Abstract
Natural selection shapes the evolution of anti-predator defences, such as camouflage. It is currently contentious whether crypsis and disruptive coloration are alternative mechanisms of camouflage or whether they are interrelated anti-predator defences. Disruptively coloured prey is characterized by highly contrasting patterns to conceal the body shape, whereas cryptic prey minimizes the contrasts to background. Determining bird predation of artificial moths, we found that moths which were dissimilar from the background but sported disruptive patterns on the edge of their wings survived better in heterogeneous habitats than did moths with the same patterns inside of the wings and better than cryptic moths. Despite lower contrasts to background, crypsis did not provide fitness benefits over disruptive coloration on the body outline. We conclude that disruptive coloration on the edge camouflages its bearer independent of background matching. We suggest that this result is explainable because disruptive coloration is effective by exploiting predators' cognitive mechanisms of prey recognition and not their sensory mechanisms of signal detection. Relative to disruptive patterns on the body outline, disruptive markings on the body interior are less effective. Camouflage owing to disruptive coloration on the body interior is background-specific and is as effective as crypsis in heterogeneous habitats. Hence, we hypothesize that two proximate mechanisms explain the diversity of visual anti-predator defences. First, disruptive coloration on the body outline provides camouflage independent of the background. Second, background matching and disruptive coloration on the body interior provide camouflage, but their protection is background-specific.
Keywords: crypsis, natural selection, anti-predator defences, colour vision, prey recognition
1. Introduction
Disruptive coloration and background matching are two techniques of camouflage that are often quoted as textbook examples of natural selection. Visually hunting predators are thought to select for highly contrasting disruptive prey coloration that disguises the body outline of the animal, preventing prey recognition (Thayer 1909; Cott 1940), or for cryptic prey coloration that matches a random sample of the background (Endler 1978). The key question for understanding the evolution of camouflage is whether the two techniques are interrelated or whether they represent alternative principles that exploit different mechanisms of prey recognition.
Cott (1940, p. 50) emphasized that disruptive coloration is contingent on background matching, stating that ‘…the effect of a disruptive pattern is greatly strengthened when some of its components closely match the background, while others differ strongly from it. Under these conditions, by the contrast of some tones and the blending of others, certain portions of the object fade out completely while others stand out emphatically.’ Subsequent studies on artificial and natural moths followed his view in treating disruptive coloration (Cuthill et al. 2005) and crypsis (Endler 1984) as interrelated techniques to deceive predators. However, Cott and other pioneers of the study of camouflage (Thayer 1909; Cott 1940) also stressed the universality of disruptive coloration, and recent experiments suggested that it might be effective above and beyond background matching (Merilaita 1998; Cuthill et al. 2005; Merilaita & Lind 2005).
To understand the evolution of crypsis and disruptive coloration, it is important to consider the constraints associated with both techniques (Merilaita & Tullberg 2005). Theory predicts that natural selection leads to reduced contrasts between prey and background, i.e. crypsis (Endler 1978, 1980; Cooper & Allen 1994). Because crypsis is background-specific, background heterogeneity is expected to compromise the effectiveness of camouflage (Merilaita et al. 1999; Lev-Yadun et al. 2004; Ruxton et al. 2004). It is thus generally assumed that camouflage by crypsis entails indirect opportunity costs because animals may forgo foraging opportunities in microhabitats in which their background matching is poor (Ruxton et al. 2004; Merilaita & Tullberg 2005).
Despite the fact that humans adopted the principle of disruptive coloration widely for military purposes, the constraints associated with that strategy are not well understood. There are only two convincing examples of the effectiveness of disruptive coloration both involving artificial prey objects. In one experiment, artificially coloured moths, which had been designed to match their background of oak trunks in colour and brightness, survived better if they sported highly contrasting patterns on the edge of their wings compared to moths with the same patterns inside their wings (Cuthill et al. 2005). Similarly, disruptive prey items with close resemblance to background survived better than some cryptic prey that exactly matched the background (Merilaita & Lind 2005). An important implication of that study is that if disruptive coloration functions independently of background matching, disruptively coloured animals might exploit a greater range of habitats than cryptic ones (Sherratt et al. 2005). Possible constraints of disruptive coloration are that contrasting patterns are commonly symmetric, which might reduce the effectiveness of camouflage (Sherratt et al. 2005; Merilaita & Lind 2006).
We compared the effectiveness of crypsis and disruptive coloration using birds as predators and artificial moths as targets on two distinct backgrounds in a temperate forest. We classified moth targets as cryptic if they resembled the background in colour and pattern (see Endler 1984) and as disruptive if they were characterized by highly contrasting markings that break up the body outline or its surface. Birds are one of the most important visually oriented predator groups, whose selective pressures are assumed to shape anti-predator defences in a range of animal taxa, most notably insects (Bond & Kamil 2002; Cuthill et al. 2005). Studies using models of avian vision concluded that natural selection by birds reduces the contrasts of prey—particularly the chromatic contrasts (Stuart-Fox et al. 2004; Håstad et al. 2005).
Using an avian eye model, we predicted fitness advantages for cryptic prey if it exhibited lower chromatic contrasts against background than did disruptive prey. We also tested whether cryptic prey had fitness advantages if it had lower achromatic contrasts than disruptive prey, but similar chromatic contrasts. To elucidate the constraints of disruptive coloration, we tested the survival of differently coloured moth pairs, each consisting of one type with disruptive markings on the edge (termed ‘edge’) and one type with the same colours and markings on the inside of the wing (termed ‘inside’). We tested the hypothesis that disruptive coloration is contingent on the background on two levels. Comparing the survival of edge forms, we predicted fitness advantages for each form on that background where its disruptive markings were highly contrasting. Similarly, we predicted fitness advantages for edge compared to inside forms on backgrounds where disruptive markings were highly contrasting. Finally, we tested in another pair of moths whether disruptive coloration is an effective anti-predator defence even if it consists of contrasting colours that are absent from natural backgrounds.
2. Material and methods
(a) Model species
We used the peach blossom (Thyatira batis), a common species in Central Europe, as a model for designing artificial disruptive moths. Cott (1940) classified this species as bearing disruptive coloration because its marginal pink spots are contrasting against most natural backgrounds. This nocturnal species rests during the day and, according to a survey of photographic records (n=60) hosted on the World Wide Web, is mainly found on green vegetation. Thyatira batis presumably relies on camouflage to avoid detection because it has no known chemical defences and camouflage is the anti-predator defence most commonly assumed for the family Thyatiridae. Using digital photographs of 20 specimens, we measured the size and distribution of individual colour patterns with the program Optimas v. 6.1 (Stemmer Imaging GmbH, Puchheim). Because the smallest marginal spot had an extension of less than 1.5 mm, we defined the outer 1.5 mm as the wing margin. Pink spots occupied 58±2% (mean±s.e.) of wing margins and 36±2% of the inside of the wings. Wing margins made up nearly half of the surface area of the wing. Pink spots therefore represented 47±2% of the overall wing colour.
Because Cuthill et al. (2005) demonstrated that disruptive coloration is more effective on the body outline, for each individual we compared the number of pink spots that touched the edge of the wing with a random distribution of these spots (i.e. a null hypothesis; see Merilaita 1998). Since wing development in butterflies is characterized by few basic elements, e.g. spots (McMillan et al. 2002), we treated each spot as a single unit. We repeated the analysis on two different grid sizes (1×1 and 0.25×0.25 mm) because the number of locations available for random placement affects the probability that random spots are located on the body outline. Regardless of grid size, pink wing spots in T. batis were more often located on the edge of the wing (outer 1.5 mm) than randomly expected (Mann–Whitney test, z=−5.816 and −5.842, both p<0.001), suggesting that this species relies on marginal disruptive coloration for camouflage.
We compared the survival performance of artificial disruptive moths with that of an artificial cryptic moth. This moth matched the prime example of crypsis in Lepidoptera (Grant et al. 1996), the white form of the peppered moth (Biston betularia), in colour contrasts against white birch trunks (see §2b).
(b) Colour measurements
We printed artificial moths using a Xerox Phaser 8200DX printer (1200 dpi) on normal paper. To assess colour objectively, including UV, we measured reflectance spectra of moths with a USB2000 spectrometer and a top sensor system deuterium–halogen DH-2000 (both Ocean Optics) as a standardized light source. We measured reflectance of two T. batis and two B. betularia specimens, 10 artificial moths of each type, 20 moss and 20 birch bark samples. Thyatira batis had very low reflectance in the UV and therefore figured as a model species for our paper moths, which also absorbed UV. Additionally, we measured 10 leaves of each of 50 plant species as the adaptation background for avian photoreceptors (see §2c). Reflectance was measured as the proportion of a standard white reference tile (top sensor systems WS-2). To collect the spectra, we used a coaxial fibre cable (QR400-7, Ocean Optics) that was mounted inside a matt black plastic tube to exclude ambient light. The angle of illumination and reflection was fixed at 45° to minimize the objects' glare. To measure ambient light, we recorded irradiance 2 m above the ground inside the forest with a cosine-corrected probe, which measured incoming light over an angle of 180°. For these measurements, the spectrometer was calibrated with a calibration lamp of known energy output (Ocean Optics LS-1-CAL).
(c) Contrast calculations
To analyse the contrasts between moths and background, we used the mean of 10 reflectance spectra of each artificial moth type and calculated the contrasts to all 40 background samples. We determined overall contrasts between moths and background according to the relative proportion of colours within the wing. We calculated the chromatic contrasts between wings and background according to Thery et al. (2005) by modelling the probability of photon catches by the four cone types of blue tits (Parus caeruleus; Hart et al. 2000). We used blue tits because they were the most common insectivorous bird species in the study plot and probably fed on the edible part of artificial moths. Following Thery et al. (2005), we calibrated the photoreceptor response of blue tits assuming that they display half their maximal response when stimulated by the adaptation background. To assess the achromatic contrasts in light intensity between moths and background, we calculated the photon catches of the double cone as detailed in Thery et al. (2005). We divided the values for moths by the values of the background, so that values greater than 1 indicate that moths are brighter than the background and values less than 1 indicate that moths are darker than the background. Values of 1 indicate that moths had the same brightness as the background.
(d) Experimental design
We used a 5×2 design, with four differently coloured disruptive moths and one cryptic moth that were glued on two different backgrounds, green and white (for humans). As the green background we chose moss-covered trunks of oak (Quercus rubra) and birch (Betula pendula), and as white background we chose birch trunks in a mixed deciduous forest of 11 ha (Mooswald, Freiburg, Germany; 48° N, 8° E). To make artificial moths attractive as prey, we glued a dead mealworm (Tenebrio molitor) underneath the wings so that less than half of the mealworm was visible as the edible body of our artificial moth. We did not account for contrasts created by mealworms because these were invariant in all moth types and therefore could not explain differences in moth mortality. Moreover, the visible part of the mealworm was small compared to the wings. Artificial moths were positioned on tree trunks with the mealworm perpendicular to the ground at a height of 1.5 m. We checked the survival of artificial moths after 4, 6, 8 and 24 h. Fourteen trials were run once a week from June until the beginning of September 2005. If birds discovered the moths, the mealworm was missing, whereas if insects or spiders fed on the mealworms they left the exoskeleton.
The four disruptive moth types comprised two pairs, each with one type that matched T. batis in the location of the spots (edge) and one type with the same overall coloration but none of the spots touching the outline of the moth (inside). The straight lines on the wing margins of inside types presumably enhance detection (Cuthill et al. 2005). We therefore assumed that differences in the survival rates of moth types should be particularly pronounced. None of the disruptive moths matched the backgrounds. The first pair of edge (n=80; numbers refer to the numbers on each background) and inside (n=70) moths resembled T. batis in colour (brown with pink spots; termed pink). According to Cuthill et al. (2005), greater contrasts of disruptive patterns enhance the effectiveness in providing camouflage. For the avian eye, pink spots had higher chromatic (t-test: t=6.77, p<0.01) and achromatic (t-test: t=7.44, p<0.0001) contrasts on moss than on birch. Comparing survival rates of pink edge and inside forms, we therefore predicted fitness advantages for edge forms on moss.
The second pair (edge: n=80, inside: n=80) had the same colours as the first one but with an inversed pattern, i.e. pink moths with brown spots (termed brown). For birds, brown spots have higher chromatic (t-test: t=8.82, p<0.0001) and achromatic (t-test: t=7.44, p<0.0001) contrasts on birch than on moss. We therefore predicted that edge forms would survive better on birch than inside forms. Finally, comparing the survival rates of edge forms, we predicted that the type with pink marginal spots would survive better than the type with brown marginal spots on moss and that the type with pink marginal spots would survive better on birch. To evaluate the effectiveness of disruptive coloration and crypsis, we used an artificial cryptic moth (n=80) which had the same contrasts to background as the mean reflectance of B. betularia specimens (all t-tests: t<1.22, p>0.22). We compared the survival performance of all disruptive moths and the cryptic moth using Cox regressions following Cuthill et al. (2005). Insect predation or disappearance of the entire target was treated as censored values in that analysis.
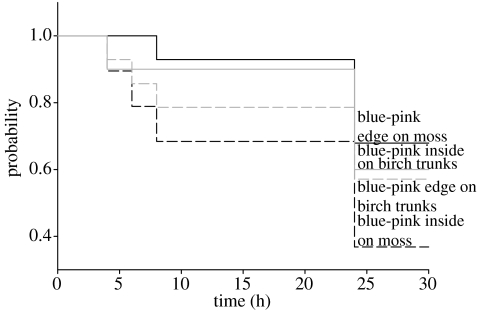
To test Cott's assumption that disruptive coloration is most effective if an animal partly matches the background, we created a third pair of edge and inside forms with pink spots and blue wing colour in a second trial. We used the colour combination blue/pink because that combination is not represented in natural backgrounds. We conducted that trial in late August, when fewer birds were present. Therefore, we had a lower number of blue moths (edge: n=58, inside: n=35) and analysed their survival probability separately.
Because data were normally distributed and were homogeneous in variance (Levene test: p>0.2), we tested for differences in the chromatic and achromatic contrasts of moths and background with one-way ANOVA and post hoc Scheffé tests.
3. Results and discussion
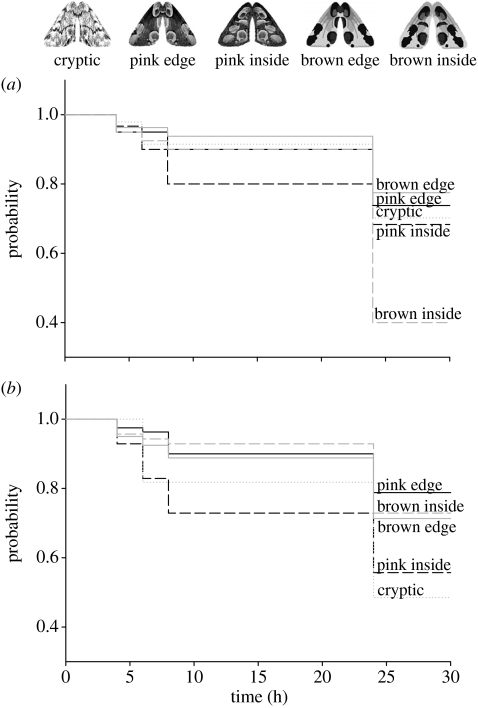
The first two pairs of artificial disruptive moths and the cryptic moth exhibited all the same achromatic contrasts (ANOVA F=1.05, p=0.35) against a background of birch. On birch trunks, moths had the same brightness as the background (table 1). However, moths differed in their chromatic contrasts (ANOVA F=14.675, p<0.001) and also in their survival probabilities (Wald=19.632, p<0.001; figure 1). Although the cryptic moth exhibited lower chromatic contrasts against birch than the other moth types (post hoc Scheffé test: p<0.001), it did not have higher survival probabilities than the two edge moth types and the inside form with pink interior spots (brown edge=pink edge=cryptic=pink inside, all Wald<1, all p>0.4). All of these moths survived better than the moth with brown interior spots and pink wing margins (brown edge versus brown inside, Wald=13.489, p<0.001; pink edge versus brown inside, Wald=10.068, p<0.01; cryptic versus brown inside Wald=5.505; pink inside versus brown inside Wald=4.21, both p<0.05).
Table 1.
Contrasts (mean±s.e.) between the chromatic colour component and the achromatic brightness component of artificial moths and birch and moss backgrounds. (Note that blue moths were tested separately and therefore not compared with other moth types. Achromatic values of 1.0 predict equal brightness of moths and background.)
| moth type | chromatic | achromatic | ||
|---|---|---|---|---|
| contrast | statistics | contrasts | statistics | |
| birch | ||||
| pink | 0.42±0.03 | pink versus brown: p=0.76 | 1.0±0.14 | pink versus brown: p=0.95 |
| brown | 0.40±0.03 | cryptic versus pink & brown: p<0.001 | 0.9±0.13 | pink versus cryptic: p=0.84 |
| cryptic | 0.29±0.02 | 0.7±0.10 | brown versus cryptic: p=0.67 | |
| blue | 0.63±0.01 | 1.0±0.14 | ||
| moss | ||||
| pink | 0.51±0.02 | pink versus brown: p=0.78 | 2.6±0.16 | pink versus brown: p=0.67 |
| brown | 0.48±0.02 | pink versus cryptic: p=0.83 | 2.4±0.15 | pink versus cryptic: p=0.09 |
| cryptic | 0.54±0.03 | brown versus cryptic: p=0.43 | 2.0±0.12 | brown versus cryptic: p<0.05 |
| blue | 0.56±0.00 | 2.7±0.17 | ||
Figure 1.
The survival probabilities of artificial moths on (a) birch trunks and (b) moss. Solid lines indicate the disruptively coloured ‘edge’ moths with marginal spots, whereas dashed lines indicate the ‘inside’ moth types with spots inside the wings. Black lines represent the edge form with pink marginal markings and its corresponding inside form with pink spots on the wing interior. Grey lines represent the edge form with marginal brown spots and its corresponding form with brown spots inside the wing. The grey spotted line represents the cryptic moth type.
All artificial moths exhibited similar chromatic (ANOVA F=0.86, p=0.43) but different achromatic contrasts (ANOVA F=5.19, p<0.01) against the background of moss. The cryptic moth had significantly lower achromatic contrasts than moths with brown spots and marginally significantly lower contrasts than moths with pink spots (post hoc Scheffé test: p<0.05 and p=0.09). The survival probabilities of moths differed (Wald=14.404, p<0.01; figure 2), but the ranking was distinct from that on birch trunks. Against moss, the two edge forms and the brown inside form survived equally well (all Wald<1, all p>0.32) and better than the cryptic and the pink inside forms (pink edge versus cryptic Wald=7.816, versus pink inside Wald=10.068, both p<0.01; brown inside versus cryptic Wald=4.733, versus pink inside Wald=4.934, both p<0.05; brown edge versus cryptic Wald=3.988, versus pink inside Wald=4.144, both p<0.05). There was no difference between cryptic and pink inside moths (Wald=0.65, p=0.8).
Figure 2.
Survival probabilities of blue artificial moths with pink spots on birch trunks (grey lines) and on moss (black lines). Solid lines indicate edge forms, whereas dashed lines indicate inside forms.
Despite its lower chromatic contrasts on birch, the cryptic form had no fitness advantage compared with three of the four disruptive moths. When the cryptic moth had equal chromatic but lower achromatic contrasts than the disruptive types (on moss), it had a lower survival rate than three of the four disruptive moths. This result challenges the basic but rarely tested assumption of signal theory, i.e. increased contrasts to background augment automatically signal efficacy. Our results demonstrate that chromatic contrasts are more important to reduce signal efficacy to predators than achromatic contrasts (but see Stevens et al. in press). This is because low chromatic contrasts of the cryptic moth reduced its mortality to the same level as disruptive prey, whereas low achromatic contrasts resulted in a higher mortality of the cryptic moth. We hypothesize that camouflage is mainly mediated by chromatic contrasts; this conjecture explains why reptiles and insects are more cryptic in the chromatic but not the achromatic aspect of their coloration according to the visual perception of their predators (Stuart-Fox et al. 2004; Thery et al. 2005).
Regarding the overall survival probabilities on both backgrounds, disruptively coloured edge forms survived better than the cryptic form (Wald=4.027, p<0.05), mainly because the cryptic moth had lower survival probabilities on the dissimilar background of moss. As predicted by theory (Merilaita et al. 2001; Ruxton et al. 2004), our result implies that the evolution of crypsis is constrained by background heterogeneity. Because the two edge forms had similar survival performance on both backgrounds as the cryptic moth on the background that matched its colour (all comparisons Wald<1, all p>0.73), disruptive patterns on the body outline (but not the body interior, see below) enable its carrier to exploit a wider range of habitats than cryptic coloration. These results, which pertain to the specific pattern that we tested, are the first to demonstrate that disruptive markings of a butterfly species provide effective camouflage independent of background matching and even though the disruptive patterns are symmetrical, as they are in most moth species (Sherratt et al. 2005; Merilaita & Lind 2006).
In contrast to disruptive edge forms, the survival rates of inside forms depended on background coloration. The brown inside form survived better on moss, whereas the pink inside form survived better on birch. Disruptive interior exhibits markings are thus more effective as anti-predator defence if uni-coloured wing margins exhibit high contrasts (pink on moss, brown on birch) and the body interior exhibits low contrasts against background. When exhibiting such contrasts, the inside forms were as protected as the cryptic form when it matched the background (all Wald<1, p>0.05). Conversely, when contrasts against background were low on the edge and high on the interior, inside forms were as vulnerable as the cryptic form on a dissimilar background (all Wald<1, all p>0.68). The cryptic moth and the inside forms had similar overall survival rates on both backgrounds (Wald=0.256, p=0.61). Thus, we conclude that disruptive markings on the body interior are an equally effective technique of camouflage in heterogeneous habitats as the cryptic coloration of B. betularia, one of the best known—albeit controversial—examples of how natural selection shapes anti-predator defence (Grant 1999; Ruxton et al. 2004). This result is particularly important since the relatively straight uni-coloured lines of our inside treatments presumably enhance the body outline and thereby increase the conspicuousness of targets (Cuthill et al. 2005).
Edge forms survived better than inside forms on the two backgrounds (Wald=17.669, p<0.001; see also Stevens et al. in press). An important implication of this result is that the locations of disruptive markings determine signal efficacy. In general, signal efficacy is characterized by four components: signal production, transmission, perception and neuronal processing of signals (Endler 1992). Since pairs of edge and inside forms had the same colour contrasts, signal production, transmission and perception are identical. Thus, we emphasize that disruptive coloration exploits the cognitive component of prey recognition in birds. This conjecture is important because it demonstrates that selection on visual signals targets signal processing, but traditionally most studies addressed signal production and perception as the targets of selection. While it is well established that natural selection might counteract the selective pressures of sexual selection by reducing the colour contrasts within prey or between prey and background (Endler 1980; Cooper & Allen 1994; Håstad et al. 2005), we suggest that natural selection might also alter the colour patterns of prey so that patterns are harder to perceive as pertaining to a sole object.
Our results document that the key prediction of Cuthill et al. (2005), i.e. that disruptive patterns are more effective on the body outline, is valid independent of background matching. However, fitness advantages of edge forms are habitat- or background-specific because they only occurred when disruptive markings were highly contrasting. Because edge forms provide camouflage independent of background colour, we failed to find the predicted differences in survival probabilities of the two edge forms.
To test whether our disruptively coloured edge forms gained protection because brown is a colour commonly encountered in backgrounds, we created blue moths with pink spots, a colour combination that is absent from natural backgrounds. If avian predators select disruptive coloration according to the contrasts of disruptive spots, we expected these blue-pink moths to survive as well as pink moths because both types had pink spots. By contrast, if disruptive coloration is contingent on the frequency with which a colour is represented in the background, blue forms should have lower survival rates on both backgrounds. The blue-pink inside and edge forms had equal survival probabilities on birch trunks (Wald=0.1, p=0.75) and did not differ from pink types (all Wald<2.4, all p>0.2). On moss, the blue-pink edge and pink edge forms survived equally well (Wald=1.396, p=0.24) and better than the two inside types (Wald=4.352 and 4.14, both p<0.05).
Because blue-pink moths showed the same pattern of survival as brown-pink forms, we conclude that disruptively coloured edge forms are freed from the constraints of background matching. Importantly, alternative explanations, such as aposematism or dietary conservatism, do not explain the differential survival rates of blue edge and inside forms on moss. In contrast to disruptively coloured edge forms, camouflage owing to disruptive patterns on the inside of the wing and crypsis is contingent on the background. These conclusions clearly pertain to the specific colour pattern that we tested and to the limited complexity of background heterogeneity in our study. Nevertheless, both the independency from background in disruptive edge forms as well as background-specific camouflage in inside and cryptic forms are proximate mechanisms to explain the diversity of visual anti-predator defences.
Acknowledgments
This study was conceived and completed independently of the parallel research by Stevens et al. (in press). We are grateful to Innes Cuthill for access to their unpublished manuscript and strongly recommend that readers consult their paper and the accompanying commentary article. We gratefully acknowledge the help of Ragna Franz, Eva Nowatschin and Isabelle Szabo in conducting the experiments. We thank Innes Cuthill, John Endler and Tim Caro for greatly improving earlier versions of the manuscript with many valuable comments. Nathan Hart kindly provided blue tit photoreceptor catches. The Zoologische Staatssammlung München generously provided access to the butterfly collection. N.S. was sponsored by a Ph.D. grant from the Cusanuswerk, M.S. by a DFG grant Scha 1008/4-1 during the writing of the manuscript.
References
- Bond A.B, Kamil A.C. Visual predators select for crypticity and polymorphism in virtual prey. Nature. 2002;415:609–613. doi: 10.1038/415609a. doi:10.1038/415609a [DOI] [PubMed] [Google Scholar]
- Cooper J.M, Allen J.A. Selection by wild birds on artificial dimorphic prey on varied backgrounds. Biol. J. Linn. Soc. 1994;51:433–446. doi:10.1006/bijl.1994.1033 [Google Scholar]
- Cott H.B. Methuen; London, UK: 1940. Adaptive coloration in animals. [Google Scholar]
- Cuthill I.C, Stevens M, Sheppard J, Maddocks T, Párraga C.A, Troscianko T.S. Disruptive coloration and background pattern matching. Nature. 2005;434:72–74. doi: 10.1038/nature03312. doi:10.1038/nature03312 [DOI] [PubMed] [Google Scholar]
- Endler J.A. A predator's view of animal color patterns. Evol. Biol. 1978;11:319–364. [Google Scholar]
- Endler J.A. Natural selection on color patterns in Poecilia reticulata. Evolution. 1980;34:76–91. doi: 10.1111/j.1558-5646.1980.tb04790.x. doi:10.2307/2408316 [DOI] [PubMed] [Google Scholar]
- Endler J.A. Progressive background matching in moths, and a quantitative measure of crypsis. Biol. J. Linn. Soc. 1984;22:187–231. [Google Scholar]
- Endler J.A. Signals, signal conditions, and the direction of evolution. Am. Nat. 1992;139:s125–s153. doi:10.1086/285308 [Google Scholar]
- Grant B.S. Fine-tuning the peppered moth paradigm. Evolution. 1999;53:980–984. doi:10.2307/2640740 [Google Scholar]
- Grant B, Owen D.F, Clarke C.A. Parallel rise and fall of melanic peppered moths in America and Britain. J. Hered. 1996;87:351–357. [Google Scholar]
- Hart N.S, Partridge J.C, Cuthill I.C, Bennett A.T.D. Visual pigments, oil droplets, ocular media and cone photoreceptor distribution in two species of passerine bird: the blue tit (Parus caeruleus L.) and the blackbird (Turdus merula L.) J. Comp. Physiol. A. 2000;186:375–387. doi: 10.1007/s003590050437. doi:10.1007/s003590050437 [DOI] [PubMed] [Google Scholar]
- Håstad O, Victorsson J, Ödeen A. Differences in color vision make passerines less conspicuous in the eyes of their predators. Proc. Natl Acad. Sci. USA. 2005;102:6391–6394. doi: 10.1073/pnas.0409228102. doi:10.1073/pnas.0409228102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lev-Yadun S, Dafni A, Flaishman A, Inbar M, Izhaki I, Katzir G, Ne'eman G. Plant coloration undermines herbivorous insect camouflage. Bioessays. 2004;26:1126–1130. doi: 10.1002/bies.20112. doi:10.1002/bies.20112 [DOI] [PubMed] [Google Scholar]
- McMillan W.O, Monteiro A, Kapan D.D. Development and evolution on the wing. Trends Ecol. Evol. 2002;17:125–133. doi:10.1016/S0169-5347(01)02427-2 [Google Scholar]
- Merilaita S. Crypsis through disruptive coloration in an isopod. Proc. R. Soc. B. 1998;265:1059–1064. doi:10.1098/rspb.1998.0399 [Google Scholar]
- Merilaita S, Lind J. Background-matching and disruptive coloration, and the evolution of cryptic coloration. Proc. R. Soc. B. 2005;272:665–670. doi: 10.1098/rspb.2004.3000. doi:10.1098/rspb.2004.3000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merilaita S, Lind J. Great tits (Parus major) searching for artificial prey: implications for cryptic coloration and symmetry. Behav. Ecol. 2006;17:84–87. doi:10.1093/beheco/arj007 [Google Scholar]
- Merilaita S, Tullberg B.S. Constrained camouflage facilitates the evolution of conspicuous warning coloration. Evolution. 2005;59:38–45. [PubMed] [Google Scholar]
- Merilaita S, Tuomi J, Jormalainen V. Optimization of cryptic coloration in heterogeneous habitats. Biol. J. Linn. Soc. 1999;67:151–161. doi:10.1006/bijl.1998.0298 [Google Scholar]
- Merilaita S, Lyytinen A, Mappes J. Selection for cryptic coloration in a visually heterogeneous habitat. Proc. R. Soc. B. 2001;268:1925–1929. doi: 10.1098/rspb.2001.1747. doi:10.1098/rspb.2001.1747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruxton G.D, Sheratt T.N, Speed M.P. Oxford University Press; Oxford, UK: 2004. Avoiding attack: the evolutionary ecology of crypsis, warning signals and mimicry. [Google Scholar]
- Sherratt T.N, Rashed A, Beatty C.D. Hiding in plain sight. Trends Ecol. Evol. 2005;20:414–416. doi: 10.1016/j.tree.2005.05.010. doi:10.1016/j.tree.2005.05.010 [DOI] [PubMed] [Google Scholar]
- Stevens, M., Cuthill, I. C., Windsor, A. & Walker, H. In press. Disruptive contrast in animal camouflage. Proc. R. Soc. B273 (doi:10.1098/rspb.2006.3614) [DOI] [PMC free article] [PubMed]
- Stuart-Fox D, Moussalli A, Johnston G.R, Owens I.P.F. Evolution of color variation in dragon lizards: quantitative tests of the role of crypsis and local adaptation. Evolution. 2004;58:1549–1559. doi: 10.1111/j.0014-3820.2004.tb01735.x. [DOI] [PubMed] [Google Scholar]
- Thayer G.H. MacMillan; New York, NY: 1909. Concealing-coloration in the animal kingdom: an exposition of the laws of disguise through color and pattern. [Google Scholar]
- Thery M, Debut M, Gomez D, Casas J. Specific color sensitivities of prey and predator explain camouflage in different visual systems. Behav. Ecol. 2005;16:25–29. doi:10.1093/beheco/arh130 [Google Scholar]