Abstract
Post-mating reproductive isolating mechanisms may be among the earliest reproductive barriers to emerge among incipient species. Trinidadian guppy, Poecilia reticulata, populations in the Caroni and Oropouche drainages in Northern Trinidad exhibit marked genetic divergence and provide an ideal system in which to search for these barriers. We inseminated virgin females with equal amounts of sperm from two males, a ‘native’ male from the female's own population and a ‘foreign’ male from the other drainage. Artificial insemination ensured that mating order and mate choice did not affect the outcome. Paternities were assigned to the resulting broods using microsatellite markers. As predicted, sperm from native males had precedence over foreign sperm. Moreover, this effect was symmetrical for both drainages. In contrast, we detected no native sperm precedence in controls, in which females received sperm from the same and another population within the same drainage. Our results show that gametic isolation can arise between geographically proximate, though genetically divergent, populations of a single species and highlight the potential role of this process in speciation.
Keywords: speciation, sperm competition, internal fertilization, genetic divergence, post-mating pre-zygotic isolation
1. Introduction
Speciation occurs when groups of organisms become reproductively isolated. However, although extant species may be prevented from interbreeding by a number of different reproductive barriers, it is not always easy to determine which of these were important during speciation. Pinpointing the origin of reproductive isolation is a major goal in evolutionary biology (Coyne & Orr 2004). Isolating mechanisms may emerge before, during or after mating, but most of the research on reproductive isolation has focused on pre-mating barriers, including behavioural isolation. This is in part not only because pre-mating barriers are amenable to study, but also because they operate early in the reproductive sequence and may therefore be important in initiating speciation. For example, pre-mating isolation in Drosophila has been found to be much stronger than post-zygotic isolation among species pairs that occur sympatrically (Coyne & Orr 1989, 1997). Other work has shown that sexual selection by female choice results in reproductive isolation and ultimately speciation (Barraclough et al. 1995; Price 1998). Although gametic isolation did not, until recently, receive much attention, its importance during reproductive isolation is becoming increasingly apparent (e.g. Price 1997; Price et al. 2000; Brown & Eady 2001). Gametic isolation includes all reproductive barriers acting between copulation and fertilization (Coyne & Orr 2004, p. 232) and may be a relatively early form of isolation to emerge (Coyne & Orr 1989). (This does not imply that gametic isolation arises quickly. The fastest documented reproductive barriers to form tend to be ecologically dependent pre- and post-zygotic isolation, such as selection against migrants (Nosil et al. 2005) and selection against hybrids (Rundle 2002).) Systems in which female choice is not consistent enough to generate strong assortative mating, or where male mating tactics override female preferences, are good candidates in which to search for gametic isolation.
Trinidadian guppies, Poecilia reticulata, have a promiscuous mating system that appears to fall into this category (Magurran 2005). Receptive females (Liley 1966) engage in consensual mating with males chosen on the basis of colour pattern and morphology (Endler & Houde 1995; Houde 1997). Although there is some consensus on the colour patterns preferred, there are also significant differences among females in the male characters they find attractive (Endler & Houde 1995; Brooks & Endler 2001). Novel partners are usually favoured (Hughes et al. 1999). Females often mate with several males during each receptive period (Evans & Magurran 2000) and switch partners between broods (Becher & Magurran 2004; Eakley & Houde 2004). This behaviour enhances both the quality and the quantity of the resultant brood (Evans & Magurran 2000; Ojanguren et al. 2005). Males, in turn, constantly pursue females and engage in sneaky matings with unreceptive females (Magurran 2005). Sperm can be successfully transferred during sneaky mating (Pilastro & Bisazza 1999) and a substantial fraction of wild females contain sperm inseminated by this means (Matthews & Magurran 2000; Evans et al. 2003a), though because all males use both mating behaviours, it is difficult to assess the success of sneaky mating in nature. Unfamiliar females generally receive more copulation attempts than familiar ones (Kelley et al. 1999). Together, these features mean that when divergent populations come into secondary contact, considerable intermating is likely to occur (Magurran 2001, 2005; Brooks 2002). Laboratory tests in which both females and males from genetically divergent populations are given the opportunity to mate support this conclusion (Endler & Houde 1995; Magurran 1998, 2005).
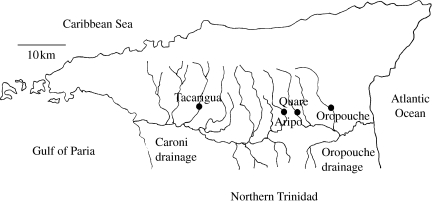
Contemporary guppy populations in Trinidad provide a valuable opportunity to investigate the evolution of reproductive isolating mechanisms. Guppies in the Caroni drainage (which drains west into the Gulf of Paria; figure 1) and the Oropouche drainage (which flows east into the Atlantic Ocean) are geographically proximate but genetically divergent (Carvalho et al. 1991; Fajan & Breden 1992; Alexander & Breden 2004). This marked divergence, which exceeds anything so far recorded within the guppy's natural range, is consistent with an extended period of allopatry (Russell & Magurran 2006), and could represent a separation of the order of 2 million years. In light of this we ask whether any gametic isolation has arisen and test the hypothesis that it takes the form of sperm precedence. Virgin females were inseminated with equal amounts of sperm from native and foreign males in a series of inter- and intra-drainage trials. Our study takes advantage of artificial insemination to exclude the effects of mating order and mating behaviour.
Figure 1.
Location of study populations in Northern Trinidad.
2. Material and methods
(a) Source of fishes
Wild guppies were collected from two localities in each of the two drainages in Trinidad: the Lower Tacarigua and Lower Aripo rivers in the Caroni drainage and the Lower Quare and Lower Oropouche in the Oropouche drainage. In all cases, these fishes co-occur with the pike cichlid, Crenicichla alta, and other significant predators of guppies. Fishes were transported to our tropical aquarium at the University of St Andrews, where they were maintained in tanks furnished with gravel, weeds and filters. Populations were strictly segregated. Virgin females were produced by separating males and females at the point of sexual maturity and housing the sexes apart thereafter. It is essential to use virgin females in this type of study, as females may store sperm from previous matings (Liley 1966).
Virgin females from Lower Aripo (Caroni drainage) and Lower Oropouche (Oropouche drainage) were inseminated with sperm from two males; one male from their own (native) population and the other one from the foreign drainage. As a control, we carried out the same procedure with a male from the female's own population and a male from the other river within the same drainage.
(b) Artificial insemination
Male guppies produce sperm packaged in bundles (spermatozeugmata), which are discrete units clearly visible under a dissecting microscope. To obtain sperm for the artificial inseminations, males were anaesthetized and placed under a microscope on a glass slide. The gonopodium was swung forward, and gentle pressure was applied on the lower abdomen at the base of the gonopodium (a detailed explanation of this procedure is provided in Matthews et al. 1997). This released the sperm bundles from the male and allowed us to count them individually. For each insemination trial, two males were stripped for sperm collection (a male from the female's native population and a male from a foreign population), and equal numbers of bundles (18–20) were obtained from each male using a Gilson micropipette (the number of sperm bundles obtained from each male was based on the size of natural ejaculates described in Evans et al. (2003b)). The bundles were added to a microtube with sterile saline solution and the sample was gently mixed. The sperm mix was then inseminated into an anaesthetized female using a machine-pulled glass micropipette with a penetration depth of approximately 2 mm. After stripping them, the males were humanely killed using an overdose of anaesthetic and preserved in ethanol for subsequent paternity analysis (a different pair of males was used in each replicate). Following artificial insemination, females were revived and isolated in 6 l tanks until they produced their first brood. Broods were counted at the second day after birth and then humanely killed, along with their mothers, before being preserved in ethanol for later analysis. This work was conducted under UK Home Office project and personal licences.
We inseminated 131 females from two river localities: the Lower Aripo river and the Lower Oropuche river. No females died during or immediately after the insemination process. However, nine females (6.87%) died a few days after the inseminations were conducted. Of the remaining 122 inseminated females (57 females from the Lower Aripo river and 65 females from the Lower Oropouche), 65 of them (53.27%) gave birth and produced broods. This figure is consistent with the level of brood production by virgin females allowed to mate naturally in controlled laboratory experiments (Evans & Magurran 2000). No differences in brood production are attributable to male ‘type’ (see Russell & Magurran 2006). Females that gave birth to one or two offspring (nine females) were not used for the paternity analyses; only broods of three or more offspring (56 broods) were used to determine the proportion of paternity by each male in the brood.
(c) Paternity analysis
Tissue samples for paternity analyses were obtained from all fishes (mother, two putative sires and offspring) immediately before the DNA extractions. Tissue samples were taken from the caudal fin and peduncle for adult males and females, and from half of the body for 2-day-old fry. Genomic DNA was extracted using the PureGene protocol (Gentra systems, Minneapolis, MN). Three polymorphic microsatellite markers (accession numbers: AF026459, AF164205, AF533589) were used to estimate each male's relative share of paternity. The PCR protocol followed Evans et al. (2003b). Amplified fragments were resolved using either one of two methods. For some samples, fragments were resolved on a 6% denatured polyacrilamide gel (by vertical electrophoresis) and ran at 1500 V. A 10 bp ladder (GibcoBRL) was used to size the alleles. The amplified loci were visualized by silver-stain following Promega's protocol. The rest of the samples followed the same PCR procedure, but one primer from each pair was end-labelled with a fluorescent dye and amplified loci were visualized using an automated genotyping eight-channel capillary sequencer (Beckman-Coulter CEQ 2000 XL). Two markers were run in duplex (AF533589, AF026459) and the forward primers were labelled with green and blue dyes (D3 and D4 Proligo, France).
Paternity was assigned to offspring according to allele sharing between putative sires, mother and offspring. In all cases, paternity was assigned unambiguously to all offspring (370 fishes in 56 broods).
(d) Statistical analysis
We used repeated measures ANOVA to test the hypothesis that the number of juveniles sired by the native male is greater than the number sired by the foreign male. Repeated measures have the advantage of considering the total number of offspring as well as the difference in number of juveniles between the two sires. In addition, the repeated measures design allows us to include female origin as a fixed factor and thus to test the relative success of the two types of male with just one p estimation. The ANOVA deals with the fact that the numbers of offspring fathered by each male are not independent. Kolmogorov–Smirnov tests confirmed that offspring numbers were normally distributed.
We also asked whether native fathers sired a greater proportion of the brood. Proportions were given an arcsin transformation and tested against the random expectation of 0.5 (which becomes 0.45 upon transformation) using a one sample t-test.
3. Results
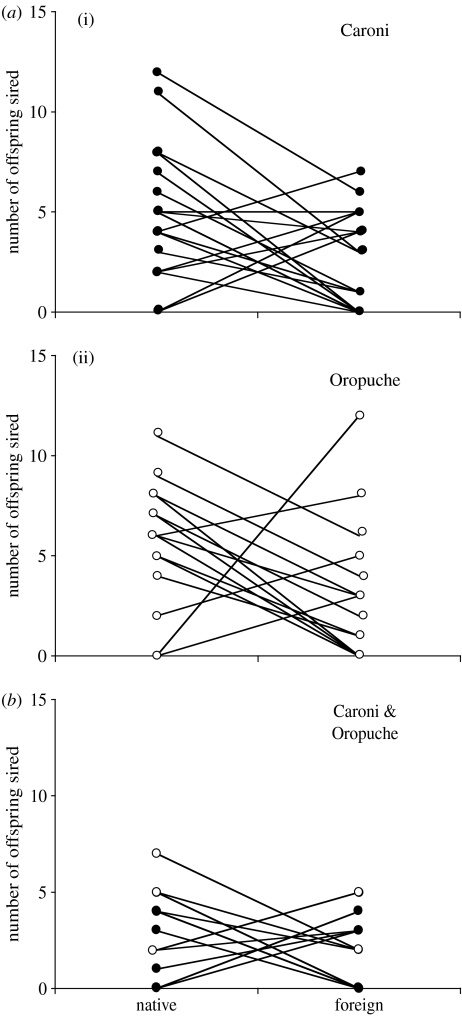
In the between-drainage trials, native fathers gained a significantly higher fraction of the paternity than foreign males (p=0.003; see table 1 and figure 2a). There was no effect of female origin (p=0.36) and no interaction between male origin and female origin (p=0.95). In contrast, there was no significant effect of male origin in the control (within-drainage) trials (p=0.75, see table 1 and figure 2b), as well as no effect attributable to female origin (p=0.08) and no interaction between male and female origin (p=0.60).
Table 1.
ANOVA tables showing the results of the between-drainage and the within-drainage crosses. (Both female origin (drainage) and male origin (native or foreign) are fixed effects. MS, mean square. See text for details.)
| source | between drainage | within drainage | ||||||
|---|---|---|---|---|---|---|---|---|
| d.f. | MS | F | p | d.f. | MS | F | p | |
| between subjects | ||||||||
| drainage (female origin) | 1 | 6.53 | 0.867 | 0.358 | 1 | 3.68 | 3.375 | 0.083 |
| error | 34 | 7.54 | 18 | 1.09 | ||||
| within subjects | ||||||||
| male origin | 1 | 101.87 | 9.946 | 0.003 | 1 | 0.68 | 0.102 | 0.754 |
| male×drainage | 1 | 0.03 | 0.003 | 0.954 | 1 | 1.88 | 0.282 | 0.602 |
| error | 34 | 10.24 | 18 | 6.64 | ||||
Figure 2.
Numbers of offspring sired in each brood by native and foreign males. (a) Cross-drainage trials (shown separately for the two types of female: (i) Caroni female n=20; (ii) Oropouche female n=16); (b) within-drainage trials (Caroni n=5 (solid dots) and Oropouche n=15 (open circles) females combined). See text for further details.
Native fathers sired a significantly greater proportion of the brood when competed against males from the foreign drainage (t35=2.76, p=0.009). However, there was no difference in the proportions of offspring fathered by native and foreign males in the within-drainage trials (t19=0.619, p=0.54).
4. Discussion
Our study provides evidence for partial gametic isolation in guppies by showing that sperm of males from the female's own population have precedence over sperm from genetically divergent males. As the effect is independent of male origin, we can conclude that the process is symmetrical. This conclusion was supported by the control trials; we found no sperm precedence among males from a different river within the same drainage. Although there were fewer control inseminations, this result seems unlikely to be explained by a reduction in power—the median proportion of offspring sired by the native male in a random draw of 20 inseminations from the between-drainage trials (repeated 10 times) is 0.78, compared with the observed value of 0.53 in the within-drainage trials. The advantage of using artificial insemination was that we could isolate the effects of sperm precedence from factors such as the relative contribution of sperm from competing males and the effects of a female's mating decisions—mating order (Evans & Magurran 2001) and a female's perception of male quality (Pilastro et al. 2004) both influence reproductive output. By eliminating the pre-copulatory elements affecting the distribution of paternity in a system with multiple mating, artificial insemination allowed us to isolate the effect of post-copulatory processes. The results are not explained by variation in sperm bundle size. Between-male variation in the size of these bundles does not exceed within-male variation, at least within a single population (Evans et al. 2003b). Moreover, even if sperm numbers vary among bundles, this will only introduce random variation and thereby reduce power, which is irrelevant, given the observed significance. Differences in sperm per packet among populations will not introduce any bias into the study because such differences would lead to asymmetric isolation, which was not observed.
Gametic isolation can take two forms (Coyne & Orr 2004). Non-competitive isolation arises when sperm from heterospecific males are less successful at fertilizing eggs. There are a variety of ways in which this can occur. For instance, fewer sperm may be transferred during heterospecific crosses (e.g. Price et al. 2001), foreign gametes may be inviable in the female's reproductive tract (e.g. Gregory & Howard 1994) and fertilization may not take place if gametes meet (e.g. Palumbi & Metz 1991). Competitive isolation, on the other hand, can only occur when sperm from both the native and foreign male are simultaneously present in the female's reproductive tract. It is manifested when a heterospecific male is disadvantaged in competition with a native male relative to his performance in a non-competitive situation. Coyne & Orr (2004) argue that conspecific sperm precedence could be an important reproductive barrier, given the prevalence of multiple mating in nature. Price (1997), for example, showed that Drosophila simulans females produce fewer than expected hybrids when the sperm of Drosophila mauritiana males compete with D. simulans sperm. To demonstrate that the higher success of native males is indeed due to gametic isolation, we need to be able to show that there is no reduction in the fecundity of females inseminated with foreign sperm. Fortunately, these tests have already been conducted. Crosses involving single-mated females drawn from the same sources as those used in this study were made as part of a test of intrinsic reproductive isolation (Russell 2004; Russell & Magurran 2006). There is no reduction, relative to within-population crosses, in the fecundity of guppy females (of either drainage) inseminated with foreign sperm (i.e. from the other drainage; fig. 3 in Russell & Magurran 2006), though fecundity was lower in F2 and backcross lines. The ready production of F1 hybrids in cross-drainage matings in both directions is consistent with competitive gametic isolation, though the reasons why foreign sperm are less successful in competition with native sperm remain to be elucidated.
To date there have been few reports of competitive gametic isolation, or ‘conspecific’ sperm precedence, between populations of a single species. One exception is Brown & Eady's (2001) study of two allopatric populations of the bruchid beetle, Callosobruchus maculatus. Reciprocal crosses between geographically isolated populations (one from Africa, the other from India) revealed that native males gained precedence over foreign males during sperm competition. Females were also more receptive to further matings with native males. Brown and Eady's work relied on natural copulations and their results therefore reflect both pre- and post-mating effects. Our study builds on this finding by showing that sperm precedence can occur solely as a result of the interactions between the gametes. Moreover, the presence of the effect in geographically proximate populations, as well as in a vertebrate, provides strong support for the idea that gametic isolation may be widespread among incipient species.
Why has competitive gametic isolation in guppies arisen? One possibility is antagonistic sexual selection. This has been implicated in speciation in insects; taxa where polyandry occurs are more species rich than those whose females mate only a single time during their life (Arnqvist et al. 2000). However, as Coyne & Orr (2004) point out, multiple mating can be associated with non-antagonistic sexual selection through female choice or male–male competition. Indeed, increasing support for cryptic female choice in guppies (Pilastro et al. 2004) suggests that this may be a plausible mechanism. It is certainly intriguing that a single male can obtain all the paternity despite sperm mixing, not only when the sperm derive from males of the same population, but even on occasion (as here) when the successful male is from a foreign drainage. The long separation of guppy populations in the Caroni and Oropouche drainages and the pronounced divergence in all of the genetic markers examined to date mean that genetic drift is another potential explanation. On the other hand, natural selection is unlikely to be important, as there are parallel selection pressures (notably predation risk and productivity levels) in both drainages that have resulted in convergence in behaviour, morphology and life history (Magurran 2005). Of course, it is always possible that a source of divergent selection has been overlooked.
Gametic isolation is not the only barrier that appears to be emerging in the guppy system. Post-zygotic isolation occurs in the form of male behavioural dysfunction in the F1 generation, while reduced fecundity and lower sperm counts are apparent in F2 generation crosses (Russell 2004; Magurran 2005; Russell & Magurran 2006). Two forms of post-mating isolation are thus appearing in tandem. We cannot be certain that the barriers that are currently developing will be the ones that eventually ensure complete isolation, but it seems reasonable to predict that this will be the case assuming that no large-scale intermixing of Caroni and Oropouche populations occurs in the interim.
Acknowledgments
We thank Jon Evans for invaluable help with the artificial insemination technique and for insightful ideas during the early phase of this project, and Jerry Coyne for highlighting the importance of gametic isolation. We also thank Isobel Maynard, Kit Magellan, Alfredo Ojanguren and Stephen Russell for assistance with fish-keeping. Charles Paxton and Alfredo Ojanguren provided statistical advice and two anonymous referees made some very helpful comments on an earlier version of the paper. This work was supported by a NERC grant to A.E.M.
References
- Alexander H.J, Breden F. Sexual isolation and extreme morphological divergence in the Cumaná guppy: a possible case of incipient speciation. J. Evol. Biol. 2004;17:1238–1254. doi: 10.1111/j.1420-9101.2004.00788.x. doi:10.1111/j.1420-9101.2004.00788.x [DOI] [PubMed] [Google Scholar]
- Arnqvist G, Edvardsson M, Friberg U, Nilsson T. Sexual conflict promotes speciation in insects. Proc. Natl Acad. Sci. USA. 2000;97:10 460–10 464. doi: 10.1073/pnas.97.19.10460. doi:10.1073/pnas.97.19.10460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barraclough T.G, Harvey P.H, Nee S. Sexual selection and taxonomic diversity in passerine birds. Proc. R. Soc. B. 1995;259:211–215. [Google Scholar]
- Becher S.A, Magurran A.E. Multiple mating and reproductive skew in Trinidadian guppies. Proc. R. Soc. B. 2004;271:1009–1014. doi: 10.1098/rspb.2004.2701. doi:10.1098/rspb.2004.2701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks R. Variation in female mate choice within guppy populations: population divergence, multiple ornaments and the maintenance of polymorphism. Genetica. 2002;116:343–358. doi:10.1023/A:1021228308636 [PubMed] [Google Scholar]
- Brooks R, Endler J.A. Female guppies agree to differ: phenotypic and genetic variation in mate-choice behavior and the consequences for sexual selection. Evolution. 2001;55:1644–1655. doi: 10.1111/j.0014-3820.2001.tb00684.x. [DOI] [PubMed] [Google Scholar]
- Brown D.V, Eady P.E. Functional incompatibility between the fertilization systems of two allopatric populations of Callosobruchus maculatus. Evolution. 2001;55:2257–2262. doi: 10.1111/j.0014-3820.2001.tb00740.x. [DOI] [PubMed] [Google Scholar]
- Carvalho G.R, Shaw P.W, Magurran A.E, Seghers B.H. Marked genetic divergence revealed by allozymes among populations of the guppy Poecilia reticulata (Poeciliidae), in Trinidad. Biol. J. Linn. Soc. 1991;42:389–405. [Google Scholar]
- Coyne J.A, Orr H.A. Patterns of speciation in Drosophila. Evolution. 1989;43:362–381. doi: 10.1111/j.1558-5646.1989.tb04233.x. doi:10.2307/2409213 [DOI] [PubMed] [Google Scholar]
- Coyne J.A, Orr H.A. ‘Patterns of speciation in Drosophila’ revisited. Evolution. 1997;51:295–303. doi: 10.1111/j.1558-5646.1997.tb02412.x. doi:10.2307/2410984 [DOI] [PubMed] [Google Scholar]
- Coyne J.A, Orr H.A. Sinauer Associates; Sunderland, MA: 2004. Speciation. [Google Scholar]
- Eakley A.L, Houde A.E. Possible role of female discrimination against ‘redundant’ males in the evolution of colour pattern polymorphism in guppies. Proc. R. Soc. B. 2004;271(Suppl. 5):S299–S301. doi: 10.1098/rsbl.2004.0165. doi:10.1098/rsbl.2004.0165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endler J.A, Houde A.E. Geographic variation in female preferences for male traits in Poecilia reticulata. Evolution. 1995;49:456–468. doi: 10.1111/j.1558-5646.1995.tb02278.x. doi:10.2307/2410270 [DOI] [PubMed] [Google Scholar]
- Evans J.P, Magurran A.E. Multiple benefits of multiple mating in guppies. Proc. Natl Acad. Sci. USA. 2000;97:10 074–10 076. doi: 10.1073/pnas.180207297. doi:10.1073/pnas.180207297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans J.P, Magurran A.E. Patterns of sperm precedence and predictors on paternity in the Trinidadian guppy. Proc. R. Soc. B. 2001;268:719–724. doi: 10.1098/rspb.2000.1577. doi:10.1098/rspb.2000.1577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans J.P, Pilastro A, Ramnarine I.W. Sperm transfer through forced matings and its evolutionary implications in natural guppy (Poecilia reticulata) populations. Biol. J. Linn. Soc. 2003a;78:605–612. doi:10.1046/j.0024-4066.2002.00193.x [Google Scholar]
- Evans J.P, Zane L, Francescato S, Pilastro A. Directional postcopulatory sexual selection revealed by artificial insemination. Nature. 2003b;421:360–363. doi: 10.1038/nature01367. doi:10.1038/nature01367 [DOI] [PubMed] [Google Scholar]
- Fajan A, Breden F. Mitochondrial DNA sequence variation among natural populations of the Trinidad guppy, Poecilia reticulata. Evolution. 1992;46:1457–1465. doi: 10.1111/j.1558-5646.1992.tb01136.x. doi:10.2307/2409949 [DOI] [PubMed] [Google Scholar]
- Gregory P.G, Howard D.J. A post-insemination barrier to fertilization isolates two closely related ground crickets. Evolution. 1994;48:705–710. doi: 10.1111/j.1558-5646.1994.tb01355.x. doi:10.2307/2410480 [DOI] [PubMed] [Google Scholar]
- Houde A.E. Princeton University Press; Princeton, NJ: 1997. Sex, color and mate choice in guppies. [Google Scholar]
- Hughes K.A, Du L, Rodd F.H, Reznick D.N. Familiarity leads to female mate preference for novel males in the guppy, Poecilia reticulata. Anim. Behav. 1999;58:907–916. doi: 10.1006/anbe.1999.1225. doi:10.1006/anbe.1999.1225 [DOI] [PubMed] [Google Scholar]
- Kelley J.L, Graves J.A, Magurran A.E. Familiarity breeds contempt in guppies. Nature. 1999;401:661. doi: 10.1038/44314. doi:10.1038/44314 [DOI] [PubMed] [Google Scholar]
- Liley N.R. Ethological isolating mechanisms in four sympatric species of poeciliid fishes. Behav. Suppl. 1966;13:1–197. [Google Scholar]
- Magurran A.E. Population differentiation without speciation. Phil. Trans. R. Soc. B. 1998;353:275–286. doi:10.1098/rstb.1998.0209 [Google Scholar]
- Magurran A.E. Sexual conflict and evolution in Trinidadian guppies. Genetica. 2001;112/113:463–474. doi:10.1023/A:1013339822246 [PubMed] [Google Scholar]
- Magurran A.E. Oxford University Press; Oxford, UK: 2005. Evolutionary ecology: the Trinidadian guppy. [Google Scholar]
- Matthews I.M, Magurran A.E. Evidence for sperm transfer during sneaky mating in wild Trinidadian guppies. J. Fish Biol. 2000;56:1381–1386. doi:10.1111/j.1095-8649.2000.tb02150.x [Google Scholar]
- Matthews I.M, Evans J.P, Magurran A.E. Male display rate reveals ejaculate characteristics in the Trinidadian guppy. Poecilia reticulata. Proc. R. Soc. B. 1997;264:695–700. doi:10.1098/rspb.1997.0099 [Google Scholar]
- Nosil P, Vines T.H, Funk D.J. Reproductive isolation caused by natural selection against immigrants from divergent habitats. Evolution. 2005;59:705–719. [PubMed] [Google Scholar]
- Ojanguren A.F, Evans J.P, Magurran A.E. Multiple mating influences offspring size in guppies. J. Fish Biol. 2005;67:1184–1188. doi:10.1111/j.0022-1112.2005.00805.x [Google Scholar]
- Palumbi S.R, Metz E.C. Strong reproductive isolation between closely tropical sea urchins (genus Echinometra) Mol. Biol. Evol. 1991;8:227–239. doi: 10.1093/oxfordjournals.molbev.a040642. [DOI] [PubMed] [Google Scholar]
- Pilastro A, Bisazza A. Insemination efficiency of two alternative male mating tactics in the guppy (Poecilia reticulata) Proc. R. Soc. B. 1999;266:1887–1891. doi:10.1098/rspb.1999.0862 [Google Scholar]
- Pilastro A, Simonato M, Bisazza A, Evans J.P. Cryptic female preference for colorful males in guppies. Evolution. 2004;58:665–669. [PubMed] [Google Scholar]
- Price C.S.C. Conspecific sperm precedence in Drosophila. Nature. 1997;388:715–719. doi: 10.1038/41753. doi:10.1038/41753 [DOI] [PubMed] [Google Scholar]
- Price T. Sexual selection and natural selection in bird speciation. Phil. Trans. R. Soc. B. 1998;353:251–260. doi:10.1098/rstb.1998.0207 [Google Scholar]
- Price C.S.C, Kim C.H, Dyer K.A, Coyne J.A. Mechanisms of conspecific sperm precedence in Drosophila. Evolution. 2000;54:2028–2037. doi: 10.1111/j.0014-3820.2000.tb01246.x. [DOI] [PubMed] [Google Scholar]
- Price C.S.C, Kim C.H, Gronlund C.J, Coyne J.A. Cryptic reproductive isolation in the Drosophila simulans clade. Evolution. 2001;55:2028–2037. doi: 10.1111/j.0014-3820.2001.tb01274.x. [DOI] [PubMed] [Google Scholar]
- Rundle H.D. A test of ecologically dependent postmating isolation between sympatric sticklebacks. Evolution. 2002;56:322–329. doi: 10.1111/j.0014-3820.2002.tb01342.x. [DOI] [PubMed] [Google Scholar]
- Russell, S.T. 2004 Evolution of reproductive isolation in the Trinidadian guppy, Poecilia reticulata, p. 142. Ph.D. thesis, University of St Andrews.
- Russell S.T, Magurran A.E. Intrinsic reproductive isolation between Trinidadian populations of the guppy. Poecilia reticulata. J. Evol. Biol. 2006;19:1294–1303. doi: 10.1111/j.1420-9101.2005.01069.x. [DOI] [PubMed] [Google Scholar]