set-up to an isolated, dark, humidity-controlled room, a new dataset was gathered: out of 51 trials in which the two male flies were present simultaneously on the food surface, 45 pairs of flies established stable dominance relationships according to the three-lunge/three-retreat rule. Of these, 30 pairs engaged in at least 30 encounters. Lunging and retreating in the first 30 encounters of these fights were analyzed in detail. Over the course of successive encounters, and beginning early in a fight, winners lunged significantly more than losers (two-factor repeated measures ANOVA, interaction P < 0.01, main effect of encounter P < 0.01, main effect of status, P < 0.01, n = 30 trials) (Fig. 1B). In contrast, losers retreated significantly more than winners (two-factor repeated measures ANOVA, interaction P < 0.01, main effect of encounter P < 0.01, main effect of status, P < 0.01, n = 30 trials) (Fig. 1C).
No major differences were observed in the ways flies fought in this series compared with earlier results (15). In the 5-h trials, the latency for both flies to appear on the surface in successful trials varied widely from 0.3 to 245 min, with a mean value of 100.8 ± 36.5 (mean ± SEM) min. In analyzing the fights, we considered T = 0 to be the time of the first encounter between the flies, regardless of the latency, to minimize effects of circadian variations in aggression and to allow standardization of the data set. On average, fights lasted 66.2 ± 29.5 (mean ± SEM) min in a 5-h trial and included 59 ± 20.6 (mean ± SEM) encounters, or ≈0.89 encounters per min.
The analysis of lunges and retreats suggested that these measures could be used reliably to assign dichotomous labels of social status. We sought to validate this method by using data from 30 trials in which there were at least 40 encounters (12 trials from the previous data set and 18 new pairings of naive male flies) by computing a discriminant function and testing its ability to separate individual flies grouped by the status designation obtained by using the three-lunge/three-retreat rule. This function was based on values of seven quantitative variables observed in the first 40 encounters of these 30 fights. The data for winners and losers were split into two groups. Each group, used to compute and test the discriminant function respectively, was made up of 30 cases of flies from 30 different fights (15 winners + 15 losers). These groups were determined by listing the fights in order of data acquisition and assigning each individual case to each group alternately. Thus, 30 cases were used to develop a model to discriminate between the two levels of status and then 30 cases were subjected to this model to test its ability to predict the status of unlabeled cases.
The seven behavioral patterns included as predictive variables in the discriminant function analysis were as follows: approach, lunge, retreat, hold, chase, wing flick, and wing threat. These were selected because they are demonstrated by an individual fly, as opposed to fencing, boxing, and tussling, which are dyadic. By using a stepwise selection algorithm, it was determined that all seven variables were significant predictors of status (approaches: Wilk's lambda = 0.599, approximate F = 18.74, P < 0.001; wing threats: Wilk's lambda = 0.428, approximate F = 18.06, P < 0.001; retreats: Wilk's lambda = 0.395, approximate F = 13.30, P < 0.001; wing flicks: Wilk's lambda = 0.378, approximate F = 10.27, P < 0.001; chases: Wilk's lambda = 0.377, approximate F = 7.93, P < 0.001; lunges: Wilk's lambda = 0.377, approximate F = 6.34, P < 0.001; holds: Wilk's lambda = 0.377, approximate F = 5.20, P < 0.001).
Discriminant function values for winners and losers, calculated by using the single standardized discriminant function [(−0.40194 retreats) + (0.0549097 lunges) + (0.297713 wing flicks) − (0.0277213 holds) + (0.0564062 chases) + (0.596997 wing threats) + (0.923701 approaches)] were significantly different (Student's t = −6.80878, P < 0.001) and overlapped in the region of 0 (Fig. 1D; see supporting information, which is published on the PNAS web site). In this standardized discriminant function, a linear combination of predictor variables, the standardized coefficient applied to each term is reflective of how each individual variable is being used to discriminate between the two groups: winners and losers. This function was statistically significant (Wilk's Lambda 0. 376549, P = 0.0012), and 28 of 30 (93.33%) of the flies included in the computation of the function were separated into the same groups arrived at by using the three-lunge/three-retreat rule. Of the 30 cases kept aside for testing the function, 27 of 30 (90%) were classified the same as the three-lunge/three-retreat rule. The five animals that were classified differently in the entire analysis (60 total cases) were winners of low-intensity fights and losers of high-intensity fights. Thus, a discriminant function based only on the frequencies of behaviors exhibited by individual flies with no information about the actions of an opponent (i) gave results that were remarkably similar to the three-lunge/three-retreat rule for classification based on dyadic interactions and (ii) differed from the three-lunge/three-retreat rule in a predictable way.
Persistence of Hierarchical Relationship and Individual Recognition.
Another striking feature of long trial periods was that the number of encounters between socially naive opponents dropped significantly after the first 10 min of interaction, compared with eight subsequent 10-min time bins (1-factor repeated measures ANOVA, P < 0.01, n = 43 trials) (Fig. 2A). We used this finding as one criterion to ask whether fruit flies remember a familiar opponent after a period of separation. After the first 90-min fight period, winning and losing flies were returned to their original isolation chambers for 30 min. We then paired them in test fights with familiar and unfamiliar opponents. Familiar opponents had significantly fewer encounters and spent less time on the food surface in the first 10 min of these second fights than naive opponents, whereas unfamiliar opponents were not different from naive flies in either category (1-factor ANOVA, P < 0.01, n = 16, 13, 7 trials) (Fig. 2B). There also was evidence of altered fighting strategy in flies depending on whether they were fighting against familiar or unfamiliar opponents. Unfamiliar opponents were flies that faced different opponents in their second fights than in their first fights. Losers paired with unfamiliar winners lunged more in the first 10 encounters of their second fights than losers paired with familiar winners [Kruskal–Wallis statistic 12.2, Gaussian approximation P < 0.05, Dunn's multiple comparison test, familiar winner/loser (fW/L) vs. unfamiliar winner/loser (ufW/L) P < 0.05] (Fig. 2C).
Strikingly, the outcomes of second fights between familiar opponents were invariably the same as the first fights (Table 1). There seemed to be a strong loser effect: whether paired with familiar winners, unfamiliar winners, or naive opponents, experienced losers did not win any second fights, although half drew fights against naive opponents. By contrast, experienced winners won, lost, and drew at comparable frequencies in second fights against other experienced winners, as did naive control flies in their first fights against each other (Table 1).
Table 1.
Outcomes of second fights and control fights
| Winner |
Loser |
Naive |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| vs. | W | fL | ufL | N | Total | L | fW | ufW | N | Total | N | W | L | Total |
| Win | 1 | 5 | 6 | 4 | 16 | 3 | 0 | 0 | 0 | 3 | 7 | 5 | 6 | 18 |
| Loss | 1 | 0 | 0 | 5 | 6 | 3 | 5 | 6 | 6 | 20 | 7 | 4 | 0 | 11 |
| Draw | 0 | 0 | 1 | 4 | 5 | 2 | 5 | 1 | 6 | 14 | 11 | 4 | 6 | 21 |
| Total | 2 | 5 | 7 | 13 | 27 | 8 | 10 | 7 | 12 | 37 | 25 | 13 | 12 | 50 |
Counts of wins, losses, and draws by experienced winner, experienced loser, and socially naive flies paired with experienced and naive opponents.
Winner and Loser Strategies.
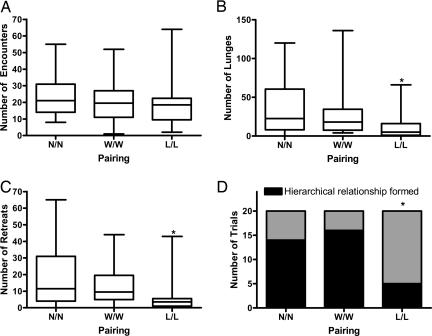
To address the question of whether both winners and losers adopt different experience-dependent fighting strategies in their second fights, we analyzed encounter number, lunges, retreats and fight outcome, in pairings of naive flies and in winner/winner, and loser/loser second fights. For this analysis, status assignment of winners and losers was based on a five-lunge/five-retreat rule as a more stringent criterion for inclusion of experienced animals in this block of experiments, aimed at testing the stability of status-dependent behaviors. For the naive/naive pairings, a set of socially naive animals were exposed alone to fight chambers for 90 min and returned to their isolation vials. After the 30-min isolation interval, pairs of naive animals, pairs of winners, and pairs of losers were introduced to novel fighting chambers for 60 min. All three groupings of flies had similar numbers of encounters in 60-min test fight periods (Kruskal–Wallis statistic 2.216, Gaussian approximation P value 0.3301) (Fig. 3A). Loser pairs exhibited significantly fewer lunges (Kruskal–Wallis statistic 8.925, Gaussian approximation P < 0.05, Dunn's multiple comparison test N/N vs. L/L, P < 0.05) (Fig. 3B) and significantly fewer retreats than naive pairs (Kruskal–Wallis statistic 7.5, Gaussian approximation P < 0.05, Dunn's multiple comparison test N/N vs. L/L, P < 0.05) (Fig. 3C), suggesting that loser fights were lower in intensity. We found no significant differences in any of these measurements between naive pairs and winner pairs. The most striking finding was that despite comparable numbers of encounters fewer loser/loser pairs formed hierarchical relationships than naive/naive and winner/winner pairs (two-tailed χ2 = 14.13, df = 2, P < 0.001) (Fig. 3D).
Discussion
The data presented here demonstrate that learning and memory play prominent roles in aggression in Drosophila. Socially naive male flies change their fighting strategy during fights, with mid-intensity lunging behavior becoming the main component shown by winners, and retreating being the main component shown by losers. Flies continue to fight for periods of time of up to 5 h, even though it takes losers longer to return to the food surface as the fight progresses. After a hierarchical relationship has been established and flies have been separated for 30 min, losing flies fight differently against familiar and nonfamiliar opponents, but lose to all opponents except other losers. In the latter pairings a loser can become a winner in a small percentage of the fights.
Whereas in many respects, intraspecific aggression in Drosophila resembles that seen in other animals, it is unusual in that losing flies continually reengage winners. This tendency may be because flies have no dangerous weapons that threaten the health of a returning loser. With resources available on the food surface and with no threat to survival, it is advantageous for losers to return to the food surface, although with retreat as their preferred behavioral strategy. Winners, by contrast, tend to remain near the headless female in the center of the food cup, where they spend most of their time, and are more likely to lunge at an approaching loser.
Our analyses demonstrate that, after a hierarchical relationship has been established, lunging is the preferred strategy of winners and retreating of losers. We would like to know how long these behavioral changes last in the absence of an opponent. At this point, we know they are retained for up to 30 min, the time period examined here, which places them in the range of short- or mid-term memory (7, 17, 18). In future tests we will alter the behavioral protocol, for example by spaced repeated pairings of flies, to determine whether the duration of the memory can be extended, as seen with other learning and memory protocols in Drosophila (6, 7, 17, 20, 21, 24).
In other species, long-term winner and loser effects have been reported, according to which winners of one fight are more likely to win and losers more likely to lose their next fights (27–29). The persistence of these effects depends on status: losers tend to “remember” their status for longer periods of time than winners. Such effects could be important for long-term survival, as losing animals engage in subsequent fights with greater caution after a loss. The half-life of behavioral effects of this type varies widely across species depending on the social environment and the number of intraspecific interactions taking place. In the studies reported here, it seems that aggression-induced behavioral changes may be longer-lasting in losers than in winners. Losers do not win second fights against any opponents other than other losers, and even here, only in a small percentage of the trials. Winners, by contrast do not seem very different from naive flies in the outcomes of second fights. Future experiments will explore further the duration of the behavioral changes triggered by the establishment of hierarchical relationships during fruit fly fights.
One consideration in evaluating the results we present here is whether within a normal population of Canton-S flies, one group has an inherently higher probability of becoming losers, whereas another group has a higher probability of becoming winners. That such effects might be found is suggested by the recent demonstration that selection techniques can be used to identify high and low aggression flies in wild type populations, and that differences in expression levels of groups of genes accompany the behavioral differences (30). The results presented here could be interpreted as suggesting that losers are losers from the start of their first fights. We believe this not to be the case, however, for several reasons. First, losers can and do lunge in first fights, although as fights progress they use this behavior less and less and use retreat behavior more and more. In second fights, losers do not lunge against familiar opponents, but do lunge against unfamiliar opponents (Fig. 2) suggesting that recognition of previous opponents influences how losers fight. Second, losers can become winners, even though at a reduced rate when compared with paired naive flies or paired winners. For this change of status to happen, losers must use mid-intensity lunging behavior (data not shown). These findings, whereas not completely ruling out that a genetic component might exist in the decision making process, point instead to an operant-like model of behavior relating to who will be the ultimate winner or loser of a fight. In this model, one fly lunges at an opponent. If the opponent retreats, the fly uses that strategy more and more often to gain control of the resources (potential mate, food). If the opponent retaliates with a lunge, which happens in a significant number of cases, the fly learns nothing relating to the outcome of a fight from using that strategy and must try other approaches to control the resources. The ultimate loser of a fight, by contrast, learns that retreat and forgoing desired resources may be a preferred route to avoid possible injury from an opponent that has just reared up and snapped down on its body.
A surprising outcome of these studies is that flies seem capable of recognizing individual conspecifics. This interpretation is suggested by the analyses of second fights in which we pair familiar and unfamiliar winners and losers. Against familiar opponents there are fewer meetings between the flies and the losers fight with different strategies than they use against unfamiliar or naive opponents. How flies recognize each other in re-pairings during fights is unknown. One possibility is that cuticular hydrocarbons may be involved (25, 26), because fencing, in which flies tap the surface of opponents, is the most prominent behavioral pattern seen during fruit fly fights and is commonly seen during the initial approach and contact between opponents. Whereas differences in the cuticular hydrocarbon profiles between individual males or females of any fly line are reportedly small (25), they still might be detectable and used for recognition purposes. This mechanism has been elegantly demonstrated in courtship conditioning experiments showing that the learning of the behavioral suppression depends on hydrocarbon olfactory cues given off by the behavioral trainers with males being able to distinguish between immature virgins, mature virgins and mature mated flies in the subsequent suppression of the behavior (26). Further studies will be required to address this issue as well.
Despite remaining unanswered questions, the basic results presented in this paper are unambiguous. Learning and memory do accompany changes in social status resulting from fights between pairs of male fruit flies. The wealth of genetic tools available for studies of learning and memory in Drosophila now can be applied in this complex social context, thereby adding greatly to the value of this model system for the study of aggression.
Materials and Methods
Animal Care.
The common laboratory Canton-S strain of D. melanogaster was used in all experiments. The flies were raised in standard vials containing food (0.9% agar medium containing 10.5% dextrose, 5% cornmeal, 2.6% bakers yeast, and 0.23% tegocept, to which active yeast is added) prepared by the laboratory of D. van Vactor at Harvard Medical School. The same food was used in all experiments. Adult flies were housed in temperature- and humidity-controlled incubators (25°C, 50% humidity) on a 12:12-h day/night cycle. In earlier experiments, male flies were isolated by aspiration immediately after eclosion and housed individually for 3–5 days in glass vials (16-mm diameter) containing ≈2 ml of food. In the later experiments, pupae were isolated before emergence. Approximately 2 days before experimentation, flies were anesthetized with CO2 and painted with a dot of colored acrylic paint on the dorsal thorax for identification in digital video records.
Chamber.
An experimental chamber described previously in ref. 15 was used in all experiments. In brief, the square chamber used in fights consisted of four clear glass half-microscope slides (3.75 cm × 2.5 cm × 1 mm) cemented together at the edges and placed in 5 mm of 2% agarose. A food cup (1.5-cm diameter, 1-cm height) containing fresh fly food with drops of apple juice on the surface was placed in the center. The chamber was covered with an inverted Petri dish lid that had small holes for ventilation and a larger hole for introduction of the flies by aspiration. An external light source was focused on the food surface through a circular piece of black filter paper with a hole cut in the center.
Animal Handling During Experiments.
In all experiments, fresh food cups were prepared and virgin Canton-S females were anesthetized with CO2, decapitated, and placed on the food surface, with the thorax and abdomen elevated. Isolated males of the same ages and similar sizes were paired randomly for trials at the start of “lights on” in the incubators. Flies were introduced simultaneously into the experimental chamber by gentle aspiration. Trials were recorded immediately after the introduction of the males on Sony Video8 digital tapes (Tape and Media Corp., Danbury, CT) with Sony Digital8 Handycams (Ritz Camera, Irvine, CA). In the first set of experiments, 5-h trials were carried out in a general laboratory area. Fights were recorded continuously for 5 h, regardless of when both flies first landed on the food surface. Trials in which two male files failed to simultaneously occupy the food surface were discarded without analysis. In these experiments, we observed considerable variability in the latency for the two flies to land on the food surface, and we observed reduced numbers of interactions per 10-min time bin compared with those reported in our earlier studies (15). For the second set of studies (the social learning paradigm), therefore, we transferred our apparatus to a light- and humidity-controlled, dedicated room. Data generated in this room closely resembled the original results (15). In this group of experiments, flies were allowed to fight for 90 min (“fight period”), then separated for 30 min (“break period”) by returning them to their home vials, then paired again with familiar or nonfamiliar opponents for an additional hour (“test period”). Trials in which both flies did not occupy the food surface simultaneously after 90 min during the fight period were discarded. The first 90-min periods also were used for calculations of changes in fight strategy after a hierarchical relationship was established. Naive male flies were not paired for fights during the first 90 min. Instead, they were introduced individually into an experimental chamber at lights on and were removed, by gentle aspiration, between 90 and 180 min later. These flies were returned to their home vials for the 30-min break period and then were paired for fights during the test period. During the test period, video recordings were made for 1 h.
Data Gathering and Statistical Analyses.
Digital videotapes were examined by using iMovie on Macintosh computers (all from Apple Computers, Inc.). Individual encounters were clipped from full-length records and these were scored for behavioral patterns by using Microsoft Excel (Microsoft Corporation) spreadsheets. Discriminant function analysis was performed by using Statgraphics centurion software (Statpoint, Inc., Herndon, VA). All other statistical analyses were performed by using GraphPad Prism 4 (GraphPad Software, Inc., San Diego, CA).
Supplementary Material
Fig. 1.
Indicators of dominant and subordinate status. (A) Within the first 30 min of interaction, losers spent significantly less time on the food cup surface than winners (two-factor repeated measures ANOVA, mean ± SEM, n = 12 trials). (B) Over the course of successive encounters, winners lunged significantly more than losers (two-factor repeated measures ANOVA, mean ± SEM, n = 30 trials). (C) Losers retreated significantly more than winners (two-factor repeated measures ANOVA, mean ± SEM, n = 30 trials). (∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001 in post hoc Bonferroni multiple comparison test.) (D) A discriminant function based on approach, lunge, retreat, hold, chase, wing flick, and wing threat in 40 encounters classified 28 of 30 flies (93.33%) the same as the within-fight three-lunge/three-retreat rule. The x axis represents computed values of the discriminant function for individual cases and the y axis represents the number of observations in each bin of discriminant function values for winners (gray bars) and losers (white bars).
Fig. 2.
Persistence of dominance relationship and individual recognition. (A) The number of encounters between socially naive opponents drops significantly after the first 10 min from the beginning of the fight (1-factor repeated measures ANOVA, mean ± SEM, n = 43 trials; ∗∗, P < 0.05 in post hoc Dunnett's multiple comparison test). (B) Familiar opponents had significantly fewer encounters in the first 10 min than naive opponents, whereas unfamiliar opponents did not (1-factor ANOVA, mean ± SEM, n = 16, 13, 7 trials; ∗, P < 0.05 in post hoc Bonferroni multiple comparison test). (C) Losers paired with unfamiliar winners lunged more in the first 10 encounters of their second fights than losers paired with familiar losers (Kruskal–Wallis statistic 12.2, Gaussian approximation P < 0.05, Dunn's multiple comparison test fW/L vs. ufW/L P < 0.05). Lunges by losers of naive/naive pairings are included as a control.
Fig. 3.
Winner and loser strategies in re-pairings. (A) Naive/naive, winner/winner, and loser/loser pairs had similar numbers of encounters in 60 min (Kruskal–Wallis statistic 2.216, Gaussian approximation P value 0.3301). (B) Loser pairs exhibited significantly fewer lunges than naive pairs (Kruskal–Wallis statistic 8.925, Gaussian approximation P < 0.05, Dunn's multiple comparison test N/N vs. L/L P < 0.05). (C) Loser pairs exhibited significantly fewer retreats than naive pairs (Kruskal–Wallis statistic 7.5, Gaussian approximation P < 0.05, Dunn's multiple comparison test N/N vs. L/L P < 0.05). (D) Fewer loser/loser pairs formed heirarchical relationships than naive/naive and winner/winner pairs (two-sided χ2 = 14.13, df = 2, P < 0.001).
Acknowledgments
We thank members of the Kravitz laboratory (S. Certel, S. Nilsen, Y.-B. Chan, J. Yew, E. Black, C. Lee, S. Chen, and A. Lee) for many helpful discussions and for careful critiquing of these studies. This work was supported by National Institutes of Health Grant GM072411, by National Science Foundation Grant IBN-0090730, and by a grant from the Mind Brain Behavior Interfaculty Initiative at Harvard University.
Abbreviations
- f
familiar
- uf
unfamiliar
- W
winner
- L
loser
- N
naive.
Footnotes
The authors declare no conflict of interest.
References
- 1.Tully T, Quinn WG. J Comp Physiol. 1985;157:263–277. doi: 10.1007/BF01350033. [DOI] [PubMed] [Google Scholar]
- 2.Wolf R, Heisenberg M. J Comp Physiol A. 1991;169:699–705. doi: 10.1007/BF00194898. [DOI] [PubMed] [Google Scholar]
- 3.Wustmann G, Rein K, Wolf R, Heisenberg M. J Comp Physiol A. 1996;179:429–436. doi: 10.1007/BF00194996. [DOI] [PubMed] [Google Scholar]
- 4.Waddell S, Quinn WG. Trends Gen. 2001;17:719–726. doi: 10.1016/s0168-9525(01)02526-4. [DOI] [PubMed] [Google Scholar]
- 5.Putz G, Heisenberg M. Learn Mem. 2002;9:349–359. doi: 10.1101/lm.50402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siwicki KK, Ladewski L. Behav Proc. 2003;64:225–238. doi: 10.1016/s0376-6357(03)00137-2. [DOI] [PubMed] [Google Scholar]
- 7.Dubnau J, Chiang A-S, Tully T. J Neurobiol. 2003;54:238–253. doi: 10.1002/neu.10170. [DOI] [PubMed] [Google Scholar]
- 8.Siegel RW, Hall JC. Proc Natl Acad Sci USA. 1979;76:3430–3434. doi: 10.1073/pnas.76.7.3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tompkins L, Siegel RW, Gailey DA, Hall JC. Behav Genet. 1983;13:565–578. doi: 10.1007/BF01076402. [DOI] [PubMed] [Google Scholar]
- 10.Kamyshev NG, Iliadi KG, Bragina JV. Learn Mem. 1999;6:1–20. [PMC free article] [PubMed] [Google Scholar]
- 11.Kamyshev NG, Smirnova GP, Kamysheva EA, Nikiforov ON, Parafenyuk IV, Pnomarenko VV. Neurosci Behav Physiol. 2002;32:401–408. doi: 10.1023/a:1015832328023. [DOI] [PubMed] [Google Scholar]
- 12.Jacobs ME. Ecology. 1960;41:182–188. [Google Scholar]
- 13.Dow MA, von Schilcher F. Nature. 1975;254:511–512. doi: 10.1038/254511a0. [DOI] [PubMed] [Google Scholar]
- 14.Hoffman AA. Anim Behav. 1987;35:807–818. [Google Scholar]
- 15.Chen S, Lee AY, Bowens NM, Huber R, Kravitz EA. Proc Natl Acad Sci USA. 2002;99:5664–5668. doi: 10.1073/pnas.082102599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nilsen SP, Chan YB, Huber R, Kravitz EA. Proc Natl Acad Sci USA. 2004;101:12342–12347. doi: 10.1073/pnas.0404693101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McGuire SE, Deshazer M, Davis RL. Prog Neurobiol. 2005;76:328–347. doi: 10.1016/j.pneurobio.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 18.Xia S, Liu L, Feng C, Guo A. Learn Mem. 1997;4:205–218. doi: 10.1101/lm.4.2.205. [DOI] [PubMed] [Google Scholar]
- 19.Heisenberg M, Wolf R, Brembs B. Learn Mem. 2001;8:1–10. doi: 10.1101/lm.8.1.1. [DOI] [PubMed] [Google Scholar]
- 20.Tully T, Preat T, Boynton SC, DelVecchio M. Cell. 1994;79:35–47. doi: 10.1016/0092-8674(94)90398-0. [DOI] [PubMed] [Google Scholar]
- 21.Dubnau J, Tully T. Annu Rev Neurosci. 1998;21:407–444. doi: 10.1146/annurev.neuro.21.1.407. [DOI] [PubMed] [Google Scholar]
- 22.Isabel G, Pascual A, Preat T. Science. 2004;304:1024–1027. doi: 10.1126/science.1094932. [DOI] [PubMed] [Google Scholar]
- 23.Vaias LJ, Napolitano LM, Tompkins L. Behav Gen. 1993;23:91–97. doi: 10.1007/BF01067558. [DOI] [PubMed] [Google Scholar]
- 24.McBride SM, Giuliani G, Choi C, Krause P, Correale D, Watson K, Baker G, Siwicki KK. Neuron. 1999;24:967–977. doi: 10.1016/s0896-6273(00)81043-0. [DOI] [PubMed] [Google Scholar]
- 25.Siwicki KK, Riccio P, Ladewski L, Marcillac F, Dartevelle L, Cross SA, Ferveur JF. Learn Mem. 2005;12:636–645. doi: 10.1101/lm.85605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ejima A, Smith BPC, Lucas C, Levine JD, Griffith LC. Curr Biol. 2005;15:194–206. doi: 10.1016/j.cub.2005.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chase ID, Bartolomeo C, Dugatkin LA. Anim Behav. 1994;48:393–400. [Google Scholar]
- 28.Whitehouse MEA. Anim Behav. 1997;53:913–923. [Google Scholar]
- 29.Hsu Y, Wolf LL. Anim Behav. 1999;57:903–910. doi: 10.1006/anbe.1998.1049. [DOI] [PubMed] [Google Scholar]
- 30.Dierick HA, Greenspan RJ. Nat Genet. 2006;38:1023–1031. doi: 10.1038/ng1864. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.