Abstract
The main tenet of Hamilton's ‘selfish herd theory’ for the evolution of group living is that individual risk of being killed upon attack by a predator is greater when relatively far from conspecifics. Here we examine the role of spacing using video analysis of encounters between redshanks, Tringa totanus, in flocks on saltmarsh, and sparrowhawks, Accipiter nisus, surprise hunting from adjacent woodland. Targeted redshanks were 35% (approx. 5 body lengths) more widely spaced than their nearest non-targeted neighbours, controlling for proximity to the hawk. Although targeted redshanks were also twice as slow to escape, the effect dropped out of a model containing spacing, which alone accounted for twice as much variation as escape delay. Redshanks were more tightly spaced on the riskiest side of the flock, suggesting they attempted to compensate for the greater risk, while birds on the edges of flocks were more widely spaced than those in the centre. Our analysis controls for most of the confounding effects associated with the edge–centre comparisons that are normally used in similar studies and provides strong support for spacing-dependent differential predation risk in the wild. In general, we suggest that positive selection for tight spacing when prey are stationary is largely due to domains of danger, but that this also leads to positive selection when prey are mobile because of predator confusion.
Keywords: selfish herd, predation, group living, confusion
1. Introduction
Understanding the costs and benefits to individuals of joining a group is key to explaining the evolution of group living. Protection from predators is one of the main benefits of group living and multiple mechanisms can be responsible for these benefits (Krause & Ruxton 2002; Caro 2005). Arguably, the most important and simplest of these is the dilution effect, where individual risk is inversely related to group size (Foster & Treherne 1981). The dilution effect assumes that risk is equal among all individuals, but this is rarely the case, and in reality differential predation risk is common (Krause & Ruxton 2002; Caro 2005). Though Hamilton's selfish herd and derived models (Hamilton 1971; Vine 1971; Morton et al. 1994; James et al. 2004) were developed to explain how gregariousness might have evolved through predation, they also describe one of the mechanisms by which differential risk among individuals can arise.
Selfish herd theory states that simply moving towards conspecifics reduces the individual's own personal ‘domain of danger’, and implies that differential predation risk can arise because of variation in spacing between individuals within groups (Hamilton 1971). The larger the area surrounding an individual that is not occupied by any other neighbour, the greater the risk of selection by a predator that attacks that position randomly. Much evidence for this type of differential predation risk has come from research on stationary groups through demonstration of ‘marginal predation’ on group edges (see reviews in Krause & Ruxton 2002; Caro 2005), where animals are generally surrounded by fewer conspecifics and by default are assumed to have greater domains of danger. This commonly occurs, for example, among sessile organisms (Okamura 1986) and in reproducing colonies (Raynor & Uetz 1993; see reviews in Krause & Ruxton 2002; Caro 2005). Aggregations of mobile animals are also widespread in nature, and yet evidence for the importance of domains of danger from this source is weak, coming only from a handful of studies on captive fish (Krause & Ruxton 2002), from studies in which the main effects are unavoidably confounded by other factors (Fitzgibbon 1990) and from descriptive studies (e.g. several cited in Hamilton 1971; Stankowich 2003). Moreover, some studies on captive animals contradict marginal predation, showing that, in fact, central and not peripheral individuals are at greater risk (Milinski 1977; Parrish 1989). Consequently, the importance of spacing and domains of danger as explanations for group living requires clarification from more studies in different systems that control for numerous, potentially confounding effects (Stankowich 2003; Caro 2005).
Stankowich (2003) suggests that lack of agreement between investigations of marginal predation, in part, comes from biases associated with distinguishing between edge and central animals. He argues that the variety of approaches that have been used can lead to very different classifications. Furthermore, for several reasons, showing a higher predation risk on group edges does not provide evidence for differential predation risk according to spacing per se. First, it makes the assumption that individuals on edges are more widely spaced than those in central positions and, second, the predator could simply be selecting prey according to the first prey encountered. Furthermore, edge–centre comparisons do not account for scenarios in which predators attack from within groups or where initial attacks to the centre serve to increase spacing between individuals (Parrish 1989). Similarly, most studies from the wild have been unable to discount the possibility that individuals on edges were selected because they were of a different phenotype to those in central positions (Fitzgibbon 1990; Balmford & Turyaho 1992). A further problem arises because it is extremely difficult to separate the effects of domains of danger from other mechanisms that depend on spacing, such as the confusion effect (Neill & Cullen 1974; Milinski 1977) and collective detection (Pulliam 1973). For example, predators may select more widely spaced individuals because these are late detectors (Hilton et al. 1999; Quinn & Cresswell 2005a) and/or to minimize the confusion effect.
We tested key predictions of Hamilton's selfish herd theory (Hamilton 1971) in flocking redshanks when attacked by sparrowhawks on Tyninghame estuary, Scotland, over three winters (2001–2004). This system allowed us to overcome many of the factors that inhibit the study of differential predation risk in wild animal groups. First, predation events in the wild are often difficult to observe, but at Tyninghame sparrowhawks regularly attack redshank flocks in an open, discrete area of saltmarsh surrounded by forest (Cresswell 1996). Second, in many predator–prey systems it is usually impossible to identify the selected individual, either because prey are too tightly packed or because the group's mobility is such that individuals are impossible to track (Krause & Ruxton 2002). Foraging redshanks at Tyninghame, however, are slow moving and occur in relatively loose flocks (Cresswell 1994a,b). Identifying individuals also allowed us to compare targeted redshanks with their nearest neighbours, thus helping to avoid the problem of selection for different phenotypes that easily arise in edge/centre comparisons. Third, our system allowed us to, at least partly, discount spatially dependent collective detection as a confounding effect—where relatively isolated group members escape slowly because they are slow to detect escaping neighbours—because we could measure individual escape delay as a surrogate for vigilance (Elgar 1989; Hilton et al. 1999).
We tested whether targeted individuals were more widely spaced compared to their nearest neighbours. In contrast to edge–centre comparisons, this approach controls for the possibility that redshanks on flock edges might have been of a different phenotype to that of central birds, and that the redshanks were being selected on the basis of this phenotype rather than on their spatial position. We then specifically tested two additional potential confounding effects. First, we examined whether any effect of spacing on targeting was a consequence of individuals being nearest to the sparrowhawk being preferentially targeted. Second, we tested whether speed of escape response might be the underlying mechanism responsible for selection of widely spaced redshanks by sparrowhawks. Finally, we tested the prediction that if risk of predation increases with spacing, redshanks should compensate for this by becoming more tightly spaced on the attacked flock edges.
2. Material and methods
Our study area consisted of saltmarsh backed by woodland and dunes at Tyninghame Estuary, East Lothian, Scotland (Whitfield 1985). Sparrowhawks belong to a genus in the Accipitridae that contain some of the main predators of small-to medium-sized birds worldwide. Redshanks are a common Northern Hemisphere wading bird, Scolopacidae, and in winter feed predominantly on coastal habitats. At Tyninghame they are resident, and juveniles suffer a high mortality because their saltmarsh feeding area is bordered by sparrowhawk-concealing woodland cover, allowing the hawks to attack by surprise and from close quarters (Cresswell 1994a). Sixty-six per cent of attacks end within one second of the redshanks taking off and 94% within five seconds (Cresswell 1996).
Attacks occur without prior warning and therefore flocks were videoed continuously throughout the day over three winter seasons, taking approximately 600 video-tape hours to capture 18 attacks (flock size 18.33±16.4 s.d.; range 2–62), in which all flock members were being videoed at the start of the attack and were clearly distinguishable as they flew away (i.e. far enough away from the camera so that all birds were in the field of view, but not so far away that individuals were not distinguishable), and the targeted bird could be seen throughout the chase on video. The direction in which the camera pointed was adjusted regularly to track the position of the walking redshank flocks and the camera was zoomed out as far as necessary to ensure that flock members were in the field of view. Attacks were videoed from the edge of the wood, about 3 m above the saltmarsh level and the camera was usually perpendicular to the line of the hawk's attack. Seven of the 18 attacks led to the targeted redshank being killed. This is inevitably biased towards successful attacks since, for the hawk to be successful and for us to identify a targeted redshank, the hawks must be close to the flocks when they begin to escape. This bias has no bearing on the spacing hypothesis being tested. In the remaining unsuccessful 11 attacks, targeted individuals could be identified because the flocks were relatively close to the camera and/or because the hawk got within 1 m of the targeted bird. We assume that there was no qualitative difference in relative individual behaviour between successful and unsuccessful attacks and, consequently, that targeting reflects actual predation risk irrespective of whether attacks were successful. Though not presented here because of insufficient power, the mean effects in the main analysis were very similar for successful and unsuccessful attacks.
Spacing between redshanks within flocks was calculated using the mean distance to the three nearest neighbour distances (NND) in bird length units (1 bird length unit≈0.2 m). Mean NND for individuals and for flock averages were 6.04±7.30 s.d. (n=330; range 1–50) and 10.41±8.94 (n=18; range 2.25–37.25). Although each video provided a two-dimensional view of the flock, any errors in measuring bird position or spacing due to foreshortening were assumed to be random with respect to any bird in the flock. This assumption is likely to hold because hawks invariably attacked horizontal to the field of view.
Previous tests of marginal predation used an edge–centre comparison, an approach that is open to substantial subjective assessment (Stankowich 2003) and that does not account for biased risk gradients caused by the predator attacking from a given direction (Bumann et al. 1997). To overcome these problems, and because half of the 18 flocks contained no central birds (as defined below for the spatial variation analysis), redshanks were classified according to their proximity to the attacking hawk (1, 2, …, n, where n is the flock size and the furthest position from the hawk) just before the first began to escape in a flock. ‘Hawk proximity’ was standardized to account for different flock sizes by dividing positions by flock size, so that, for example, values for the most proximate redshanks to hawks in flocks of size 2 and 50 were 0.5 and 0.02, respectively. Therefore, less weight is given to the first bird being targeted in a flock of only 2. Individuals within the same flock that were equally close to the hawk were given an equal measure.
We used variation in escape response delay among individuals to test whether selection for redshanks on the basis of spacing could be influenced or confounded by two other effect: individual vigilance and collective detection. Escape response delay is potentially a good surrogate for vigilance, which is otherwise effectively impossible to assess directly (Lima & Bednekoff 1999). However, delay is also strongly correlated with collective detection because widely spaced individuals delay their escape response for longer (Hilton et al. 1999; Quinn & Cresswell 2005a) because, it is assumed, proximity to conspecifics facilitates the visual detection of the departure of conspecifics, which is important for intraflock cohesion in Charadriiformes (Brooke 1998). Any measured correlation between selection or spacing on escape response delay could therefore indicate a combination of vigilance and collective predator detection effects. Escape response delay was measured relative to the first individual that began to escape and taken directly from digital video, frame by frame (filmed with a resolution of 25 frames s−1). An individual was said to have escaped when it began to take flight, as indicated by any movement of otherwise folded wings. Escape delay for an individual was taken as the time elapsed since frame 0 (frame 0 is when the first individual(s) took flight). On average, individuals took 0.57±0.04 s.e. seconds to take flight (n=330, 18 flocks).
There was insufficient power in the 18 attacks to test for differential spacing and escape delays according to position throughout the flock. We therefore tested these ideas in a separate analysis by adding similar data taken from superfluous escape responses made in 2001–2003, bringing the total number of flock escape responses to 61 (n=1152 individual responses; see Quinn & Cresswell 2005a). Superfluous escape responses were caused by the sudden appearance of a harmless species or conspecific, presumably mistaken for a predator, or for no apparent reason (see Quinn & Cresswell 2005a,b). We assumed these superfluous responses were similar to those made to an attacking sparrowhawk. Proximity to the wood was used in place of hawk proximity in the analyses of all responses, irrespective of whether a hawk was present or not. Though attacks occurred from other directions, they predominantly came from the woods (Cresswell 1996), which therefore represented the riskiest direction. In addition to spacing, birds were classified as being in central positions if they were entirely enclosed by one ‘layer’ of other group members (see Stankowich 2003), i.e. if we construct a minimum-area polygon that encompasses all group members, then edge individuals were at the vertices of this polygon and centre individuals were inside it.
The main spacing analyses, comparing targeted and non-targeted nearest neighbour, were done using general linear models, with spacing as the response variable, targeted/non-targeted neighbour (two-level factor) and proximity to hawk (covariate) as main effects, and flock as a factor to control for non-independence of observations from individuals within the same flock (Sokal & Rohlf 1981). Logistic regression was used when separating the effect of escape delay and spacing, with targeted/non-targeted neighbour as the binary response variable. Generalized linear mixed modelling (GLMM) was used in the second analysis (incorporating ‘superfluous escape responses’) of variation in spacing and escape delays within flocks, with frame number of flight as response variable and flock as a random effect. This controls for non-independence of observations from individuals within a given escape response and avoids pseudoreplication (Genstat v. 6.2; VSN Intl 2003). Significance levels in GLMMs were established with the Wald statistic, which is tested against a χ2 distribution.
3. Results
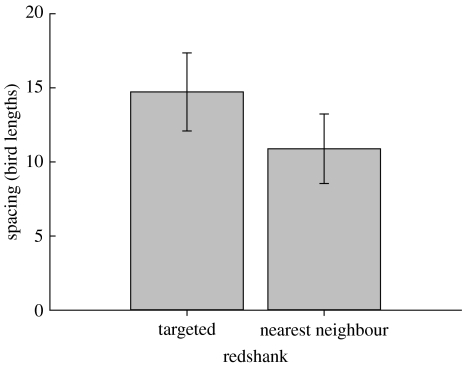
Targeted redshanks were more widely spaced compared to their nearest neighbours (F1,16=6.88, p<0.018, adjusted R2=0.82, B=0.257±0.098 for targeted individual and 0 for nearest neighbour; spacing0.33; figure 1). They were also closer to the attacking sparrowhawk compared to the rest of the flock (proximity for targeted bird versus mean proximity for all others: F1,17=19.54, p<0.001, adjusted R2=0.45, B=0 for targeted, B=0.291±0.066 for rest of flock; untransformed data), but not compared to their nearest single neighbours (F1,17=0.41, p=0.53). Consequently, the difference in spacing between targeted and nearest neighbours remained (p=0.022) after controlling for hawk proximity.
Figure 1.
Difference in spacing between the targeted redshank and its nearest neighbour. n=17 in both treatments (attack excluded when flock size=2).
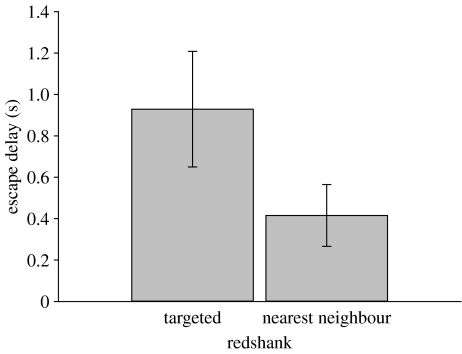
Targeted redshanks also had longer escape delays than their nearest non-targeted neighbours (F1,17=5.983, p=0.026, adjusted R2=0.12, B=0.323±0.132 s.e. for targeted bird, 0 for nearest neighbour; figure 2). Combining all three explanatory variables in the same model showed that the effects of hawk proximity and escape delay on the probability of a redshank being targeted over its nearest neighbour were encompassed by spacing, which alone remained significant in the model (table 1).
Figure 2.
Difference in escape delay (s) between the targeted redshank and its nearest neighbour. Analysis done on best transformed data (log delay+0.08); this deviated from normality (Shapiro–Wilk=0.925, d.f.=36, p=0.017), but non-parametric test on transformed data confirmed difference between targeted and nearest neighbour (Wilcoxon signed-rank test, Z=−2.157, p=0.031). n=18 for both treatments.
Table 1.
Logistic binary regression of targeted/non-targeted (binary) redshank against three main variables. (Nagelkerke R2=0.52 (0.54 when only spacing is in the model). n=17 attacks and 34 observations; attack with flock size=2 excluded because spacing is identical for both birds.)
| B | s.e. | Wald | d.f. | p | |
|---|---|---|---|---|---|
| spacing (bird lengths) | 0.832 | 0.341 | 5.938 | 1 | 0.015 |
| escape delay (s) | −0.442 | 1.023 | 0.187 | 1 | 0.666 |
| hawk proximity | 12.506 | 8.608 | 2.111 | 1 | 0.146 |
| flock | 6.564 | 16 | 0.981 | ||
| constant | −4.311 | 2.597 | 2.756 | 1 | 0.097 |
Spacing between redshanks decreased with proximity to the high-risk woodlands, but this effect was only true for individuals on the edges of flocks and not for those in the centre (proximity to wood×position; statistics and graphic in figure 3). Escape delay was also influenced by spacing (W=14.89, d.f.=1, p<0.001, B=0.11±0.03 s.e.), but not by proximity to the high-risk wood (W=2.57, d.f.=1, p=0.11, B=0.11±0.068) or by edge–centre position (W=0.91, d.f.=1, p=0.34; B=−0.05474±0.057 for centre, B=0 for edge).
Figure 3.
Variation in spacing between redshanks in flocks with respect to proximity to the high-risk woodland adjacent to the saltmarsh relative to other flock members, plotted separately for edge (open circles, top line fit) and centre (dots, bottom line fit) position within flocks. Analysis is a GLMM with normal errors, flock as a random effect: position×proximity to wood, W=19.86, p<0.001, B=−0.5444±0.12, B=0 for interaction term with edge position; position, B=−0.57±0.05 s.e.; proximity to wood, B=0.77±0.10 s.e.; constant, B=1.82±0.09; d.f.=1, n=61 flock and 1152 individual redshank responses (mean flock size=18.89±11.82 s.d., range 2–54).
4. Discussion
Targeted redshanks were on average 35% more widely spaced from other redshanks compared to their nearest non-targeted neighbours, equivalent to a difference of 5 body lengths (figure 1). This is strong evidence for the role of spacing in determining predation risk in groups, because the analysis controls for several, potentially confounding, effects and it is based on a completely natural system, where behaviourally complex and mobile group-living individuals (Cresswell 1993, 1994b) are attacked by a sophisticated and widespread generalist predator (Newton 1986; Quinn & Cresswell 2004). Although hawk proximity and escape delay were correlated to spacing and were also related to the probability of being targeted, spacing alone remained significant when the three variables were included in the same analysis and on its own accounted for far more variation. Furthermore, our analysis was based on a comparison of the targeted individual with that of its immediate neighbour, so it was unlikely that the effect of spacing on the targeting behaviour of sparrowhawks was confounded by phenotype-dependent segregation in the flock (e.g. birds in poorer condition being at the edge of the flock).
Hamilton's domain of danger hypothesis provides a plausible explanation for why spacing was important in our system. Nevertheless, the confusion effect remains a potentially important mechanism because being closer to neighbours before an attack occurs may ‘confuse’ the predator once an escape response has been initiated (Milinski 1977). Sparrowhawks usually attack redshanks by surprise (88%, n=517; Cresswell 1996), almost never change targets during an attack and almost always catch redshanks on the ground or after a very short chase (Cresswell 1996). This suggests that while they may not be ‘confused’ when initially deciding which redshank to target, confusion may be more important in later stages of an attack and this could be why spacing is important when first deciding which redshank to target. In general, we suggest that the relative importance of confusion is likely to increase with predator–prey system complexity, but that domains of danger alone may well explain spacing effects when targets are permanently stationary (e.g. in sessile organisms). Separating the two effects has never been achieved either in captivity or in the wild and is likely to remain a challenging prospect for mobile predator–prey systems due to the technical constraints associated with measuring spacing accurately in mobile, fast-moving flocks. If confusion is important, then prey capture rate is likely to be affected by variation in spacing during the attack and the predator would be expected to change targets to widely spaced individuals; if domains of danger are solely important, then there should be no strong effects of spacing on capture rates and targeting behaviour after the initial animal has been targeted.
Irrespective of the underlying mechanisms, our results provide strong support for the hypothesis that selection on spacing between individuals in close proximity can lead to the evolution of group living and refute the assumption of equal risk made by other hypotheses, such as the dilution effect (Calvert et al. 1979; Foster & Treherne 1981) and collective detection (e.g. Pulliam 1973; Elgar 1989). Optimal spacing between animals in groups generally is likely to be determined by a trade-off between the detrimental effects of competition and the beneficial effects of reducing predation mortality. There is evidence that this was also the case in our system where it has recently been shown that interference competition increases with flock size and causes some redshanks to become more widely spaced (cf. Stillman et al. 1996; Minderman et al. 2006). Redshanks in our system therefore trade-off predation risk with starvation risk when moving away from neighbours to increase intake rate. Even though redshanks had lower spacing when foraging close to high-risk areas (figure 3), there clearly remained considerable variation in spacing among these individuals. Again, this could be due to differences in competitive ability, random effects, or perhaps due to some other factor, such as individual personality type which, independently of competitive ability, is also known to influence risk-taking behaviour and predation risk (Sih et al. 2004; Ward et al. 2004; Quinn & Cresswell 2005b). Incorporating these prey effects into mechanistic studies of targeting behaviour by predators remains a major challenge for understanding predation risk and the evolution of group living in natural systems.
Acknowledgements
J.L.Q. was supported by a Leverhulme Trust Research Fellowship, W.C. by a Royal Society University Research Fellowship. We thank East Lothian District Council, the Tyninghame Estate, M. Yasue and T. Smith for logistical help. The following are thanked for helpful discussion or constructive criticism: three anonymous referees, A. Charmantier, C. Andrews, J. Chapman, C. Devereux, D. Garant, K. Jones, J. Krause, S. Patrick, T. Pizzari, B. Sheldon, M. Szukin, C. Cornwallis and especially from J. Brown who improved the analytical approach substantially.
References
- Balmford A, Turyaho M. Predation risk and lek-breeding in Uganda kob. Anim. Behav. 1992;44:117–127. doi:10.1016/S0003-3472(05)80761-4 [Google Scholar]
- Brooke M.D. Ecological factors influencing the occurrence of ‘flash marks’ in wading birds. Funct. Ecol. 1998;12:339–346. doi:10.1046/j.1365-2435.1998.00204.x [Google Scholar]
- Bumann D, Krause J, Rubenstein D. Mortality risk of spatial positions in animal groups: the danger of being in the front. Behaviour. 1997;134:1063–1076. [Google Scholar]
- Calvert W.H, Hedrick L.E, Brower L.P. Mortality of the monarch butterfly (Danaus plexippus L.): avian predation at five overwintering sites in New Mexico. Science. 1979;204:847–851. doi: 10.1126/science.204.4395.847. [DOI] [PubMed] [Google Scholar]
- Caro T.M. The University of Chicago Press; Chicago, IL: 2005. Antipredator defenses in birds and mammals. [Google Scholar]
- Cresswell W. Escape responses by redshanks, Tringa totanus, on attack by avian predators. Anim. Behav. 1993;46:609–611. doi:10.1006/anbe.1993.1231 [Google Scholar]
- Cresswell W. Age-dependent choice of redshank (Tringa totanus) feeding location: profitability or risk? J. Anim. Ecol. 1994a;63:589–600. [Google Scholar]
- Cresswell W. Flocking is an effective anti-predation strategy in redshanks, Tringa totanus. Anim. Behav. 1994b;47:433–442. doi:10.1006/anbe.1994.1057 [Google Scholar]
- Cresswell W. Surprise as a winter hunting strategy in sparrowhawks Accipiter nisus, peregrines Falco peregrinus and merlins F. columbarius. Ibis. 1996;138:684–692. [Google Scholar]
- Elgar M.A. Predator vigilance and group size in mammals and birds: a critical review of the evidence. Biol. Rev. 1989;64:13–33. doi: 10.1111/j.1469-185x.1989.tb00636.x. [DOI] [PubMed] [Google Scholar]
- Fitzgibbon C.D. Why do hunting cheetahs prefer male gazelles? Anim. Behav. 1990;40:837–845. doi:10.1016/S0003-3472(05)80984-4 [Google Scholar]
- Foster W.A, Treherne J.E. Evidence for the dilution effect in the selfish herd from fish predation on a marine insect. Nature. 1981;293:466–467. doi:10.1038/293466a0 [Google Scholar]
- Hamilton W.D. Geometry for the selfish herd. J. Theor. Biol. 1971;31:295–311. doi: 10.1016/0022-5193(71)90189-5. doi:10.1016/0022-5193(71)90189-5 [DOI] [PubMed] [Google Scholar]
- Hilton G.M, Cresswell W, Ruxton G.D. Intra-flock variation in the speed of response on attack by an avian predator. Behav. Ecol. 1999;10:391–395. doi:10.1093/beheco/10.4.391 [Google Scholar]
- James R, Bennett P.G, Krause J. Geometry for mutualistic and selfish herds: the limited domain of danger. J. Theor. Biol. 2004;228:107–113. doi: 10.1016/j.jtbi.2003.12.005. doi:10.1016/j.jtbi.2003.12.005 [DOI] [PubMed] [Google Scholar]
- Krause J, Ruxton G.D. Oxford University Press; Oxford, UK: 2002. Living in groups. [Google Scholar]
- Lima S.L, Bednekoff P.A. Back to the basics of antipredatory vigilance: can nonvigilant animals detect attack? Anim. Behav. 1999;58:537–543. doi: 10.1006/anbe.1999.1182. doi:10.1006/anbe.1999.1182 [DOI] [PubMed] [Google Scholar]
- Milinski M. Do all members of a swarm suffer the same predation? Z. Tierpsychol. 1977;43:311–325. [Google Scholar]
- Minderman J, Lind J, Cresswell W. Behaviourally mediated indirect effects: interference competition increases predation mortality in foraging redshanks. J. Anim. Ecol. 2006;75:713–723. doi: 10.1111/j.1365-2656.2006.01092.x. doi:10.1111/j.1365-2656.2006.01092.x [DOI] [PubMed] [Google Scholar]
- Morton T.L, Haefner J.W, Nugala V, Decino R.D, Mendes L. The selfish herd revisited: do simple movement rules reduce relative predation risk. J. Theor. Biol. 1994;167:73–79. doi:10.1006/jtbi.1994.1051 [Google Scholar]
- Neill S.R.St.J, Cullen J.M. Experiments on whether schooling by their prey affects the hunting behaviour of cephalopods and fish predators. J. Zool. Lond. 1974;172:549–569. [Google Scholar]
- Newton I. T. & A. D. Poyser; London, UK: 1986. The sparrowhawk. [Google Scholar]
- Okamura B. Group living and the effects of spatial position in aggregations of Mytilus edulis. Oecologia. 1986;69:341–347. doi: 10.1007/BF00377054. doi:10.1007/BF00377054 [DOI] [PubMed] [Google Scholar]
- Parrish J.K. Reexamining the selfish herd—are central fish safer? Anim. Behav. 1989;38:1048–1053. [Google Scholar]
- Pulliam H.R. On the advantages of flocking. J. Theor. Biol. 1973;38:419–422. doi: 10.1016/0022-5193(73)90184-7. doi:10.1016/0022-5193(73)90184-7 [DOI] [PubMed] [Google Scholar]
- Quinn J.L, Cresswell W. Predator behaviour and prey vulnerability. J. Anim. Ecol. 2004;73:143–154. doi:10.1046/j.0021-8790.2004.00787.x [Google Scholar]
- Quinn J.L, Cresswell W. Escape response delays in wintering redshank, Tringa totanus, flocks: perceptual limits and economic decisions. Anim. Behav. 2005a;69:1285–1292. doi:10.1016/j.anbehav.2004.10.007 [Google Scholar]
- Quinn J.L, Cresswell W. Personality, anti-predation behaviour and behavioural plasticity in the chaffinch Fringilla coelebs. Behaviour. 2005b;142:1377–1402. doi:10.1163/156853905774539391 [Google Scholar]
- Raynor L.S, Uetz G.W. Ontogenic shifts within the selfish herd—predation risk and foraging trade-offs change with age in colonial web-building spiders. Oecologia. 1993;95:1–8. doi: 10.1007/BF00649499. [DOI] [PubMed] [Google Scholar]
- Sih A, Bell A.M, Johnson J.C, Ziemba R.E. Behavioral syndromes: an integrative overview. Q. Rev. Biol. 2004;79:241–277. doi: 10.1086/422893. doi:10.1086/422893 [DOI] [PubMed] [Google Scholar]
- Sokal R.R, Rohlf F.J. Freeman; New York, NY: 1981. Biometry. [Google Scholar]
- Stankowich T. Marginal predation methodologies and the importance of predator preferences. Anim. Behav. 2003;66:589–599. doi:10.1006/anbe.2003.2232 [Google Scholar]
- Stillman R.A, Goss-Custard J.D, Clarke R.T, Durell S.E.A.Le V. Dit. Shape of the interference function in a foraging vertebrate. J. Anim. Ecol. 1996;65:813–824. [Google Scholar]
- Vine I. Risk of visual detection and pursuit by a predator and the selective advantage of flocking behaviour. J. Theor. Biol. 1971;30:405–422. doi: 10.1016/0022-5193(71)90061-0. doi:10.1016/0022-5193(71)90061-0 [DOI] [PubMed] [Google Scholar]
- VSN Intl. VSN International Ltd; Oxford, UK: 2003. Genstat, version 7.1. [Google Scholar]
- Ward A.J.W, Thomas P, Hart P.J.B, Krause J. Correlates of boldness in three-spined sticklebacks (Gasterosteus aculeatus) Behav. Ecol. Sociobiol. 2004;55:561–568. doi:10.1007/s00265-003-0751-8 [Google Scholar]
- Whitfield D.P. Raptor predation on wintering waders in southeast Scotland. Ibis. 1985;127:544–548. [Google Scholar]