Abstract
It has been a long-held assumption that the innate immune system of insects causes self-harm when used to combat an immune insult. We show empirically that this assumption is correct. Invertebrate innate immunity relies heavily on effector systems which, on activation, produce cytotoxins that kill pathogens. Reliance on these robust, fast-acting, generic killing mechanisms ensures a potent and rapid response to pathogen invasion, but has the potential disadvantage of causing self-damage. We show that the innate immune response against an immune insult produces measurable phenotypic and functional damage to self-tissue in the beetle Tenebrio molitor. This type of self-harm (autoreactivity) and the life-history implications that arise from it are important to understand evolutionary phenomena such as the dynamics between hosts and parasites as well as the nature of immune system costs.
Keywords: autoreactivity, innate immunity, phenoloxidase, Tenebrio molitor, Malpighian tubules
1. Introduction
Invertebrates rely on a suite of cytotoxin-producing immune effector systems that rapidly kill pathogens by reacting with intracellular targets including proteins, lipids and DNA (e.g. Bains et al. 1997; Brey & Hultmark 1998; Siva-Jothy et al. in press). The drawback of these effector systems is that they are likely (see Nappi & Vass 1993; Nappi et al. 1995; Read & Allen 2000; Moret 2003) to be equally reactive towards the host's own tissues. If such ‘autoreactive’ self-harm occurs, it would be an important component in the cost structure of immune systems. This has evolutionary implications (Kraaijeveld & Godfray 1997; Moret & Schmid-Hempel 2000) because the nature of these costs underpins our understanding of the dynamics between hosts and parasites as well as the life-history decisions underpinning several aspects of fitness.
Insects and other invertebrates rely heavily on enzyme cascades (Ashida & Brey 1998) to provide fast acting ‘constitutive’ (i.e. ready to act immediately) immunity. Of particular importance, in this context in insects, is the phenoloxidase cascade (Söderhall 1982; Sugumaran et al. 2000), which catalyses the production of melanin and, thereby, phenols, quinones and other cytotoxins (Nappi & Vass 1993; Nappi et al. 1995; Sugumaran et al. 2000). Because insects express this cascade in a single pervasive body cavity in which the organs are suspended, the potential for cytotoxic self-damage is further elevated. There are several ways in which insects may protect themselves from the potential negative consequences of using these cascades; for example, it is likely that the basal lamina (a membrane that lines the haemocoel; Chapman 1998) functions as a protective barrier. Cytotoxin-producing effector systems are also usually multi-level enzyme cascades (the most ubiquitous of which is phenoloxidase; Söderhall 1982; Ashida & Brey 1998; Sugumaran et al. 2000), a feature that facilitates close spatial and temporal control over the cytotoxin generating component(s). In combination, these two protective mechanisms are likely to shield most tissues and organs. However, the Malpighian tubules (an insect's ‘kidneys’) are intimately associated with the haemolymph, extend throughout the body cavity (Chapman 1998) and, because of functional necessity (Chapman 1998), cannot be covered by an impermeable protective membrane. This tissue filters waste products from the haemolymph and, when combined with its spatial disposition, is likely to make the functionally active cell monolayer highly susceptible to autoreactive damage.
If the activation of the phenoloxidase cascade in the vicinity of an immune insult results in costly autoreactive damage, we predict three measurable consequences in insects. First, we expect to see melanization of self-tissue as the phenotypic consequence of phenoloxidase-derived self-harm. Second, given a degree of evolved protection against autoreactivity, there should be higher levels of melanization in self-tissue, proximal to the insult, compared with tissue further away. Third, we predict reduced function in tissues subjected to autoreactive melanization.
We used a simple isogenic graft technique, alongside appropriate controls, and co-implanted living tissue with a standardized immune insult to test these predictions in the mealworm beetle, Tenebrio molitor.
2. Material and methods
(a) Insect cultures
Tenebrio molitor were maintained at 26±2 °C, with ad libitum access to rat chow. They received apple supplement twice a week. All larvae were maintained at a density of 150 larvae in a 30×15×10 cm box. Outbred stocks were initiated and maintained by mixing individuals from out-sourced cultures with additional mixing at each generation. Inbred isofemale lines were initiated from brother–sister matings in the offspring from a monogamous pairing and maintained by the monogamous pairings at the start of each generation for at least 13 generations prior to use in this experiment. Pupae were removed from the cultures, weighed, sexed and then stored individually until imaginal eclosion and subsequent use in experiments. Only adults derived from pupae with a wet weight of 0.10–0.11 g on the day of pupal eclosion were used.
(b) Measuring graft viability
If we damage or kill the transplanted Malpighian tubules, then any immunological response directed towards them may be a response to necrotic tissue rather than an autoreactive consequence of the response towards the nylon insult. To exclude this possibility, we harvested, photographed, transplanted for 24 h and then retrieved a Malpighian tubule from a recipient of the same inbred isofemale line as the donor and also retrieved one of the recipient's own Malpighian tubules. We then measured the physiological function of both Malpighian tubules using a modified ‘oil drop’ technique (Maddrell & Overton 1990; Neufeld & Leader 1998). This technique assays the ability of isolated Malpighian tubules to transport saline across their active cell wall into the tubule lumen, thereby, giving a functional estimate of their physiological capacity. Assayed Malpighian tubules had 0.5 mm length cut from the open end (to standardize the size and condition of the open end) before being placed in a 70 μl drop of sterile T. molitor saline (containing 0.05% w/v phenol red (to aid visualization) and 0.1 mM l−1 dibutyryl cyclic AMP). The whole preparation was then covered with mineral oil. The cut end of the tubule was pulled out of the saline droplet using a fine dissecting pin that secured the open end of the tube under mineral oil. The closed end, and most of the length of the tubule, remained in the saline droplet. A droplet of secreted fluid forms at the cut end of functional tubules. After 4 h, the volume of the secreted droplet, as well as the length of tubule within the saline (i.e. the amount of tubule across which fluid transport occurred), was measured from digital images and analysed with Optimas v. 6.1 digital image analysis software. Data were analysed using SPSS v. 11 for Macintosh.
(c) Response directed at the nylon insult
To examine whether the immune response towards the nylon insult was modified by the presence of the grafted Malpighian tubule, we conducted an experiment in which beetles were allocated to one of the three treatments. The ‘control’ group received a single sterile nylon implant. Beetles in the ‘distal insult’ group received a nylon implant on one side of the abdomen and a Malpighian tubule graft from a donor of the same inbred isofemale line on the other side of the abdomen. Beetles in the ‘proximal insult’ group received a nylon insult attached to a Malpighian tubule graft from a donor of the same inbred isofemale line as the recipient. The nylon implants were harvested 24 h after the treatment, and the volume of the cell mass encapsulating the nylon was measured (see Siva-Jothy & Thompson (2002) for details).
(d) Autoreactive melanization associated with immune insult
We quantified ‘autoreactive’ melanization in virgin adult beetles. Malpighian tubules were derived from donor insects and experimentally transplanted into a recipient beetle in the vicinity of a controlled synthetic immune insult (a nylon implant which generates an immune response in the recipient; Siva-Jothy & Thompson 2002). Insects were allocated to one of the three implant treatment groups. Beetles in the ‘control’ group received a single Malpighian tubule implant into the abdominal haemocoel. Beetles in the ‘distal insult’ group received a nylon implant in the haemocoel on one side of the abdomen and a Malpighian tubule implant into the haemocoel on the other side of the abdomen. Finally, beetles in the ‘proximal insult’ group received an implant consisting of a Malpighian tubule joined to a nylon implant, by inserting the cut end of the tubule into a longitudinal cut at the end of the nylon which was then sealed with a heated needle. This process was repeated next to Malpighian tubule implants that were not attached to nylon (‘control’ and ‘distal insult’). Malpighian tubules and nylon were inserted into the haemocoel of the recipient through a small hole in the pleural membrane between the third and the fourth abdominal sternites. All ‘proximal insult’ implants were inserted into the sealed end of the nylon first, so that the Malpighian tubule would lie alongside the nylon in the haemocoel. All procedures were carried out under aseptic conditions.
All recipient beetles were sourced from the same inbred isofemale lines as the donors. Donor beetles were dissected under sterile conditions and a length of Malpighian tubule was removed and placed in filter-sterilized T. molitor saline (274 mM NaCl, 19 mM KCl, 9 mM CaCl2, 5 mM glucose and 5 mM HEPES in 500 ml distilled water at pH 7) and implanted into a recipient within 5 min of harvesting.
(e) Quantifying autoreactive melanization in Malpighian tubules
Because Malpighian tubules vary in darkness among individuals, we had to assess their darkness before, as well as after, our experimental treatments. Immediately prior to implantation, digital images of all Malpighian tubule grafts were captured and the weighted average luminescence of each graft was quantified using Optimas v. 6.1 digital imaging software. Malpighian tubules were harvested 24 h after implantation and their darkness re-measured as described earlier. The reduction in weighted average luminescence (darker pixels have lower values than lighter ones) over 24 h represents the degree of melanization in the Malpighian tubule graft. The data were square root transformed and analysed with a univariate general linear model using SPSS v. 11 for Macintosh.
(f) Measuring physiological function in tissue exposed to autoreactive damage
All beetles in this experiment were derived from our outbred stock cultures. Experimental beetles were paired according to age, gender and size. One beetle in the pair had a nylon insult implanted into the abdominal haemocoel, while the other received an abdominal puncture of the size required to insert the nylon (the control). We assayed Malpighian tubule function (as outlined earlier) from three tubules harvested from each animal 24 h after treatment.
3. Results
Malpighian tubules remained functional after being harvested, photographed, transplanted for 24 h and then retrieved from the recipient. Their secretion rate (2.86±1.92×10−3 μl mm−1 h−1, mean±s.d.) did not differ significantly from that of the recipients' unmanipulated tubules (3.94±4.25×10−3 μl mm−1 h−1; T18=0.733, p=0.47). We conclude that our transplantation protocol does not result in Malpighian tubule death, or significantly impair Malpighian tubule function in the time frame of our experiment.
The presence of Malpighian tubules had no effect on the cellular encapsulation response directed towards the nylon monofilament (ANOVA, F2,53=0.51, p=0.61, mean response (±s.d.)=3.56×10−3±0.9×10−3 mm3, n=56) indicating that the graft does not affect the recipient's immune response towards the nylon insult.
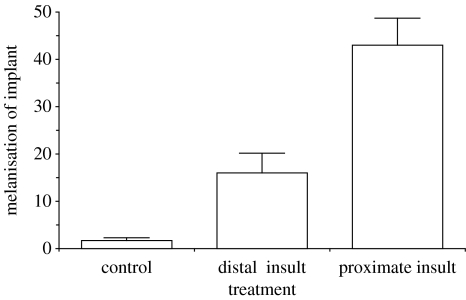
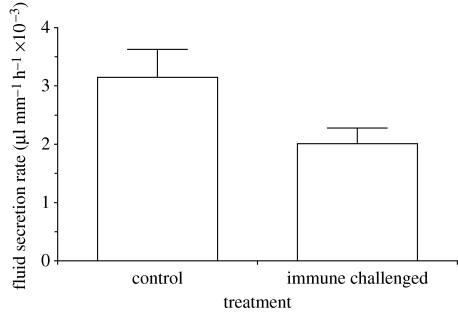
There was a highly significant effect of the presence and proximity of the nylon insult on the degree of melanization in the grafted Malpighian tubules (figure 1; ANOVA, F2,32=24.00, p<0.001) with significant differences between autoreactive melanization in each treatment (figure 1; Bonferroni-corrected pairwise t-test, p<0.01). Malpighian tubules in proximity to nylon were more melanized than tubules distal to the insult, and tubules from both these groups were more melanized than tubules in the control group. Beetles receiving a nylon insult showed a significant (T14=−3.4, p=0.004) reduction (−22±13%) in Malpighian tubule function compared with the controls (figure 2).
Figure 1.
Melanization of Malpighian tubule implants (mean+1 s.e.) measured as reduction in weighted average luminescence in the tubule between implantation and harvesting after 24 h (n=12 for each treatment). There is a highly significant effect of treatment (ANOVA, F2,32=24.00, p<0.001) and all treatment groups differ significantly from each other (Bonferroni-corrected pairwise t-test, p<0.01).
Figure 2.
Malpighian tubule function 24 h after treatment. The fluid secretion rate in control animals is significantly (T14=−3.4, p=0.004) higher than in animals subjected to a single nylon implant for 24 h (mean+1 s.e.).
We conclude that the immune response stimulated by, and directed towards, an experimental nylon insult results in a significant phenotypic and physiological damage to the Malpighian tubules in the vicinity of the insult.
4. Discussion
Our results show that Malpighian tubules in the vicinity of an immune insult are negatively affected by the host's response. Our data show that the expression of a cytotoxin generating enzyme cascade stimulated by non-self has an autoreactive effect on self-tissue. This phenomenon is therefore likely to form a part of the ‘cost of immunity’, one of the evolutionary pressures driving immune system function (Moret & Schmid-Hempel 2000; Armitage et al. 2003; Moret 2003; Rolff & Siva-Jothy 2003; Schmid-Hempel 2003). Reduced function in Malpighian tubules will lead to an impaired ability to maintain water balance and an increase in waste products inside the haemocoel; the former attribute of Malpighian tubules is vital for insect survival in drying environments (Maddrell & Overton 1990).
Apoptosis (Clarke & Clem 2003) does not qualify as ‘self-harm’ in an ultimate (i.e. evolutionary) sense since the process of cellular ‘suicide’ has demonstrable fitness advantages to the organism; the autoreactive damage, we quantified, will result in a fitness cost. A recent study on T. molitor (Armitage et al. 2003) using the same type of nylon implant protocol resulted in a 15% reduction in longevity in insulted insects despite the ideal resource conditions of the study (Armitage et al. 2003). Our results suggest that some of these fitness costs arose from self-harm to Malpighian tubules as a consequence of responding to the immune insult. Phenoloxidase is not the only cytotoxin generating system that insects use to defend themselves against pathogens (e.g. Bains et al. 1997; Nappi et al. 2000). Reactive nitrogen and oxygen intermediates are produced and released by haemocytes in response to an immune insult (Luckhart et al. 1998; Whitten & Ratcliffe 1999). These products cause pathogen cell death and damage (Wang et al. 2001) and are detrimental through reactions with many intracellular targets, including proteins, lipids and DNA (e.g. Siva-Jothy et al. in press). Moreover, recent work on Salmonella infections in Drosophila (Brandt et al. 2004) suggests that eiger, a Tumour Necrosis Factor (TNF) homologue, may also be involved in the production of autoreactive damage. It is, therefore, likely that the functional consequences of autoreactivity, we measured, were caused by the action of additional immune effector systems.
‘Autoreactive’ damage probably contributes to a range of immune system costs uncovered in evolutionary studies (Moret & Schmid-Hempel 2000; Armitage et al. 2003). It is important to point out that this type of self-harm is mechanistically different from ‘autoimmunity’, which results from malfunctions in the acquired immune system (e.g. Shi et al. 2001, but see Sarvetnick & Ohashi 2003) that are unique to the jawed vertebrates. Invertebrates do not posses the mechanisms of acquired immunity (Hoffmann & Reichart 2002), but instead rely solely on innate immunity. Our results show that self-harm (a well-documented phenomenon in vertebrate immunity; see Graham et al. in press) also results from the use of the innate immune system and, consequently, implies that an important general cost of using agonistic defence mechanisms is self-harm.
We demonstrate and quantify the phenotypic and functional consequences of phenoloxidase cascade-induced autoreactivity in an insect. The phenotypic consequences are enhanced with proximity to the insult and we predict that this is likely to be the case for the functional consequences as well. Our results confirm the long-held notion (Nappi & Vass 1993; Nappi et al. 1995; Bains et al. 1997; Read & Allen 2000; Moret 2003; Rolff & Siva-Jothy 2003; Schmid-Hempel 2003; Nappi et al. 2005) that autoreactivity is an important cost associated with the insect immune response.
Acknowledgments
We thank Shelly Adamo, Sophie Armitage, Yannick Moret, Klaus Reinhardt and Jens Rolff for their experimental critiques, advice and comments that improved the manuscript, and Richard Naylor for his logistic support. M.S.-J. was supported by grants from The Royal Society, The Leverhulme Trust and the NERC. B.M.S. was supported by a LEA grant.
Footnotes
Present address: Ecology & Evolution, ETH Zentrum, CHN K14, 8092 Zurich, Switzerland.
References
- Armitage S.A.O, Thompson J.W, Rolff J, Siva-Jothy M.T. Examining costs of induced and constitutive immune investment in Tenebrio molitor. J. Evol. Biol. 2003;16:1038–1044. doi: 10.1046/j.1420-9101.2003.00551.x. doi:10.1046/j.1420-9101.2003.00551.x [DOI] [PubMed] [Google Scholar]
- Ashida M, Brey P.T. Recent advances in research on the insect prophenoloxidase cascade. In: Brey P.T, Hultmark D, editors. Molecular mechanisms of immune responses in insects. Chapman and Hall; London, UK: 1998. p. 135. [Google Scholar]
- Bains J.S, Kakkar R, Sharma S.P. Increased longevity, reduced fecundity, and delayed development in fruitfly (Zaprionus paravittiger) fed on butylated hydroxy anisole. Proc. Soc. Exp. Biol. Med. 1997;215:237–242. doi: 10.3181/00379727-215-44133. [DOI] [PubMed] [Google Scholar]
- Brandt S.M, Dionne M.S, Khush R.S, Pham L.N, Vigdal T.J, Schneider D.S. Secreted bacterial effectors and host-produced eiger/TNF drive death in a Salmonella-infected fruit fly. PLOS Biol. 2004;2:2067–2075. doi: 10.1371/journal.pbio.0020418. doi:10.1371/journal.pbio.0020418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brey P.T, Hultmark D. Chapman and Hall; London, UK: 1998. Molecular mechanisms of immune responses in insects. [Google Scholar]
- Chapman R.F. University of Cambridge Press; Cambridge, UK: 1998. The insects: structure and function. [Google Scholar]
- Clarke T.E, Clem R.J. Insect defenses against virus infection: the role of apoptosis. Int. Rev. Immunol. 2003;22:401–424. doi: 10.1080/08830180305215. doi:10.1080/08830180305215 [DOI] [PubMed] [Google Scholar]
- Graham, A. L., Allen, J. E. & Read, A. F. In press Evolutionary causes and consequences of immunopathology. Ann. Rev. Ecol. Evol. Syst.36, 373–397.
- Hoffmann J.A, Reichart J.M. Drosophila innate immunity: an evolutionary perspective. Nat. Immunol. 2002;3:121–126. doi: 10.1038/ni0202-121. doi:10.1038/ni0202-121 [DOI] [PubMed] [Google Scholar]
- Kraaijeveld A.R, Godfray H.C.J. Trade-off between parasitoid resistance and larval competitive ability in Drosophila melanogaster. Nature. 1997;389:278–280. doi: 10.1038/38483. doi:10.1038/38483 [DOI] [PubMed] [Google Scholar]
- Luckhart S, Vodovotz Y, Cui L, Rosenberg R. The mosquito Anopheles stephensi limits malaria parasite development with inducible synthesis of nitric oxide. Proc. Natl Acad. Sci. USA. 1998;95:5700–5705. doi: 10.1073/pnas.95.10.5700. doi:10.1073/pnas.95.10.5700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddrell S.H.P, Overton J.A. Transport in insect malpighian tubules. Methods Enzymol. 1990;192:617–632. doi: 10.1016/0076-6879(90)92099-y. [DOI] [PubMed] [Google Scholar]
- Moret Y. Explaining variable costs of the immune response: selection for specific versus non-specific immunity and facultative life history change. Oikos. 2003;102:213–216. doi:10.1034/j.1600-0706.2003.12496.x [Google Scholar]
- Moret Y, Schmid-Hempel P. Survival for immunity: the price of immune system activation for bumblebee workers. Science. 2000;290:1166–1168. doi: 10.1126/science.290.5494.1166. doi:10.1126/science.290.5494.1166 [DOI] [PubMed] [Google Scholar]
- Nappi A.J, Vass E. Melanogenesis and the generation of cytotoxic molecules during insect cellular immune reactions. Pigment Cell Res. 1993;6:117–126. doi: 10.1111/j.1600-0749.1993.tb00590.x. [DOI] [PubMed] [Google Scholar]
- Nappi A.J, Vass E, Frey F, Carton Y. Superoxide anion generation in Drosophila during melanotic encapsulation of parasites. Euro. J. Cell Biol. 1995;68:450–456. [PubMed] [Google Scholar]
- Nappi A.J, Vass E, Frey F, Carton Y. Nitric oxide involvement in Drosophila immunity. Nitric Oxide Biol. Chem. 2000;4:423–430. doi: 10.1006/niox.2000.0294. doi:10.1006/niox.2000.0294 [DOI] [PubMed] [Google Scholar]
- Neufeld D.S, Leader J.P. Cold inhibition of cell volume regulation during the freezing of insect malpighian tubules. J. Exp. Biol. 1998;201:227–236. doi: 10.1242/jeb.201.14.2195. [DOI] [PubMed] [Google Scholar]
- Read A.F, Allen J.E. The economics of immunity. Science. 2000;290:1104–1105. doi: 10.1126/science.290.5494.1104. doi:10.1126/science.290.5494.1104 [DOI] [PubMed] [Google Scholar]
- Rolff J, Siva-Jothy M.T. Invertebrate ecological immunology. Science. 2003;301:473–475. doi: 10.1126/science.1080623. doi:10.1126/science.1080623 [DOI] [PubMed] [Google Scholar]
- Sarvetnick N, Ohashi P.S. Autoimmunity. Curr. Opin. Immunol. 2003;15:647–650. doi: 10.1016/j.coi.2003.09.019. doi:10.1016/j.coi.2003.09.019 [DOI] [PubMed] [Google Scholar]
- Schmid-Hempel P. Variation in immune defence as a question of evolutionary ecology. Proc. R. Soc. B. 2003;270:357–366. doi: 10.1098/rspb.2002.2265. doi:10.1098/rspb.2002.2265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi F, Ljunggren H, Sarvetnick N. Innate immunity and autoimmunity: from self-protection to self-destruction. Trends Immunol. 2001;22:97–101. doi: 10.1016/s1471-4906(00)01821-4. doi:10.1016/S1471-4906(00)01821-4 [DOI] [PubMed] [Google Scholar]
- Siva-Jothy M.T, Thompson J.W. Short-term nutrient deprivation affects immune function. Physiol. Entomol. 2002;27:206–212. doi:10.1046/j.1365-3032.2002.00286.x [Google Scholar]
- Siva-Jothy, M. T., Moret, Y. & Rolff, J. In press Insect immunity: an evolutionary ecology perspective. Adv. Insect Physiol.32, 1248.
- Söderhall K. Prophenoloxidase activating system and melanization—a recognition mechanism of arthropods? A review. Dev. Comp. Immunol. 1982;6:601–611. [PubMed] [Google Scholar]
- Sugumaran M, Nellaiappan K, Valivittan K. A new mechanism for the control of phenoloxidase activity: inhibition and complex formation with quinone isomerase. Arch. Biochem. Biophys. 2000;379:252–260. doi: 10.1006/abbi.2000.1884. doi:10.1006/abbi.2000.1884 [DOI] [PubMed] [Google Scholar]
- Wang Y, Oberley L.W, Murhammer D.W. Antioxidant defense systems of two lipidopteran insect cell lines. Free Radic. Biol. Med. 2001;30:1254–1262. doi: 10.1016/s0891-5849(01)00520-2. doi:10.1016/S0891-5849(01)00520-2 [DOI] [PubMed] [Google Scholar]
- Whitten M.M.A, Ratcliffe N.A. In vitro superoxide activity in the hemolymph of the West Indian leaf cockroach, Blaberus discoidalis. J. Insect Physiol. 1999;45:667–675. doi: 10.1016/s0022-1910(99)00039-6. doi:10.1016/S0022-1910(99)00039-6 [DOI] [PubMed] [Google Scholar]