Abstract
Camouflage typically involves colour patterns that match the background. However, it has been argued that concealment may be achieved by strategic use of apparently conspicuous markings. Recent evidence supports the theory that the presence of contrasting patterns placed peripherally on an animal's body (disruptive coloration) provides survival advantages. However, no study has tested a key prediction from the early literature that disruptive coloration is effective even when some colour patches do not match the background and have a high contrast with both the background and adjacent pattern elements (disruptive contrast). We test this counter-intuitive idea that conspicuous patterns might aid concealment, using artificial moth-like targets with pattern elements designed to match or mismatch the average luminance (lightness) of the trees on which they were placed. Disruptive coloration was less effective when some pattern elements did not match the background luminance. However, even non-background-matching disruptive patterns reduced predation relative to equivalent non-disruptive patterns or to unpatterned controls. Therefore, concealment may still be achieved even when an animal possesses markings not found in the background. Disruptive coloration may allow animals to exploit backgrounds on which they are not perfectly matched, and to possess conspicuous markings while still retaining a degree of camouflage.
Keywords: camouflage, disruptive coloration, crypsis, predation, visual search, animal coloration
1. Introduction
Most animals are under persistent risk of predation, and many animals have evolved a range of defensive colour patterns, of which a primary example is camouflage. Camouflage has most frequently been considered to be mediated through background pattern matching (termed crypsis by Endler (1981)), where an animal represents a random sample of the background where the risk of predation is greatest (Cott 1940; Edmunds 1974; Endler 1978, 1981, 1984, 1988, 1991; Ruxton et al. 2004). With the exception of countershading (Poulton 1890), almost all early discussions of camouflage were of the background-matching type (Wallace 1889; Poulton 1890; Beddard 1895) until the pioneering work of Thayer (1909) and Cott (1940). A potential flaw in the strategy of background matching, identified by the latter two authors, was that the outline of an animal's body would always reveal its presence due to discontinuities between the boundary of the animal and the background. Thayer (1909) instead proposed a different form of camouflage: a theory of disruptive (‘ruptive’) coloration, which was extended and formalized by Cott (1940). Disruptive theory argues that the placement of adjacent, highly contrasting markings near the edge of the body will serve to break up the animal's outline, giving the impression of a series of distinct and apparently unrelated objects (Thayer 1909; Cott 1940; Merilaita 1998; Cuthill et al. 2005; Merilaita & Lind 2005, 2006; Stevens et al. in press a). If an animal or object possesses markings which match a random sample of the background (crypsis), then some markings will sometimes intersect the outline of the body in a disruptive fashion purely by chance. However, the theory of disruptive coloration predicts that markings are statistically more likely to be located at the periphery of the body than would be expected if the distribution of patterns simply matched that found in the background. Additionally, Thayer's alternative term ‘dazzle coloration’ makes it clear that he did not see disruptive coloration as merely a type of background matching, and Poulton (1890) also argued that apparently ‘brilliant tints’ of colour may actually aid concealment. More specifically, Cott (1940) proposed two key tenets of disruptive theory: first, ‘differential blending’, where some patches on an individual stand out from the background while other patches blend in; and second, ‘maximum disruptive contrast’, where adjacent pattern elements are highly contrasting in tone and some are different from the background. These factors are effective in disruptive camouflage because they exploit edge detection mechanisms that function in early visual processing (Stevens & Cuthill 2006). The principles of disruptive coloration have only recently been carefully tested, and have received both indirect (Merilaita 1998) and direct support (Cuthill et al. 2005; Merilaita & Lind 2005, 2006).
In previous field experiments involving avian predators, we showed that artificial moth-like targets with differentially blending disruptive patterns survived significantly better than targets with background-matching patterns (Cuthill et al. 2005). Furthermore, targets with highly contrasting disruptive patterns survived significantly better than equivalent low-contrast patterns, supporting the disruptive contrast hypothesis. However, this work left a major question unanswered (Sherratt et al. 2005). All the colour elements in these artificial prey were chosen from colours common in the background, so that the experiments in Cuthill et al. (2005) cannot be considered a test of what we might call the strong theory of maximum disruptive contrast. In this study, we directly test whether all components of camouflage need to match the background for concealment to be effective or whether, as the early literature proposed (Poulton 1890; Thayer 1909; Cott 1940), some colour patches should be highly conspicuous. Thayer (1909) argued that disruptive coloration may allow animals found on a range of different backgrounds to achieve camouflage on each, and further, enable them to combine camouflage with other potentially conspicuous forms of coloration (such as warning colours and sexually selected colour patterns).
2. Material and methods
The experiment follows the same procedure as in Cuthill et al. (2005). We created triangular artificial targets, 50 mm wide by 25 mm long, using waterproof paper (Hewlett Packard LaserJet Tough Paper) printed with specific patterns on a Hewlett Packard LaserJet 4050N printer. These were not intended to mimic any real species of moth, but the markings were designed to match the visual texture of mature oak bark. Patterns were samples of digital photos of oak tree trunks at 1 : 1 reproduction, taken with a Nikon Coolpix 5700 camera, calibrated to linearize the relationship between radiance and the greyscale in each colour channel (C. A. Párraga 2003, unpublished work; Westland & Ripamonti 2004; Stevens et al. in press b), and saved as uncompressed TIFF files. Images were converted using Image J (Rasband 1997–2006; Abràmoff et al. 2004) to greyscale and thresholded at 50% to binary (black/white) images to provide, when printed onto paper, two-tone bark-like dark and light spatial variations (figure 1). Different samples, from different trees, were used for each replicate target.
Figure 1.
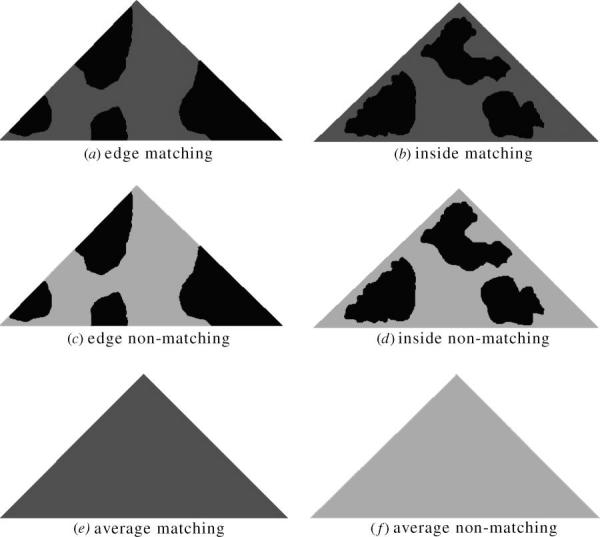
Sample stimuli used in the experiment: (a) edge matching; (b) inside matching; (c) edge non-matching; (d) inside non-matching; (e) average matching (average of the two tones in the matching treatments); and (f) average non-matching (average of the two tones in the non-matching treatments). In the experiment, each replicate for the two-tone treatments had a unique pattern.
Unlike Cuthill et al. (2005), we used grey-and-black rather than brown-and-black targets, and the key experimental manipulation was whether the luminance (perceived lightness) of the grey components matched, or mismatched, the background bark. The black components matched with the dark shadows between ridges of bark, and therefore blended with the background. There were several reasons for using greyscale targets. First, when paired with black, light brown patches that contrast with the background would tend towards the common warning (aposematic) coloration of yellow and black. We wanted to create colour patches that contrasted with the background but were not typical warning colours. Second, calibration of the printer for luminance matching was, at the time of the experiment, easier than for colour matching. Third, there is some evidence that for birds, as with humans, visual tasks involving textural discriminations are more strongly influenced by luminance than colour contrast (Jones & Osorio 2004). Fourth, it is at present uncertain whether luminance-based tasks in birds are subserved by the double cones or a weighted sum of single cone output (Jones & Osorio 2004; Osorio & Vorobyev 2005). Furthermore, there are species and intra-retinal differences in the effective spectral sensitivity of double cones (due to differences in oil droplet concentration; Hart 2001). Use of greyscale targets rendered all manipulations of target luminance virtually identical regardless of the assumptions about predator species or retinal mechanism. The above considerations, combined with the fact that the browns present in oak bark are relatively unsaturated colours, gave us confidence that our greyscale targets would be quite cryptic in those treatments where the grey matched the luminance of oak bark. Personal experience confirmed this (without accurate maps, the targets would have been very hard to relocate). We do not claim to have held chromatic contrast constant across treatments. Because the paper on which patterns were printed absorbs UV, the targets were not ‘bird grey’ (i.e. on the achromatic locus in bird colour space; Vorobyev et al. 1998; Kelber et al. 2003). Therefore, the chromatic contrast between the target and bark will have been higher in our non-matching treatments, just as with the luminance.
The luminance of oak bark was assessed using spectrophotometry combined with calculation of the photon catches of avian photoreceptors when viewing the samples under various natural illuminants (Vorobyev et al. 1998; Kelber et al. 2003; Endler & Mielke 2005). Reflectance of bark (samples from n=30 trees from the study area), as described in Cuthill et al. (2006), was measured normal to the image plane using a Zeiss MCS 230 UV–NIR diode array photometer, with illumination by a Zeiss CLX 111 Xenon lamp (Carl Zeiss Group, Jena, Germany) held at 45° to normal. Five replicate measurements were taken from randomly selected regions of each sample, recorded in 1 nm intervals from 300 to 700 nm, and expressed relative to a Spectralon 99% white reflectance standard (Labsphere, Congleton, UK). This was followed by modelling of predicted photon catches (following Maddocks et al. (2001)) of a typical woodland passerine bird, the blue tit's (Parus caeruleus) double cone photoreceptors (Hart et al. 2000), using irradiance spectra collected at our field site using an Ocean Optics USB2000 spectrometer fitted with a cosine corrector. While the exact mechanism for luminance-based tasks is not fully characterized in birds, available evidence suggests that they are mediated by double cones (Osorio et al. 1999a,b; Jones & Osorio 2004; Osorio & Vorobyev 2005). For example, Jones & Osorio (2004) showed that domestic fowl (Gallus gallus domesticus) chicks could not discriminate textures that were isoluminant for double cones, even when colour information was present. The experiment was conducted between January and May, usually under overcast skies, so that the calculations were repeated for leafless and full leaf canopy conditions, equivalent to Endler's (1993) classifications ‘large gaps’ and ‘woodland shade’.
The mean perceived luminance (double cone photon catch) of the oak bark samples was 12% of that for the white standard. Therefore, for treatments where the grey components of targets were designed to match the average luminance of the bark, a printed grey was chosen that would also produce a 12% photon catch (measurements and calculations as for the bark samples). The black pattern components corresponded to a 4% cone-catch, the darkest black that we could print. For the treatments with non-background-matching pattern elements, we made the grey two standard deviations higher in luminance than the average oak bark values, equivalent to a 31% cone-catch. Calibration of the printer was necessary because a printer's greyscale (here 8 bit, or 0–255) is rarely linearly related to perceived luminance for a human, far less a bird (Westland & Ripamonti 2004; Stevens et al. in press b). ‘Ramp’ images (22 cm long) were printed with grey values increasing from left to right from 0 to 255 and reflectance values were measured on a 21-point transect across the ramp, followed by modelling of avian double cone photon catches (as above). Least-squares regression was used to fit a power function to the relationship between cone-catch and grey value, and the fitted curve was used for calibration (r2 values greater than 0.98). Stimulus grey values were then scaled to correspond to the required luminance values when printed; then the printed stimuli were double-checked via spectrophotometry and photon catch modelling. Calculations were repeated for different daylight illuminants (cloudy and blue sky) to assess the robustness of our estimates, and this made little difference (less than or equal to 1 point on the 256-point greyscale). The calibration procedure was repeated each time a new set of stimuli were printed, as the level of print toner affected the image grey value that produced a correct match.
There were six treatments (figure 1): (a) edge matching, where both the black (4% cone-catch) patches and grey (12% cone-catch) patches matched avian perceived luminance values of oak bark/shadow, and the dark patches overlapped the edge of the ‘wings’ in a disruptive fashion; (b) inside matching, where the colour patches matched the bark/shadow luminance as for the previous treatment, and the shape of the pattern elements matched those in the oak background (as did their general distribution), except that the dark pattern elements did not overlap the wing edges (non-disruptive); (c) edge non-matching (disruptive), where the dark markings (4% cone-catch) matched the luminance of shadow and were placed at the edge of the wings, but the grey components were much lighter than bark (31% cone-catch); (d) inside non-matching (non-disruptive), which had the same luminance patches as treatment (c) but the patches did not overlap the wing edges; (e) average matching, which was a uniformly coloured treatment (no background-matching pattern) where the luminance of the grey was the average of the matching treatment patch luminances (i.e. a 50 : 50 blend of the grey values corresponding to the 4 and 12% cone-catches since the dark and light markings were found in approximately equal amounts on each moth); and (f) average non-matching, which was the average value of the non-matching treatment patterns (a 50 : 50 blend of grey values corresponding to the 4 and 31% cone-catch values).
Targets were pinned onto oak trees in the mixed deciduous Leigh Woods National Nature Reserve, North Somerset, UK (2°38.6′ W, 51°27.8′ N) and their ‘survival’ checked at ca 2, 4, 24 and 48 h. An edible component for birds was pinned to each target, consisting of a dead (frozen overnight at −80 °C, then thawed) mealworm (Tenebrio molitor larva). Avian predation was revealed by complete or nearly complete disappearance of the mealworm. The study site contains a range of avian predators and we have witnessed a range of birds taking the ‘prey’ in various similar experiments (see Cuthill et al. (2006) for more details). Other forms of predation could also be identified: spiders sucked fluids out leaving a hollow exoskeleton, and slugs left slime trails. Non-avian predation, the disappearance of the whole target, or survival to 48 h were treated as ‘censored’ values in survival analysis (see below).
The experiment followed a randomized block design, with a total sample size of 720 spread over 12 blocks. In any one block, 10 replicates of each treatment were randomly allocated, one per tree, along a nonlinear transect of ca 1.5 km by 20 m (targets were placed upon fewer than 5% of available trees), subject to the constraints that no lichen covered the trunk and no young trees of trunk circumference less than 0.9 m were used. The lichen criterion was because some species of lichen reflect ultraviolet light (Majerus et al. 2000), whereas plain oak bark (Cuthill et al. 2006) and our printed stimuli (I. C. Cuthill and M. Stevens, unpublished data) did not. Each block took place on different days and in different locations of the field site, so as to minimize any learning and search image effects. The low density of targets and the individually distinct patterns on each replicate also served to minimize this possibility.
Survival analysis for both experiments was performed with Cox proportional hazards regression (Cox 1972; Lawless 2002; Klein & Moeschberger 2003), which is ideally suited for censored data and the non-uniform change in predation risk with respect to time of day that are evident in such data (Cuthill et al. 2006). Significance was tested with the Wald statistic (abbreviated W with d.f. as a subscript) and pairwise contrasts were used to compare specific treatments.
3. Results
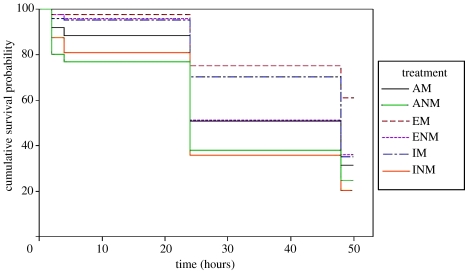
Treatments differed significantly in survival (W5=58.502, p<0.001; figure 2). The edge matching treatment survived better than the inside matching treatment (W1=8.081, p=0.004) and the latter in turn tended to survive better than the average matching control, although this difference was not significant (W1=3.365, p=0.067). Importantly, the edge non-matching treatment survived better than the inside non-matching treatment (W1=9.597, p=0.002) and the latter survived similarly to the average non-matching control (W1=0.021, p=0.884). Indeed, the edge non-matching treatment survived similarly to the inside treatment that matched background luminance (W1=1.142, p=0.285). That said, disruptive targets with luminance matching patterns survived better than edge non-matching targets (W1=14.263, p<0.001). The inside luminance matching treatment survived better than the inside non-matching treatment (W1=17.254, p<0.001). In all, 266 replicates were censored in the analysis (36.9%): 79 from invertebrate predation, 10 never relocated and the 177 surviving unpredated to the end of the 48 h trials.
Figure 2.
Survival plot of the experimental treatments (top to bottom: average matching (AM), average non-matching (ANM), edge matching (EM), edge non-matching (ENM), inside matching (IM), inside non-matching (INM)). Curves are the probability of surviving bird predation as a function of time (in minutes), based on Kaplan–Meier estimates to account for censoring due to non-avian predation and survival to the end of the study period. Long gaps without reduced survival correspond to overnight, when targets were not checked.
There were highly significant differences in average survival between blocks (W11=80.353, p<0.001), but as we have no means of identifying their origins (e.g. area, seasonal, weather or bird density differences) and they are probably not relevant to the hypotheses under test, they are not discussed further.
4. Discussion
The higher survival of disruptive (edge) treatments than the non-disruptive (inside) treatments of equivalent background-matching luminance confirms the findings of Cuthill et al. (2005); disruptive markings provide a significant survival advantage against avian predators compared to targets with background-matching patterns that are not placed disruptively (see also Schaefer & Stobbe (in press)). The disruptive and background-matching treatments had higher survival when both the pattern elements matched the background luminance than when the grey component was brighter than the background. However, the targets with the disruptive non-matching patterns survived as well as the non-disruptive matching treatment. This indicates that, as Thayer (1909) and Cott (1940) predicted, disruptive patterns are still effective when some of the pattern elements do not match the background (see also Schaefer & Stobbe (in press)), although it does appear that disruptive patterns are maximally effective when all components match elements present in the background. It should be noted that there may be other differences between the inside and edge treatments in that the inside treatments are more likely to have a greater concentration of markings close to the target midline (Cuthill et al. in press), and that the inside treatment can result in straight lines at the edge of the body which may aid detection (Cuthill et al. 2005).
Another factor to bear in mind is that because the mealworm was placed on top of each target, the contrast between the patterns on the ‘wings’ may also be important. The average luminance of the mealworms, estimated as the double cone photon catch expressed as a percentage of that for the white standard, was 19.5% (s.d.=3.7, n=9 mealworms, each comprising the mean of five measurements). This lies roughly as an intermediate between the luminance of the grey patches in the matching (12%) and non-matching (31%) treatments, suggesting that mealworm–wing luminance difference was similar across these treatments. It is notable that the mealworm–wing contrast in the average non-matching treatment (19.5 versus 17.5%) was far smaller than in the average matching treatment (19.5 versus 8%), and yet the survival of the latter treatment was higher (figure 1). This suggests that wing : background contrast was more important in prey detection than mealworm : wing contrast, an effect entirely expected from the relative areas of the two prey components (wing area: 625 mm2, average mealworm area: 65 mm2, measured from photographs using Image J). The area of the pinhead was negligible (less than 2 mm2).
From our reading of Thayer (1909), and some of Cott's (1940) diagrams (e.g. p. 50, fig. 6), the prediction was that the non-matching disruptive treatment would have the highest survival of all since these two colour elements had the highest contrast and therefore the strongest disruptive effect. This was not the case; the disruptive treatment where both pattern elements matched the background, survived best. However, when one colour element did not match the background, disruptive placement of colour patches did significantly improve survival over non-disruptive placement. The non-matching non-disruptive treatment survived as poorly as the monochrome treatment of the same average luminance. This suggests that when an animal benefits from having some conspicuous markings (e.g. for display), then by twinning them with background-matching colours and placing the conspicuous elements disruptively, it can gain partial camouflage. This relates to the theory that colour patterns can serve a dual function, where a potentially conspicuous pattern (e.g. aposematic coloration) at one distance, or in one context, is compatible with crypsis at another (Endler 1978; Tullberg et al. 2005). It also accords with Thayer's (1909) idea that disruptive coloration may be a particularly important method of concealment in species which are found on a range of backgrounds and so cannot be perfectly matched to any one situation (see also compromise crypsis; Merilaita et al. 1999, 2001).
Because our targets did not match the colour of bark, the result that targets with two-tone background-matching patterns suffer less predation than monochrome targets without such patterns is an indirect demonstration that pattern detection in birds involves luminance information (Osorio et al. 1999a; Jones & Osorio 2004). Any additional advantage conferred by chromatic information remains to be tested, as does the existence of such an effect against backgrounds with more saturated colours than bark. Matching the average background colour may be of general importance, but colour may have limited effect in terms of the spatial pattern. Therefore, it would be intriguing to know if there are differences in the survival of disruptively marked individuals and background-matching individuals when the targets are isoluminant and the differences in pattern are solely chromatic.
Overall, our results provide further support for the theory of disruptive coloration, and show that it is a method of concealment far more resilient to potentially negative factors, such as non-background-matching components, than is crypsis alone. Our results also indicate that optimal concealment is likely to be achieved by twinning crypsis and disruptive coloration since both these forms of camouflage were effective in reducing the risk of predation compared to either non-matching or non-patterned treatments. Disruptive coloration may enable animals to exploit backgrounds and environments towards which they have only a partial resemblance, and to bear conspicuous markings without paying the full cost of reduced crypsis.
Acknowledgements
This study was conceived and completed independently of the parallel research by Schaefer & Stobbe (in press). We are grateful to Martin Schaefer Research Group for access to their unpublished manuscript and strongly recommend that readers consult their paper and the accompanying commentary article. The research was supported by a BBSRC grant to I.C.C., T. Troscianko and J. C. Partridge, and by a BBSRC studentship to M.S. We thank Tom Troscianko and Alejandro Párraga for their helpful advice. M.S. and I.C.C. conceived the experiment, designed the stimuli and wrote the manuscript; all authors took an equal role in the field experiment.
References
- Abràmoff M.D, Magalhäes P.J, Ram S.J. Image processing with Image J. Biophot. Int. 2004;7:36–43. [Google Scholar]
- Beddard F.E. 2nd edn. Swan Sonnenschein; London, UK: 1895. Animal coloration: an account of the principal facts and theories relating to the colours and markings of animals. [Google Scholar]
- Cott H.B. Methuen & Co. Ltd; London, UK: 1940. Adaptive coloration in animals. [Google Scholar]
- Cox D.R. Regression models and life-tables. J. R. Stat. Soc. 1972;34:187–220. [Google Scholar]
- Cuthill I.C, Stevens M, Sheppard J, Maddocks T, Párraga C.A, Troscianko T.S. Disruptive coloration and background pattern matching. Nature. 2005;434:72–74. doi: 10.1038/nature03312. doi:10.1038/nature03312 [DOI] [PubMed] [Google Scholar]
- Cuthill I.C, Hiby E, Lloyd E. The predation costs of symmetrical cryptic coloration. Proc. R. Soc. B. 2006;273:1267–1271. doi: 10.1098/rspb.2005.3438. doi:10.1098/rspb.2005.3438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthill, I. C., Stevens, M., Windsor, A. M. M. & Walker, H. J. In press. The effects of pattern symmetry on detection of disruptive and background matching coloration. Behav. Ecol
- Edmunds M. Longman Group Ltd; Harlow, UK: 1974. Defence in animals. [Google Scholar]
- Endler J.A. A predator's view of animal color patterns. Evol. Biol. 1978;11:319–364. [Google Scholar]
- Endler J.A. An overview of the relationships between mimicry and crypsis. Biol. J. Linn. Soc. 1981;16:25–31. [Google Scholar]
- Endler J.A. Progressive background matching in moths, and a quantitative measure of crypsis. Biol. J. Linn. Soc. 1984;22:187–231. [Google Scholar]
- Endler J.A. Frequency-dependent predation, crypsis and aposematic coloration. Phil. Trans. R. Soc. B. 1988;319:505–523. doi: 10.1098/rstb.1988.0062. [DOI] [PubMed] [Google Scholar]
- Endler J.A. Interactions between predators and prey. In: Krebs J.R, Davis N.B, editors. Behavioural ecology: an evolutionary approach. 3rd edn. Blackwell Scientific Publisher; Oxford, UK: 1991. pp. 169–196. [Google Scholar]
- Endler J.A. The color of light in forests and its implications. Ecol. Monogr. 1993;63:1–27. doi:10.2307/2937121 [Google Scholar]
- Endler J.A, Mielke P.W. Comparing entire colour patterns as birds see them. Biol. J. Linn. Soc. 2005;86:405–431. doi:10.1111/j.1095-8312.2005.00540.x [Google Scholar]
- Hart N.S. The visual ecology of avian photoreceptors. Prog. Retin. Eye Res. 2001;20:675–703. doi: 10.1016/s1350-9462(01)00009-x. doi:10.1016/S1350-9462(01)00009-X [DOI] [PubMed] [Google Scholar]
- Hart N.S, Partridge J.C, Cuthill I.C, Bennett A.T.D. Visual pigments, oil droplets, ocular media and cone photoreceptor distribution in two species of passerine: the blue tit (Parus caeruleus L.) and the blackbird (Turdus merula L.) J. Comp. Physiol. A. 2000;186:375–387. doi: 10.1007/s003590050437. doi:10.1007/s003590050437 [DOI] [PubMed] [Google Scholar]
- Jones C.D, Osorio D. Discrimination of orientated visual textures by poultry chicks. Vision Res. 2004;44:83–89. doi: 10.1016/j.visres.2003.08.014. doi:10.1016/j.visres.2003.08.014 [DOI] [PubMed] [Google Scholar]
- Kelber A, Vorobyev M, Osorio D. Animal colour vision—behavioural tests and physiological concepts. Biol. Rev. 2003;78:81–118. doi: 10.1017/s1464793102005985. doi:10.1017/S1464793102005985 [DOI] [PubMed] [Google Scholar]
- Klein J.P, Moeschberger M.L. Springer; New York, NY: 2003. Survival analysis: techniques for censored and truncated data. [Google Scholar]
- Lawless J.F. Wiley; New York, NY: 2002. Statistical models and methods for lifetime data. [Google Scholar]
- Maddocks S.A, Church S.C, Cuthill I.C. The effects of the light environment on prey choice by zebra finches. J. Exp. Biol. 2001;204:2509–2515. doi: 10.1242/jeb.204.14.2509. [DOI] [PubMed] [Google Scholar]
- Majerus M.E.N, Brunton C.F.A, Stalker J. A bird's eye view of the peppered moth. J. Evol. Biol. 2000;13:155–159. doi:10.1046/j.1420-9101.2000.00170.x [Google Scholar]
- Merilaita S. Crypsis through disruptive coloration in an isopod. Proc. R. Soc. B. 1998;265:1059–1064. doi:10.1098/rspb.1998.0399 [Google Scholar]
- Merilaita S, Lind J. Background-matching and disruptive coloration, and the evolution of cryptic coloration. Proc. R. Soc. B. 2005;272:665–670. doi: 10.1098/rspb.2004.3000. doi:10.1098/rspb.2004.3000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merilaita S, Lind J. Great tits (Parus major) searching for artificial prey: implications for cryptic coloration and symmetry. Behav. Ecol. 2006;17:84–87. doi:10.1093/beheco/arj007 [Google Scholar]
- Merilaita S, Toumi J, Jormalainen V. Optimization of cryptic coloration in heterogeneous habitats. Biol. J. Linn. Soc. 1999;67:151–161. doi:10.1006/bijl.1998.0298 [Google Scholar]
- Merilaita S, Lyytinen A, Mappes J. Selection for cryptic coloration in a visually heterogeneous environment. Proc. R. Soc. B. 2001;268:1925–1929. doi: 10.1098/rspb.2001.1747. doi:10.1098/rspb.2001.1747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osorio D, Vorobyev M. Photoreceptor spectral sensitivities in terrestrial animals: adaptations for luminance and colour vision. Proc. R. Soc. B. 2005;272:1745–1752. doi: 10.1098/rspb.2005.3156. doi:10.1098/rspb.2005.3156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osorio D, Miklósi A, Gonda Z. Visual ecology and perception of colouration patterns by domestic chicks. Evol. Ecol. 1999a;13:673–689. doi:10.1023/A:1011059715610 [Google Scholar]
- Osorio D, Vorobyev M, Jones C.D. Colour vision in domestic chicks. J. Exp. Biol. 1999b;202:2951–2959. doi: 10.1242/jeb.202.21.2951. [DOI] [PubMed] [Google Scholar]
- Poulton E.B. The International Scientific Series. 2nd edn. Kegan Paul, Trench Trübner & Co. Ltd; London, UK: 1890. The colours of animals: their meaning and use. Especially considered in the case of insects. [Google Scholar]
- Rasband W.S. National Institutes of Health; Bethesda, MD: 1997–2006. Image J.http://rsb.info.nih.gov/ij/ [Google Scholar]
- Ruxton G.D, Sherratt T.N, Speed M.P. Oxford University Press; Oxford, UK: 2004. Avoiding attack. [Google Scholar]
- Schaefer, H. M. & Stobbe, N. In press. Disruptive colouration provides camouflage independent of background matching. Proc. R. Soc. B273 (doi:10.1098/rspb.2006.3615) [DOI] [PMC free article] [PubMed]
- Sherratt T.N, Rashed A, Beatty C.D. Hiding in plain sight. Trends Ecol. Evol. 2005;20:414–416. doi: 10.1016/j.tree.2005.05.010. doi:10.1016/j.tree.2005.05.010 [DOI] [PubMed] [Google Scholar]
- Stevens M, Cuthill I.C. Disruptive coloration, crypsis and edge detection in early visual processing. Proc. R. Soc. B. 2006 doi: 10.1098/rspb.2006.3556. doi:10.1098/rspb.2006.3556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens, M., Cuthill, I. C., Párraga, C. A. & Troscianko, T. In press a The effectiveness of disruptive coloration as a concealment strategy. In Progress in brain research (ed. J.-M. Alonso, S. Macknik, L. Martinez, P. Tse & S. Martinez-Conde). Amsterdam, The Netherlands: Elsevier. [DOI] [PubMed]
- Stevens, M., Párraga, C. A., Cuthill, I. C., Partridge, J. C. & Troscianko, T. S. In press b Using digital photography to study animal coloration. Biol. J. Linn. Soc
- Thayer G.H. Macmillan; New York, NY: 1909. Concealing-coloration in the animal kingdom: an exposition of the laws of disguise through color and pattern: being a summary of Abbott H. Thayer's discoveries. [Google Scholar]
- Tullberg B.S, Merilaita S, Wiklund C. Aposematism and crypsis combined as a result of distance dependence: functional versatility of the colour pattern in the swallowtail butterfly larva. Proc. R. Soc. B. 2005;272:1315–1321. doi: 10.1098/rspb.2005.3079. doi:10.1098/rspb.2005.3079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorobyev M, Osorio D, Bennett A.T.D, Marshall N.J, Cuthill I.C. Tetrachromacy, oil droplets and bird plumage colours. J. Comp. Physiol. A. 1998;183:621–633. doi: 10.1007/s003590050286. doi:10.1007/s003590050286 [DOI] [PubMed] [Google Scholar]
- Wallace A.R. Macmillan; London, UK: 1889. Darwinism. An exposition of the theory of natural selection with some of its applications. [Google Scholar]
- Westland S, Ripamonti C. Wiley; Chichester, UK: 2004. Computational colour science using MATLAB. [Google Scholar]