Abstract
It is widely accepted that natal philopatry is a prerequisite for the evolution of sociality. The life-history hypothesis maintains that longevity of adults results in extended territory tenure and thus limits breeding vacancies for offspring, which makes natal philopatry more likely. Here, we tested the importance of longevity for natal philopatry in females of a basal primate, the grey mouse lemur (Microcebus murinus). This species is regarded as being solitary due to its foraging habits but while males disperse, female offspring in this species forgo dispersal and form long-term sleeping groups with their mothers. We tested whether high adult survival could be a cause for natal philopatry of female offspring. In addition, we assessed costs and benefits associated with space sharing between mothers and daughters and whether mothers actively increase survival of daughters by beqeauthal of territories, information transfer about resources or thermoregulation. Contrary to our predictions, adult females had low-survival rates. Space sharing appeared to improve survival of both, mothers and daughters. This could be a result of information transfer about sleeping sites and thermoregulatory benefits. Our results cast doubt on the idea that longevity predisposes species for social traits and provide support for benefits of philopatry.
Keywords: Microcebus murinus, natal philopatry, annual survival, sociality, cooperative breeding
1. Introduction
Delayed dispersal of offspring is thought to be one of the key factors which may lead to the evolution of sociality (Hatchwell & Komdeur 2000). Although a number of hypotheses have been proposed to explain natal philopatry (e.g. Kokko & Johnstone 1999; Pen & Weissing 2000), two have been the most influential. Only differing in the emphasis on the cost of leaving or the benefits of staying the ‘ecological constraints hypothesis’ (Emlen 1984) and ‘benefits of philopatry hypothesis’ (Stacey & Ligon 1987, 1991) are widely accepted as explanations for the evolution of delayed dispersal. In both cases, the chances for the successful establishment of independent breeding territories for offspring are constrained due to a lack of vacancies, mates or high mortality of dispersers. Alternatively, the ‘life-history hypothesis’ suggests that high-adult survival rates cause low territorial turnover thus limiting the availability of breeding territories (Brown 1987; Rowley & Russell 1990). Like these, most models aiming to explain natal philopatry and the evolution of sociality have been developed for cooperatively breeding species. However, cooperative breeding is a rare and advanced form of sociality that entails natal philopatry, delayed reproduction and care for others' offspring (Solomon & French 1997). This can potentially limit the generalization of such models (Emlen 1997) and tests in species with less advanced sociality are urgently needed to evaluate the validity of these hypotheses for explaining natal philopatry in general.
Patterns of philopatry and dispersal are often sex-specific and Greenwood (1980) has suggested that this is related to the mating system of a species (i.e. resource defence versus mate defence). He argues that the sex, which competes for resources for reproduction (females in most mammals) should be philopatric while the other sex disperses. Natal philopatry often leads to a kin-structured population and could thus be a precursor for the evolution of sociality.
An offspring's decision to remain philopatric may require the consent of a parent. Delayed dispersal could lead to resource competition between parent and offspring (Wolff 1992; Johnson et al. 2001) and as a result impair a parent's survival and reproductive success (Lambin et al. 2001). However, the costs of a philopatric offspring to a parent may vary with the reproductive value of a parent (Hamilton & May 1977) and tolerance should increase with the age of a parent (Ronce et al. 2000). Age was indeed an important variable for decisions concerning breeding dispersal in the North American red squirrel (Tamiasciurus hudsonicus) with older mothers being more likely to move their territory (Berteaux & Boutin 2000). Extended parental investment appears to be particularly advanced in red squirrels as mothers in this species obtain resources for offspring long before parturition (Boutin et al. 2000). However, this constitutes an extreme form of parental investment and it should be more likely that a parent shares resources with an offspring if it increases offspring survival without additional costs to the parent (Waser & Jones 1983). This appears to be the case for females in species like kangaroo rats (Dipodomys spectabilis, Jones 1984) and bushy-tailed woodrats (Neotoma cinerea, Moses & Millar 1994). Resource sharing with offspring can lead to the kin-structured groups characteristic of a number of primates (de Ruiter & Geffen 1998; Boinski et al. 2005). However, in callitrichids, a family of South American primates, female natal philopatry entails delayed reproduction of offspring (French 1997) and family groups form cooperatively breeding units. Nevertheless, female offspring may still benefit by inheriting a parent's territory after the mother's death (e.g. Goldizen & Terbourgh 1989; Heymann 1998; Lazaro-Perea et al. 2000). Isbell (2004) suggested that kin grouping is beneficial as long as it does not impair a mother's future reproduction and successful dispersal for a daughter is unlikely. These assumptions parallel those of the benefits of philopatry and life-history hypotheses suggesting that the application of these hypotheses to a primate model could produce useful insights in the evolution of natal philopatry.
The aim of this study was to test predictions derived from the benefits of philopatry and the life-history hypotheses in a basal primate with primitive social tendencies—the grey mouse lemur (Microcebus murinus). Grey mouse lemurs are endemic to Madagascar and similar to other small mammals they exhibit a nocturnal lifestyle, solitary foraging and one to four young per litter (Mittermeier et al. 1994). Females form sleeping groups that consist of closely related individuals (e.g. mothers and daughters, Radespiel et al. 2001a) and raise their offspring in communal nests (communal breeders sensu Solomon & French 1997; Eberle & Kappeler 2003, H. Lutermann, unpublished data) possibly gaining thermoregulatory benefits for their young (Perret 1998). Females produce up to two litters per season (Schmelting et al. 2000). Due to altricial offspring and the use of higher quality sleeping sites (i.e. tree holes) compared to males, sleeping sites are considered a key resource for female reproduction (Radespiel et al. 1998). Grey mouse lemurs have male-biased dispersal patterns and most males leave the natal site before their first mating season (Radespiel et al. 2003). Thus, populations show female kin clustering on a local and micro-geographical scale (Wimmer et al. 2002; Fredsted et al. 2004). We tested the following predictions regarding natal philopatry in grey mouse lemurs.
The annual survival of adult females should be high, thus creating long-territory tenures.
Philopatric offspring should benefit by territory bequeathal and successful mothers (i.e. females with surviving female offspring) are likely to shift their home ranges. Bequeathal involves active, strategic dispersal by breeding females. Evidence for territory bequeathal is limited (Lambin 1997) and offspring may instead benefit by taking over their mothers' territory after her death.
Alternatively, information transfer about resources could lead to increased survival of female offspring. In this latter case, we would expect higher survival rates for female offspring until their first breeding season when their mothers survive between two breeding seasons (surviving mothers) than for daughters that lost their mothers.
Resource competition with philopatric daughters can potentially incur costs for mothers leading to increased mortality. In this case, we would expect higher annual survival for females without daughters.
2. Material and methods
(a) Study site and estimation of survival at the population level
Details of the study site and field methods have been provided elsewhere (Radespiel et al. 2001b, 2003). Briefly, the study site was a 30 ha plot of dry deciduous forest in the Reserve forestière d'Ampijoroa (46°48′ E, 16°19′ S) about 120 km southeast of Mahajanga, northwestern Madagascar. Field data were collected over the period 1995–2000. We calculated individual survival separately for the two sexes from mark-recapture data using the Kaplan–Meier estimate (Krebs 1999):
| 2.1 |
Where di=number of mouse lemurs that disappeared between time i and time i+1, and ri= number of mouse lemurs alive at time i. All first captures of a year were assigned to the birth cohort of the former year. As births occur from late November through to March (Schmelting et al. 2000) we defined December of the year prior to an individuals first capture as the assumed birth month. We assumed that only the percentage of the population that was recaptured was present in the study site and defined animal attrition from the study population as being the result of death. This assumption is supported by the observation that no animal has ever reappeared in the capture population after being absent for two or more years (B. Schmelting, unpublished data). With an estimate of 25%, mouse lemurs have the highest predation rate known for any primate species (Goodman et al. 1993). We could account for some mortality in our study population by the loss of some of our radio collared animals to predators. The radio collars of two females eaten by snakes (Leioheterodon madagascariensis and Sanzinia madagascariensis, respectively) were still working and the remains of four other individuals suggested predation by fossa (Cryptoprocar ferrox) or one of the raptor species present in the area. Despite the high predation rate, we probably underestimated life expectancy for the dispersing sex (i.e. males). Females are known to disperse occasionally (less than 14%). However, their dispersal distances never exceeded two home range diameters, which was well below the maximum distance of 1000 m that was detected for males in our study area (Radespiel et al. 2003). We are thus confident that the survival rates estimated from capture data constitute an accurate measure, particularly for the females this study focuses on.
(b) Space use of mother–daughter pairs
We established mother–daughter dyads by using microsatellite data obtained from tissue samples collected during captures. These data were available for animals captured between 1995 and 1998 and analyses for mother–daughter dyads were restricted to this period. Details of the nuclear markers, molecular methods and calculation of relatedness are described elsewhere (Radespiel et al. 2001a,b). Only mothers with daughters at the onset of their first breeding season (yearling daughters) were included. To address the possibility that mothers bequeath space to yearling daughters we compared space use of females with and without yearling daughters in consecutive years and space partition between mothers and daughters. We examined the spatial distribution of individuals with two different methods. First, we established activity centres of mothers and daughters by averaging all X and Y coordinates of capture places of both animals and compared distances between their activity centres. The estimated activity centres correspond well with those obtained by radiotelemetry (B. Schmelting, unpublished data). This method allows us to assess spatial relationships of a greater proportion of the study population. We also calculated home range overlap from 100% minimum convex polygons using Trackasc (Ganzhorn 1996) and Ranges V software (Kenward & Hodder 1996) from 25 females radio collared tracked for a total of about 1300 h at the onset of the breeding season. Each individual was located by triangulation at 40–60 min intervals during four to ten successive nights per month. All animals radio collared in a given month were tracked simultaneously. During 1995 and 1996, 4 h tracking sessions were evenly distributed throughout the nightly activity phase of the mouse lemurs. In 1997 and 1999 tracking sessions alternated between the first (1800–2400) and last 6 h of the night (2400–0600) on consecutive nights while in 1998 telemetry sessions lasted from dusk till dawn.
(c) Survival of parent–offspring associations
Sharing space with a yearling daughter may impose costs on mothers in terms of decreased survival and/or fitness. Therefore, we compared annual survival of females with yearling daughters to that of females without yearling daughters in their vicinity. Vicinity was defined as less than 138 m apart on the basis of telemetric or capture data with 138 m corresponding to the diameter of an average female home range (median 1.5 ha). We assessed potential survival benefits for daughters depending on the presence of their mother at the onset of their first breeding season. This was accomplished by comparing the number of yearling daughters at the onset of the mating season with and without surviving mothers. Every potential mother was included only once for these analyses to avoid possible errors due to pseudo-replication.
(d) Analysis
We assessed differences in space use of females with a t-test after testing for normal distribution (Kolmogorov–Smirnov test, p>0.05). To test for effects of the study year an ANOVA was performed using SPSS 11.5 with study year as covariate. Home range overlap data were arcsine-transformed to achieve normality (Sokal & Rolff 1995) and then used with the same tests.
3. Results
(a) Population dynamics and annual survival
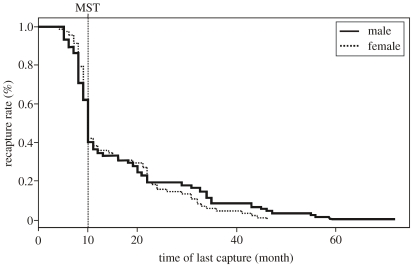
We captured 320 individuals (210 males, 110 females) in the study area between 1995 and 2000. Yearly turnover rates were high and recapture rates between years ranged from 26 to 45%. The survival analysis revealed that both sexes were equally likely to survive to their first breeding season at the age of nine month (males: 61%; females: 62%, figure 1). Survival rates decreased for both sexes until the second breeding season (males: 23%, females: 27%) and only 7% of the females survived to the onset of the third breeding season as opposed to 14% of the males. No female lived until a fourth breeding season while 6% of the males were still present and the oldest so far was 6 years of age. The median survival time (i.e. the time to loss of 50% of the individuals) for both sexes was 10 months only. Thus, most females never achieved reproduction.
Figure 1.
Survival analyses of male and female grey mouse lemurs with median survival time (MST) from 1995 to 2000.
(b) Spatial interaction of mother–daughter dyads
Activity centre data were available for successive years of 21 mother–daughter dyads. The distance between activity centres of mother–daughter pairs was on average 71.8±47.6 m (table 1) and was not affected by study year (F=1.28, d.f.=2, p=0.30). For female yearlings that had lost their mothers at the onset of the breeding season the activity centre was compared with their mother's from the former year. Mother–daughter distances ranged from 0 to 52.1 m (±0.2, table 1). Distances between activity centres of mother–daughter dyads differed significantly depending on whether the putative mother was still present in the population (F=9.14, d.f.=1, p=0.005, table 1). It thus appeared that yearling daughters resided closer to their mother's former activity centre after the mother disappeared from the population than when they were still present. In contrast, mothers did not shift their activity centres in consecutive years depending on the presence of a yearling daughter (N=9, 34.2±25.6 m) in comparison with females without yearling daughters (N=10, 44.4±20.4, t-test: T=0.97, d.f.=17, p=0.35). Hence, mothers showed no indication of territory bequeathal to yearling daughters.
Table 1.
Distances between activity centres and home range overlap of mother–daughter dyads 1996–1998 depending on the presence of the mother. (Present, mother present in captured population; absent, mother not present in captured population.)
| distance between activity centres (m) | overlap between home ranges (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| year | N | present | N | absent | N | present | N | absent |
| 96 | 3 | 104.1±50.1 | 3 | 30.4±18.4 | 2 | 75.3±7.4 | — | |
| 97 | 7 | 71.9±60.6 | 2 | 52.1±0.2 | 2 | 60.7±38.8 | 1 | 31.5 |
| 98 | 11 | 60.7±20.6 | 2 | 0 | 7 | 60.0±24.6 | — | |
| 21 | 71.8±47.6 | 7 | 24.5±24.2 | 11 | 66.7±23.3 | |||
Home range overlap data were available for eleven putative mother–yearling dyads with the mother still present in the population (table 1). Mean home range overlap was 66.7% (±23.3, N=11) and this overlap was not affected by study year (F=0.95, d.f.=3, p=0.47). One yearling female lost her mother before the onset of the breeding season and the overlap with her mother's home range from the preceding year was 31.5%. A comparison of females with (N=5, 56.8%±22.6) and without (N=3, 73.1%±26.3) yearling daughters yielded no significant difference in inter-year home range overlap between the two groups (t-test, T=0.94, d.f.=6, p=0.35). Thus, females did not appear to shift their home ranges when yearling daughters were present.
(c) Costs and benefits of mother–daughter association
Females with a yearling daughter tended to show higher survival (52.9%) until the next breeding season than those without (26.7%) but this difference was not significant (N=47, χ2=3.25, p=0.072). Hence, yearling daughters incurred no survival costs to mothers. A mother's survival may vary depending on the number of offspring present but our limited data (for only three of the mothers considered more than one yearling daughter was present) precluded a test of this possibility. Daughters of surviving mothers were more likely to survive to the onset of their first breeding season than those whose mothers died (N=47, χ2=4.11, p=0.043, non-surviving: 29.4%, surviving: 61.5%). Consequently, the presence of mothers appeared to increase the survival of their daughters to their first breeding season.
4. Discussion
Contrary to the assumptions of the life-history theory we found high yearly turnover rates in grey mouse lemurs with an average of about one third of the study population being recaptured in consecutive years. Both sexes demonstrated a marked increase of mortality at the age of maturity. This may be a result of increased energy expenditure due to sexual maturation (Promislow 1991). Sex-specific differences in survival only become apparent for animals that survived their first breeding season and in contrast to many mammals (e.g. Efford 1998; Loison et al. 1999), females had a shorter life expectancy than males. Since males are the dispersing sex (Radespiel et al. 2003), the differences in sex-specific survival are likely to be even more pronounced. Male-biased mortality in mammals is largely a result of the physiological costs associated with sexual size dimorphism (Promislow 1992, 2003). As there is no such dimorphism found in grey mouse lemurs and a correlation between female survival rate and female reproductive investment has previously been shown for mammals (e.g. Harvey & Clutton-Brock 1985) we suggest physiological costs incurred by higher reproductive investment of females that breed may explain their reduced longevity.
As a result of strict seasonal reproduction (Schmelting et al. 2000) and high mortality, most females never reproduce and the majority of the remainder will only have one reproductive season during their lifetime. This poses a strong selective pressure for the adoption of strategies that increase offspring survival and/or reproductive success of females. Territory bequeathal is assumed to improve offspring survival and has been suggested to occur in a number of mammal species (Lambin 1997). However, compelling evidence for this phenomenon is limited (e.g. Berteaux & Boutin 2000). Our data do not suggest active maternal bequeathal of space as grey mouse lemur mothers remained in the same area regardless of the presence of yearling daughters. In combination with the observed survival benefits for both mothers and daughters, this supports Lambin's (1997) notion that bequeathal is unlikely when environmental conditions allow the formation of beneficial associations between relatives. Furthermore, these benefits may extend beyond the non-breeding season our study focuses on. Communal nesting in mouse lemurs could entail thermoregulatory and predator defence benefits as a result of pooled litters and a reduction in the time offspring remain unattended (Glatston 1986). Thus, maternal bequeathal is unlikely to provide substantial advantages. However, in accordance with the assumptions of the benefits of philopatry hypothesis (Stacey & Ligon 1991) daughters can benefit by inheriting their mother's home ranges after a mother's death as observed in our study population.
Given the solitary foraging habit of grey mouse lemurs and the distinct activity centres of mother–daughter dyads, information transfer about food resources can probably be ruled out as a maternal effect on offspring survival. This is supported by behavioural observations conducted in 1998–1999. During more than 500 h of observations, social interactions were largely restricted to male conspecifics and only one brief encounter of a mother–daughter dyad was recorded (H. Lutermann, unpublished data). In contrast, sleeping sites (mostly tree holes; Radespiel et al. 1998) are permanently shared by mother–daughter dyads (between 70 and 100% of the days for individual mother–daughter pairs during the study period) and are likely to be a critical resource for female mouse lemurs in relation to reproduction, predator defence and thermoregulation (Radespiel et al. 1998). Insulation capacities of such sites determine the potential for energy savings (Schmid 1998) and have been shown to strongly affect the population turnover in mouse lemurs (Ganzhorn & Schmid 1998). If, as with other species (Kerth & Reckhardt 2003), mothers transfer knowledge about high-quality sleeping sites, daughters could gain thermoregulatory benefits. Indeed, there are indications that mothers do transfer such knowledge as behavioural data suggest that mothers tend to arrive first at a sleeping site and similar to other mouse lemur species (Braune et al. 2005) mothers attract their daughters by ‘gathering calls’. However, data on this phenomenon are limited and we cannot exclude the possibility that daughters recruit new sleeping sites.
Improved offspring survival by resource sharing with a parent has been shown previously for other species (e.g. Berteaux & Boutin 2000; Hatchwell & Komdeur 2000) and our data provide evidence of survival benefits for yearling daughters as well as mothers as a result of natal philopatry. This could be due to thermoregulatory benefits that both, mothers and daughters, accrue from sharing sleeping sites during the cold dry season (Perret 1998). Thus, mothers should actively encourage their daughters to remain philopatric as it benefits their survival and probably future reproduction. The benefits observed in this study could be a strong evolutionary force facilitating the evolution of natal philopatry and social grouping in grey mouse lemur females and could account for the sex-biased dispersal pattern in grey mouse lemurs.
In conclusion, contrary to the assumptions of the life-history hypothesis female natal philopatry in grey mouse lemurs does not appear to be a result of high adult survival since mortality is high. In accordance with the benefits of philopatry hypothesis survival benefits are obtained by the presence of the mother and philopatric daughter, respectively, and daughters may inherit their mother's home range. We suggest that the observed survival benefits are probably due to sharing of non-food resources, most likely sleeping sites. This study highlights that species with a dispersed kin-based grouping pattern, such as the grey mouse lemur, form excellent models to investigate different hypotheses on the evolution of female natal philopatry as a route to sociality. This is in accordance with West Eberhard's (1975) suggestion that kin selection has played a role in the evolution of sociality.
Acknowledgments
We thank the Commission Tripartite of the Malagasy government and the DEF, the ANGAP and CI for the permission to work in Ampijoroa. We are indebted to Prof B. Rakotosamimanana (University of Antananarivo) for the institutional help and a number of volunteers for help during data collection. The comments of M. Scantlebury and two anonymous referees improved the manuscript considerably. We also thank N. C. Bennett and P. W. Bateman for suggestions on earlier drafts. The study was funded by the German Research Council (Zi 345/12), the DAAD (HSP II to H.L. and P.E., HSP III to U.R., TG to B.S.) and the Casanuswerk (B.S.).
References
- Berteaux D, Boutin S. Breeding dispersal in female North American red squirrels. Ecology. 2000;81:1311–1326. doi:10.2307/177210 [Google Scholar]
- Boinski S, Kauffmann L, Ehmke E, Schet S, Vreedzaam A. Dispersal patterns among three species of squirrel monkeys (Saimiri oerstedii, S. boliviensis and S. sciureus): I. Divergent costs and benefits. Behaviour. 2005;142:525–632. doi:10.1163/1568539054352888 [Google Scholar]
- Boutin S, Larsen K.W, Berteaux D. Anticipatory parental care: acquiring resources for offspring prior to conception. Proc. R. Soc. B. 2000;267:2081–2085. doi: 10.1098/rspb.2000.1252. doi:10.1098/rspb.2000.1252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braune P, Schmidt S, Zimmermann E. Spacing and group coordination in a nocturnal primate, the golden brown mouse lemur (Microcebus ravelobensis): the role of olfactory and acoustic signals. Behav. Ecol. Sociobiol. 2005;58:587–596. doi:10.1007/s00265-005-0944-4 [Google Scholar]
- Brown J.L. Princeton University Press; Princeton, NJ: 1987. Helping and cooperative breeding in birds. [Google Scholar]
- de Ruiter J.R, Geffen E. Relatedness of matrilines, dispersing males and social groups in long-tailed macaques (Macaca fascicularis) Proc. R. Soc. B. 1998;265:79–87. doi: 10.1098/rspb.1998.0267. doi:10.1098/rspb.1998.0267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberle M, Kappeler P.M. Cooperative breeding in grey mouse lemurs (Microcebus murinus) Folia Primatol. 2003;74:367. doi:10.1159/000074749 [Google Scholar]
- Efford E. Demographic consequences of sex-biased dispersal in a population of brushtail possums. J. Anim. Ecol. 1998;67:503–517. doi:10.1046/j.1365-2656.1998.00222.x [Google Scholar]
- Emlen S.T. Cooperative breeding in birds and mammals. In: Krebs J.R, Davies N.B, editors. Behavioural ecology. Blackwell Scientific; Oxford, UK: 1984. pp. 305–339. [Google Scholar]
- Emlen S.T. Predicting family dynamics in social vertebrates. In: Krebs J.R, Davies N.B, editors. Behavioural ecology—an evolutionary approach. Blackwell Scientific; Oxford, UK: 1997. pp. 228–253. [Google Scholar]
- Fredsted T, Pertoldi C, Olesen J.M, Eberle M, Kappeler P.M. Microgeographic heterogeneity in spatial distribution and mtDNA variability of gray mouse lemurs (Microcebus murinus, primates: Cheirogaleidae) Behav. Ecol. Sociobiol. 2004;56:393–404. doi:10.1007/s00265-004-0790-9 [Google Scholar]
- French J.A. Proximate regulation of singular breeding in callitrichid primates. In: Solomon N.G, French J.A, editors. Communal breeding in mammals. Cambridge University Press; Cambridge, UK: 1997. pp. 34–75. [Google Scholar]
- Ganzhorn, A. 1996 Trackasc [3.0]. Götlingen, Germany.
- Ganzhorn J.U, Schmid J. Different population dynamics of Microcebus murinus in primary and secondary deciduous dry forests of Madagascar. Int. J. Primatol. 1998;19:785–796. doi:10.1023/A:1020337211827 [Google Scholar]
- Glatston A.R. The influence of other females on maternal behaviour and breeding success in the lesser mouse lemur (Microcebus murinus) In: Else J.G, Lee P.C, editors. Primate ontogeny, cognition and social behaviour. Cambridge University Press; Cambridge, UK: 1986. pp. 355–361. [Google Scholar]
- Goldizen A.R, Terborgh J. Demography and dispersal patterns of a tamarin population: possible causes of delayed breeding. Am. Nat. 1989;134:208–224. doi:10.1086/284976 [Google Scholar]
- Goodman S.M, OConner S.O, Langrand O. A review of predation on lemurs: implications for the evolution of social behavior in small, nocturnal primates. In: Kappeler P.M, Ganzhorn J.U, editors. Lemur social systems and their ecological basis. Plenum Press; New York, NY: 1993. pp. 51–65. [Google Scholar]
- Greenwood P.J. Mating systems, philopatry and dispersal in birds and mammals. Anim. Behav. 1980;28:1140–1162. [Google Scholar]
- Hamilton W.D, May R.M. Dispersal in stable habitats. Nature. 1977;269:578–581. doi:10.1038/269578a0 [Google Scholar]
- Harvey P.H, Clutton-Brock T.H. Life history variation in primates. Evolution. 1985;39:559–581. doi: 10.1111/j.1558-5646.1985.tb00395.x. doi:10.2307/2408653 [DOI] [PubMed] [Google Scholar]
- Hatchwell B.J, Komdeur J. Ecological constraints, life history traits and the evolution of cooperative breeding. Anim. Behav. 2000;59:1079–1086. doi: 10.1006/anbe.2000.1394. doi:10.1006/anbe.2000.1394 [DOI] [PubMed] [Google Scholar]
- Heymann E.W. Sex differences in olfactory communication in a primate, the moustached tamarin, Saguinus mystax (Callitrichinae) Behav. Ecol. Sociobiol. 1998;43:37–43. doi:10.1007/s002650050464 [Google Scholar]
- Isbell L.A. Is there no place like home? Ecological bases of female dispersal and philopatry and their consequences for the formation of kin groups. In: Chapais B, Berman C.M, editors. Kinship and behavior in primates. Oxford University Press; Oxford, UK: 2004. pp. 71–108. [Google Scholar]
- Johnson C.N, Clinchy M, Taylor A.C, Krebs C.J, Jarman P.J, Payne A, Ritchie E.G. Adjustment of offspring sex ratios in relation to the availability of resources for philopatric offspring in the common brushtail possum. Proc. R. Soc. B. 2001;268:2001–2005. doi: 10.1098/rspb.2001.1723. doi:10.1098/rspb.2001.1723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones W.T. Natal philopatry in bannertailed kangaroo rats. Behav. Ecol. Sociobiol. 1984;15:151–155. doi:10.1007/BF00299383 [Google Scholar]
- Kenward R.E, Hodder K.H. Institute of Terrestrial Ecology; Wareham, UK: 1996. Ranges V, an analysis system for biological location data. [Google Scholar]
- Kerth G, Reckardt K. Information transfer about roosts in female Bechstein's bats: an experimental field study. Proc. R. Soc. B. 2003;270:511–515. doi: 10.1098/rspb.2002.2267. doi:10.1098/rspb.2002.2267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokko H, Johnstone R.A. Social queuing in animal societies: a dynamic model of reproductive skew. Proc. R. Soc. B. 1999;266:571–578. doi:10.1098/rspb.1999.0674 [Google Scholar]
- Krebs C.J. Harper & Row; New York, NY: 1999. Ecological methodology. [Google Scholar]
- Lambin X. Home range shifts by breeding female Townsend's voles (Microtus townsendii): a test of the territory bequeathal hypothesis. Behav. Ecol. Sociobiol. 1997;40:363–372. doi:10.1007/s002650050352 [Google Scholar]
- Lambin X, Aars J, Piertney S.B. Dispersal, intraspecific competition, kin competition and kin facilitation: a review of the empirical evidence. In: Clobert J, Danchin E, Dhondt A.A, Nichols J.D, editors. Dispersal. Oxford University Press; Oxford, UK: 2001. pp. 110–122. [Google Scholar]
- Lazaro-Perea C, Castro C.S.S, Harrison R, Araujo A, Arruda M.F, Snowdon C.T. Behavioral and demographic changes following the loss of the breeding female in cooperatively breeding marmosets. Behav. Ecol. Sociobiol. 2000;48:137–146. doi:10.1007/s002650000215 [Google Scholar]
- Loison A, Festa-Bianchet M, Gaillard J.M, Jorgenson J.T, Jullien J.-M. Age-specific survival in five populations of ungulates: evidence of senescence. Ecology. 1999;80:2539–2554. doi:10.2307/177239 [Google Scholar]
- Mittermeier R.A, Tattersall I, Konstant W.R, Meyers D.M, Mast R.B. Conservation International; Washington, DC: 1994. Lemurs of Madagascar. [Google Scholar]
- Moses R.A, Millar J.S. Philopatry and mother–daughter associations in bushy-tailed woodrats: space use and reproductive success. Behav. Ecol. Sociobiol. 1994;35:131–140. doi:10.1007/s002650050079 [Google Scholar]
- Pen I, Weissing F.J. Towards a unified theory of cooperative breeding: the role of ecology and life history re-examined. Proc. R. Soc. B. 2000;267:2411–2418. doi: 10.1098/rspb.2000.1299. doi:10.1098/rspb.2000.1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perret M. Energetic advantage of nest-sharing in a solitary primate, the lesser mouse lemur (Microcebus murinus) J. Mammal. 1998;79:1093–1102. [Google Scholar]
- Promislow D.E.L. Senescence in natural populations of mammals: a comparative study. Evolution. 1991;45:1869–1887. doi: 10.1111/j.1558-5646.1991.tb02693.x. doi:10.2307/2409837 [DOI] [PubMed] [Google Scholar]
- Promislow D.E.L. Costs of sexual selection in natural populations of mammals. Proc. R. Soc. B. 1992;247:203–210. [Google Scholar]
- Promislow D.E.L. Mate choice, sexual conflict, and evolution of senescence. Behav. Genet. 2003;33:191–201. doi: 10.1023/a:1022562103669. doi:10.1023/A:1022562103669 [DOI] [PubMed] [Google Scholar]
- Radespiel U, Cepok S, Zietemann V, Zimmermann E. Sex-specific usage patterns of sleeping-sites in grey mouse lemurs. Am. J. Primatol. 1998;46:77–84. doi: 10.1002/(SICI)1098-2345(1998)46:1<77::AID-AJP6>3.0.CO;2-S. doi:10.1002/(SICI)1098-2345(1998)46:1<77::AID-AJP6>3.0.CO;2-S [DOI] [PubMed] [Google Scholar]
- Radespiel U, Sarikaya Z, Zimmermann E, Bruford M.W. Socio-genetic structure in a free-living nocturnal primate population: sex-specific differences in the grey mouse lemur (Microcebus murinus) Behav. Ecol. Sociobiol. 2001a;50:493–502. doi:10.1007/s002650100402 [Google Scholar]
- Radespiel U, Funk S.M, Zimmermann E, Bruford M.W. Isolation and characterization of microsatellite loci in the grey mouse lemur (Microcebus murinus) and their amplification in the family Cheirogaleidae. Mol. Ecol. Notes. 2001b;1:16–18. [Google Scholar]
- Radespiel U, Lutermann H, Schmelting B, Bruford M.W, Zimmermann E. Patterns and dynamics of sex-biased dispersal in a nocturnal primate, the grey mouse lemur, Microcebus murinus. Anim. Behav. 2003;65:709–719. doi:10.1006/anbe.2003.2121 [Google Scholar]
- Ronce O, Gandon S, Rousset F. Kin selection and natal dispersal in an age-structured population. Theor. Popul. Biol. 2000;58:143–159. doi: 10.1006/tpbi.2000.1476. doi:10.1006/tpbi.2000.1476 [DOI] [PubMed] [Google Scholar]
- Rowley I, Russell E.M. Splendid-fairy wrens: demonstrating the importance of longevity. In: Stacey P.B, Koenig W.D, editors. Cooperative breeding in birds: long-term studies of ecology and behavior. Cambridge University Press; Cambridge, UK: 1990. pp. 1–30. [Google Scholar]
- Schmelting B, Ehresmann P, Lutermann H, Randrianambinina B, Zimmermann E. Reproduction of two sympatric mouse lemur species (Microcebus murinus and M. ravelobensis) in north-west Madagascar: first results of a long term study. In: Lourenco W.R, Goodman S.M, editors. Diversité et Endémisme à Madagascar. Mémoires de la Sociéte de Biogeographie; Paris, France: 2000. pp. 1–12. [Google Scholar]
- Schmid J. Tree holes used for resting by gray mouse lemurs (Microcebus murinus) in Madagascar: insulation capacities and energetic consequences. Int. J. Primatol. 1998;19:797–809. doi:10.1023/A:1020389228665 [Google Scholar]
- Sokal R.R, Rohlf F.J. W.H. Freeman and Company; New York, NY: 1995. Biometry. [Google Scholar]
- Solomon N.G, French J.A. Cambridge University Press; Cambridge, UK: 1997. Cooperative breeding in mammals. [Google Scholar]
- Stacey P.B, Ligon J.D. Territory quality and dispersal options in the acorn woodpecker, and a challenge of the habitat saturation model of cooperative breeding. Am. Nat. 1987;130:654–676. doi:10.1086/284737 [Google Scholar]
- Stacey P.B, Ligon J.D. The benefits-of-philopatry hypothesis for the evolution of cooperative breeding: variation in territory quality and group size effects. Am. Nat. 1991;137:831–846. doi:10.1086/285196 [Google Scholar]
- Waser P.M, Jones W.T. Natal philopatry among solitary mammals. Q. Rev. Biol. 1983;58:355–390. doi:10.1086/413385 [Google Scholar]
- West Eberhard M.J. The evolution of social behaviour by kin selection. Q. Rev. Biol. 1975;50:1–33. doi:10.1086/408298 [Google Scholar]
- Wimmer B, Tautz D, Kappeler P.M. The genetic population structure of the gray mouse lemur (Microcebus murinus), a basal primate from Madagascar. Behav. Ecol. Sociobiol. 2002;52:166–175. doi:10.1007/s00265-002-0497-8 [Google Scholar]
- Wolff J.O. Parents suppress reproduction and stimulate dispersal in opposite-sex juvenile white-footed mice. Nature. 1992;359:409–410. doi: 10.1038/359409a0. doi:10.1038/359409a0 [DOI] [PubMed] [Google Scholar]