Abstract
Young vertebrates have limited capacity to synthesize antibodies and are dependent on the protection of maternally transmitted antibodies for humoral disease resistance early in life. However, mothers may enhance fitness by priming their offspring's immune systems to elevate disease resistance. Transgenerational induced defences have been documented in plants and invertebrates, but maternal priming of offspring immunity in vertebrates has been essentially neglected. To test the ability of mothers to stimulate the immune systems of offspring, we manipulated maternal and offspring antigen exposure in a wild population of birds, pied flycatchers (Ficedula hypoleuca). We show that immunization of the mother before egg laying apparently stimulates a transgenerational defence against pathogens by elevating endogenous offspring antibody production. If the disease environments encountered by mothers and offspring are similar, this transgenerational immune priming may allow young to better cope with the local pathogen fauna.
Keywords: antibody transmission, immunocompetence, maternal effects, maternal antibodies, pied flycatcher, transgenerational immunity
1. Introduction
Inducible defences allow organisms to generate a defence only when advantageous, and thus minimize costs associated with investment in the defence (Tollrian & Harvell 1999). Inducible defences provide protection from parasites, predators and herbivores across a range of taxa. For example, a chemical cue from predators elicits formation of a protective helmet in Daphnia (Tollrian & Von Elert 1994) and herbivory increases the production of chemical defences in the leaves of wild radish (Raphanus raphanistrum), decreasing future susceptibility to herbivores (Agrawal et al. 1999). In plants and invertebrates, inducible defences have been demonstrated to act across generations providing offspring with enhanced protection early in life (Agrawal et al. 1999; Moret & Schmid-Hempel 2001; Little et al. 2003). Transgenerational induced defences are examples of adaptive maternal effects, whereby females respond to environmental information and alter offspring phenotype to enhance net reproductive success (Mousseau & Fox 1998; Agrawal et al. 1999).
The most important inducible defence in vertebrates is the specific immune response to pathogens (Frost 1999). The immune response is an effective defence against pathogens and influences fitness (Lochmiller & Deerenberg 2000), but it also entails costs (Frost 1999; Schmid-Hempel 2003). Furthermore, the specific immune response may be an example of a transgenerational defence. Since young vertebrates have limited ability to synthesize antibodies endogenously (Brambell 1970; Solomon 1971; Lawrence et al. 1981), maternally derived antibodies provide the primary form of humoral (antibody-mediated) immune defence for offspring early in life (Brambell 1970; Grindstaff et al. 2003).
The production of maternal antibodies is dependent on stimulation of the maternal immune system by external antigens (Roitt et al. 1998; Lemke et al. 2003). Collectively these antibodies represent the cumulative antigen exposure of females over their lifetime (Lemke et al. 2003). If females are not exposed to particular antigens prior to egg formation, they will not transfer antibodies to those antigens, leaving their offspring susceptible to infection (Heller et al. 1990; Leitner et al. 1990). Besides providing passive immune protection early in life, maternal antibodies may also have persistent effects on the offspring immune response (Lemke et al. 2003). Therefore, maternal antibodies may prime offspring to the current disease environment and provide one means of transgenerational phenotypic plasticity (Mousseau & Fox 1998; Agrawal et al. 1999).
In order to experimentally simulate disease exposure in the field, we injected female pied flycatchers with either lipopolysaccharide (LPS) or saline (controls). Lipopolysaccharide is a component of the outer coat of Gram-negative bacteria. We chose to use LPS because it is a component of bacteria that flycatchers naturally encounter in the wild and is therefore ecologically relevant. Furthermore LPS is a thymus-independent antigen (TI-1), which means that it can elicit antibody production without T-cell help (Janeway & Travers 1996). Since T-cells are not required, responses against TI-1 antigens occur earlier, enabling females to mount a response shortly after immunization. We then investigated the effects of the maternal treatment on offspring growth and humoral immunity by measuring LPS-specific antibodies and the level of total antibodies in 5- and 14-day-old nestlings.
2. Material and Methods
(a) Field methods
The experiment was conducted in a nest-box breeding population of pied flycatchers (Ficedula hypoleuca) located in a pine forest (Vombs Fure) in southern Sweden. The pied flycatcher is a small (ca 12–14 g) passerine bird that nests in natural cavities and nest-boxes throughout Northern and Eastern Europe. Clutch sizes range from 4 to 8 eggs (mean of 6 eggs). Females incubate alone for approximately 14 days. Both sexes contribute to nestling feeding and young fledge at around 16 days post-hatching (Lundberg & Alatalo 1992).
Nest-boxes were visited at least once a week to monitor clutch initiation, clutch size, hatching date, offspring growth and fledging success. Females were allowed to lay one complete clutch of eggs and were then captured on day 1–2 of incubation. At the time of capture, females were ringed, weighed, blood sampled and immunized (with LPS (n=23) or saline (n=23)). In addition, all females were also injected intramuscularly (pectoral muscle) with diphtheria–tetanus vaccine (Statens Serum Institute, Copenhagen, Denmark) for another study. We collected all of the eggs and removed the existing nest to insure that breeding was delayed enough for females to generate an antibody response as a result of the immunization. We then located the females' new nests. All of the data presented here on offspring growth and immune responses are from young in the replacement clutches (in total, 14 broods of control females and 16 broods of LPS-immunized females). The number of monitored broods differs from the number of treated females because replacement nests of some females were not located. On day 5 after hatching (day 0=hatching day), offspring were ringed, weighed, blood sampled and immunized (LPS or saline control; see below). We collected approximately 50 μl of blood from the jugular vein of offspring on days 5 and 14. To monitor offspring growth, we measured the body mass and wing length of offspring on days 5, 11 and 14. Fledging success was determined by checking all nest-boxes at the completion of the breeding season for nestling bodies and rings.
(b) Lipopolysaccharide immunization methods
On the day the first clutch was collected, one half of the females were immunized with LPS (Salmonella typhimurium LPS, Sigma, Cat. No. L-7261) and the other half received a control treatment of phosphate buffered saline (PBS). Lipopolysaccharide is a potent antigen and we therefore took great care to use a dose of LPS that would not induce any negative effects on female behaviour. Lipopolysaccharide immunized females received 50 μl of LPS suspended in PBS (concentration=0.1 mg kg body weight−1) by intraperitoneal injection. This dose is similar to or lower than low doses used previously in domestic and wild birds (Parmentier et al. 1998; Koutsos & Klasing 2001; Bonneaud et al. 2003). Control females received 50 μl of PBS by intraperitoneal injection. Female treatment was randomized with respect to incubation initiation date of the first clutch. There were no significant differences in body mass or first clutch size between female treatment groups (body mass: t=1.02, d.f.=28, p=0.32; clutch size: t=0.32, d.f.=28, p=0.75). To measure background antibody levels, we collected a blood sample from females immediately prior to immunization. The two experimental groups did not differ in LPS specific antibody levels prior to immunization (t=1.0, d.f.=28, p=0.33).
Within each brood, half of the young were immunized with LPS and the other half received the control treatment. To avoid any size bias by treatment group, all young in a brood were measured first and a size hierarchy created. Treatment was then assigned based on size, with the lightest nestling in a brood assigned first. The next heaviest nestling received the opposite treatment and so on until all young were assigned. We alternated the treatment of the lightest nestling between broods. Half of the young in a brood received 25 μl of LPS suspended in PBS (concentration=0.1 mg kg body weight−1), the other half received 25 μl of PBS by intraperitoneal injection. To try to measure maternally derived antibody levels in young, we collected a blood sample immediately prior to treatment on day 5. We then collected another blood sample from young on day 14 (i.e. 9 days after immunization) to assess early offspring antibody production.
(c) ELISA methods
We quantified humoral immunity in females and offspring in two ways: (i) total antibody levels, and (ii) LPS-specific antibody production. Both measures of immunity were determined using enzyme-linked immunosorbent assays (ELISA). Details of ELISA methods are included in the online electronic supplementary material for this manuscript. Given the relatively short time period between immunization and initiation of the replacement clutch (3–10 days), we were unable to detect diphtheria- or tetanus-specific antibodies in offspring serum as this was a primary antibody response in females. Therefore, we report below only antibody responses to LPS.
(d) Statistics
Data were analysed using mixed models (Proc Mixed, SAS v. 9.1) in which nest identity and day by nest identity were treated as random factors. The covariance structures between repeated measurements of the same individual were explicitly modelled. When there were only two measurements of the same individual we used compound symmetric covariance, and when there were three measurements of the same individual we used first-order autoregressive covariance based on selection that minimized Akaike information criterion (AIC) (Littell et al. 1996). To account for the blocked assignment of offspring to treatment groups by body mass, we explicitly included offspring block within female identity in all models. Each block contained two nestlings. In cases where there were an odd number of nestlings, the last nestling was allocated to the last block which then contained three nestlings. The results remain the same if, instead of including blocks, we include only treatment means within broods. Denominator degrees of freedom were approximated by the Satterthwaite method. Offspring survival was tested using generalized linear models (macro glimmix; Littell et al. 1996) using a logit link with binomial error. We adjusted for over-dispersion with the p-scale option. We checked for normality of residuals and homogeneity of variance. Lipopolysaccharide values were log transformed to achieve normality.
3. Results
(a) No effect of immunization on timing of replacement clutch or clutch size
There was no difference in the number of days to re-initiate egg laying after treatment between control and LPS-immunized females (LPS=5.88±0.34 s.e. days, PBS=6.29±0.34 s.e. days; t=0.852, d.f.=28, p=0.40). This delay in clutch replacement closely approximates production of replacement clutches after experimental removal in another population of pied flycatchers (mean=5.96 days, range=5–9 days; Bauchau & Seinen 1997). This suggests that females in our study re-initiated breeding as quickly as physiologically possible and were not further delayed by any negative effects of LPS immunization. Furthermore, we found no effect of maternal immunization on the size of the replacement clutch (t=0.37, d.f.=28, p=0.71) or the difference in size between the first and the replacement clutches (t=0.56, d.f.=28, p=0.58).
(b) Effects of maternal and offspring immunization on offspring antibody production
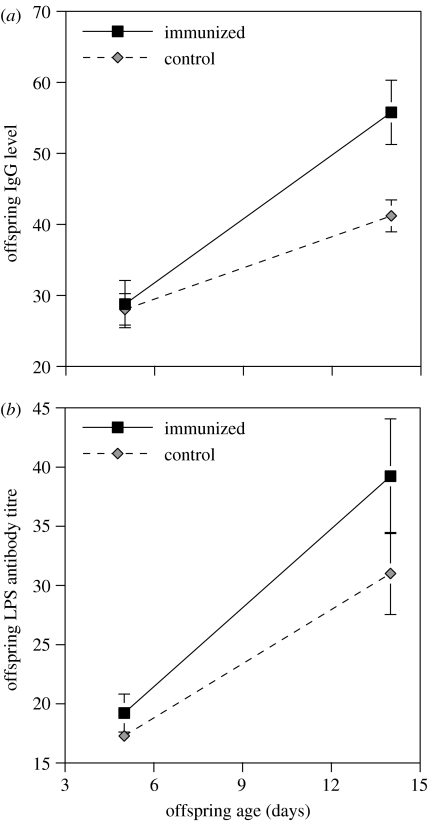
At five days of age, offspring did not vary by maternal treatment either in terms of LPS-specific antibody levels (F1,29.3=0.07, p=0.79) or total antibody levels (F1,28.4=0.04, p=0.83). On day 14, most offspring (84%) had higher total antibody levels than on day 5 (paired samples t-test: t=9.9, d.f.=58, p<0.0001). This increase in offspring antibody levels was dependent on maternal treatment, such that offspring of LPS-immunized mothers exhibited a significantly greater increase in total antibody levels from day 5 to day 14 than the offspring of control mothers (table 1, figure 1a). There was no direct effect of offspring treatment on offspring antibody production and no interaction effect between maternal and offspring treatments (table 1). Although offspring of LPS mothers did not have significantly higher LPS-specific antibody levels than offspring of control mothers, we found the same general pattern that offspring of LPS-immunized mothers seemed to have higher LPS-specific antibody production (table 1, figure 1b). Offspring treatment did not have a significant effect on LPS-specific antibody levels (table 1). Further, there was no significant interaction between maternal and offspring treatments on the production of LPS-specific antibodies. Including the number of days between immunization and re-laying as a covariate had no qualitative effect on the results.
Table 1.
Effects of maternal and offspring treatments (lipopolysaccharide (LPS) or control) and day during the nestling period on total antibody levels and LPS-specific antibody responses measured in offspring.
| dependent variable | total antibody levels | LPS antibody responses | ||||
|---|---|---|---|---|---|---|
| source | d.f. | F | p | d.f. | F | p |
| maternal treatment | 1, 28.8 | 1.69 | 0.20 | 1, 28.5 | 0.32 | 0.58 |
| offspring treatment | 1, 86.8 | 0.07 | 0.80 | 1, 103 | 1.36 | 0.25 |
| maternal treatment by offspring treatment | 1, 86.8 | 2.53 | 0.12 | 1, 103 | 1.37 | 0.24 |
| day | 1, 28.4 | 66.32 | <0.0001 | 1, 26.5 | 64.75 | <0.0001 |
| day by maternal treatment | 1, 28.4 | 7.16 | 0.01 | 1, 26.5 | 2.26 | 0.14 |
| day by offspring treatment | 1, 124 | 0.21 | 0.65 | 1, 132 | 0.89 | 0.35 |
| day by maternal treatment by offspring treatment | 1, 124 | 0.19 | 0.66 | 1, 132 | 0.12 | 0.73 |
Figure 1.
Effect of maternal immunization with LPS on (a) offspring total antibody levels and (b) LPS-specific antibody responses. Offspring antibody levels were measured from blood samples collected 5 and 14 days post-hatching. Black squares correspond to the offspring of LPS-immunized females and grey diamonds correspond to the offspring of control females. Bars represent standard errors.
(c) No effect of maternal or offspring immunization on offspring growth
On day 5 post-hatch, offspring of LPS-immunized and control mothers did not differ in body mass (F1,26.5=2.02, p=0.17) or wing length (F1,26.5=2.82, p=0.11). Furthermore, offspring of LPS-immunized and control mothers grew at the same rate throughout the nestling period (body mass: F2,55.2=0.94, p=0.40; wing length: F2,54.1=0.26, p=0.77; table 2). Offspring treatment also did not significantly affect body mass gain or wing growth (table 2). There were no significant interaction effects between maternal and offspring treatments for either body mass gain or wing growth (table 2). Finally, on day 14 the offspring of LPS-immunized mothers did not differ significantly in either body mass (F1,27.2=0.02, p=0.90) or wing length (F1,27.7=0.22, p=0.64) from offspring of control mothers.
Table 2.
Effects of maternal and offspring treatments (lipopolysaccharide (LPS) or control) and day during the nestling period on mass gain and wing growth of offspring.
| dependent variable | body mass | wing length | ||||
|---|---|---|---|---|---|---|
| source | d.f. | F | p | d.f. | F | p |
| maternal treatment | 1, 27.8 | 0.48 | 0.49 | 1, 26.5 | 2.01 | 0.17 |
| offspring treatment | 1, 86.2 | 0.83 | 0.36 | 1, 87.8 | 0.06 | 0.81 |
| maternal treatment by offspring treatment | 1, 86.2 | 0.11 | 0.74 | 1, 87.8 | 1.52 | 0.22 |
| day | 2, 55.2 | 443.09 | <0.0001 | 2, 54.1 | 5521.28 | <0.0001 |
| day by maternal treatment | 2, 55.2 | 0.94 | 0.40 | 2, 54.1 | 0.26 | 0.77 |
| day by offspring treatment | 2, 233 | 0.51 | 0.60 | 2, 229 | 0.49 | 0.61 |
| day by maternal treatment by offspring treatment | 2, 233 | 0.44 | 0.64 | 2, 229 | 1.22 | 0.30 |
(d) No effect of treatment on offspring survival to fledging
At least one nestling died between day five and fledging in 25% of the nests in the study population and mean fledging success was 89.7%. We found no significant effect of either maternal or offspring treatment on offspring survival from day five to fledging (p>0.45). Furthermore, we found no significant effect of maternal treatment on the total proportion of young fledged, taking into account unhatched eggs and offspring that died before day 5 (F1,28=0.02, p=0.89).
(e) No effect of treatment on return rates of females or offspring
Although few females and young returned to the study site in subsequent years, we found no indication of treatment-specific differences in return rate (four LPS females and three control females returned, as did two LPS nestlings and one control nestling).
4. Discussion
We found that a low dose injection with a non-pathogenic antigen (LPS) that mimics a bacterial infection in the maternal generation can apparently stimulate a transgenerational defence against pathogens by elevating early antibody production in offspring. This suggests that there may be adaptive phenotypic plasticity across generations in the immune response of vertebrates. Offspring of immunized and control females were equally likely to survive at least to fledging. However, maternal immunization may affect the survival probability of offspring during the post-fledging period, as early immune function can be an important predictor of offspring recruitment (Christe et al. 1998; Gonzalez et al. 1999).
(a) Development of offspring immunity
On day 5, there was no difference in offspring total antibody levels between maternal treatment groups. By day 14, nearly all offspring had higher antibody levels than on day 5. In most birds, maternal transfer of antibodies occurs during egg formation (Brambell 1970), and the level of circulating maternally derived antibodies in offspring declines rapidly within the first few days after hatching (Watanabe & Nagayama 1979; Boa-Amponsem et al. 1997). Hence, the increase in offspring antibody levels from day 5 to day 14 must reflect the beginning of the offspring's own antibody production. It was previously unknown when altricial birds begin to produce antibodies endogenously (Apanius 1998). On day 5, antibody levels may potentially be a mix of maternally derived and endogenously produced antibodies. However, note that immunoglobulin-secreting cells are not detectable in precocial chickens until at least 6 days after hatching (Lawrence et al. 1981) and protective levels of antibodies are not generated until at least two weeks post-hatch (Smith et al. 1994). Further, in their analysis of maternal antibody transmission in two different genetic strains of chickens, Watanabe & Nagayama (1979) found differences in serum IgG levels between strains on the day of hatch, but by 5 days post-hatch these differences were no longer detectable. Hence, by 5 days post-hatch, any initial difference in maternal antibody levels due to maternal treatment may have disappeared.
(b) Maternal immunization affects offspring antibody production
We found that between days 5 and 14, the offspring of immunized mothers increased antibody production significantly more than offspring of control mothers. This indicates that the maternal LPS immunization primed the offspring immune response apparently through elevated antibody transmission from mothers to offspring, thus enhancing the offspring's own antibody production early in life.
An alternative explanation of the elevation in antibody production by offspring of immunized mothers is that the LPS immunization made mothers ill and hence reduced maternal condition. If so, LPS-immunized mothers may have provisioned offspring less efficiently, leaving the young susceptible to infection. According to this scenario, offspring of LPS-immunized mothers produced more antibodies because they were more infected than offspring of control mothers. However, this explanation is unlikely for two reasons: (i) it fails to account for the equivalent growth rates of the offspring of LPS-immunized and control mothers, and (ii) it is highly unlikely given the dose of LPS used and the time-scale over which birds are affected by a LPS immunization. First, the hypothesized scenario would predict that if offspring of LPS-immunized mothers are more heavily infected than offspring of control mothers they should have reduced growth during the period of antibody production. Second, the hypothesized scenario requires that females remain in reduced condition for weeks after LPS immunization. In fact, because LPS is a non-replicating antigen, the effects of immunization are confined to a short period of time after immunization, generally within the first 12 h post-immunization (Koutsos & Klasing 2001). Immune responses to LPS have been shown to peak 4 days after immunization (Roitt et al. 1998). To minimize any potential negative effects on female behaviour we also used a low dose of LPS, which would be unlikely to induce any effects on female behaviour that would persist for weeks. In our study females delayed production of a replacement clutch for, on average, 6 days after immunization. Females then laid up to eight eggs and incubated them for 14 days. Therefore, in order for the above scenario to account for the effects on offspring antibody production, females would have to have been negatively impacted by the immunization for at least 24 days. This seems highly unlikely. The most parsimonious explanation of our results is, therefore, that females immunized with LPS elevated antibody transmission to offspring relative to control females, and these antibodies then stimulated offspring antibody production.
(c) Mechanisms of priming
An example of a possible mechanism of this priming effect of maternal antibodies comes from immunological studies of the ontogeny of immune function in laboratory animals. In response to receiving maternal antibodies to respiratory syncytial virus, neonatal mice mount an antibody response against the maternal antibodies that results in the production of both anti-virus antibodies and antibodies identical to the maternally derived antibodies (Okamoto et al. 1989). Maternal transmission of active immunity may occur if antibodies (Ab1) are transmitted to offspring and young mount a primary response to these antibodies to produce Ab2. As a result of complementary binding, a subset of the Ab2s will mimic the original antigen (so-called internal image antibodies (Ab2β)). Now the offspring has non-pathogenic copies of the original antigen, which will prime the offspring immune system for future encounters with the antigen (Anderson 1995; Carlier & Truyens 1995). Consequently, maternal antibodies may provide dual benefits to offspring: immediate protection against infection and priming of their own immune system for subsequent exposure to the antigen (Lemke et al. 2003).
Moreover, this priming may be non-specific as we found a strong effect of maternal treatment on offspring production of total antibodies. There are at least two explanations for the non-specific priming that we found. First, the transfer of antibodies from the mother may have a general priming effect on the development of the humoral immune response in offspring, irrespective of specificity. This may occur through increased antigen opsonization (i.e. binding of antibodies to the antigen) by maternally derived antibodies. Such opsonization results in improved ability of macrophages to recognize antigens (Janeway & Travers 1996). The presence of antigens during ontogeny is important for immune cell development, as shown by the impaired immune function of animals raised in germ-free environments (Mitsuyama et al. 1986; Freitas et al. 1991). The role of maternally derived antibodies may therefore be to enhance antigen presentation to the developing immune system of offspring, resulting in generally improved immune function. Second, at high concentrations, LPS stimulates proliferation and differentiation of all B-cell clones, regardless of antigen specificity (Janeway & Travers 1996). The maternal response to immunization may therefore be comprised of a broad repertoire of antibodies, which are transferred to the offspring, eliciting a broad but specific priming of the offspring immune response (Lemke et al. 2003). Most importantly, regardless of the mechanism involved, our data imply that exposure to an antigen in the maternal generation can stimulate a transgenerational defence against pathogens by elevating early antibody production in offspring.
(d) Immunity across generations
If maternal disease exposure is a good predictor of the pathogens that offspring are likely to encounter early in life, then maternal infections may induce adaptive changes in egg provizioning to prime offspring for the current disease environment through priming of the offspring immune system. Two previous studies provide support for the hypothesis that maternal antigen exposure may adaptively modulate offspring phenotype. Heeb et al. (1998) manipulated ectoparasite exposure of female great tits (Parus major) during egg production and found that offspring of ectoparasite-exposed females had lower levels of parasitism, increased growth and increased survival and recruitment in comparison to the offspring of unexposed females. A subsequent study revealed that the maternal effect on offspring growth arose before hatching (Buechler et al. 2002). Similarly, in a study of the effects of the intestinal nematode Heligmosomoides polygyrus on the laboratory mouse (Mus musculus), Kristan (2002) found that offspring of parasitized mothers were able to eliminate the infection and had higher growth rates than offspring of unparasitized mothers. However, in neither case was there any inference of the mechanisms involved.
In order for an inducible transgenerational defence to be favoured over a constitutive defence, there must be some cost to maintaining the defence in the absence of pathogens (Tollrian & Harvell 1999). In this study, we have shown that an antigenic cue in the maternal generation can stimulate enhanced antibody responses in their offspring; however, our experiment did not directly address potential costs of generating the defence for either mothers or offspring or the efficacy of the defence in offspring after challenge with a replicating pathogen. As is true for the adaptive immune response in general (Frost 1999), there are likely to be costs associated with generating a transgenerational defence. Costs for offspring may include a reduction in the diversity of antibody idiotypes transferred or trade-offs with other egg constituents (Blount et al. 2002). In females, elevated antibody responses may be associated with a correlated decline in the responsiveness of other components of the immune response (Biozzi et al. 1982; Ubosi et al. 1985) and declines in reproductive output (Martin et al. 1990; Grindstaff et al. 2003), as has been demonstrated in chickens artificially selected for elevated specific antibody responses, and thus enhanced maternal antibody transmission (Boa-Amponsem et al. 1997).
5. Conclusions
Transgenerational defences may provide offspring with enhanced protection against pathogens during a period of vulnerability to parasitism. Maternal antibody transmission provides humoral immune defence to offspring during a period when parasite pressure may be high and offspring have limited immune capacity. Maternally induced defences allow offspring to avoid one primary cost of inducible defences: the lag phase in the production of the defence (Agrawal et al. 1999). Moreover, maternally transferred antibodies apparently also induce a transgenerational priming of the offspring immune system, allowing young to better cope with the local pathogen fauna, particularly pathogens encountered by their mothers. Clearly, more research is needed on the influence of maternal effects on offspring survival as well as more mechanistic studies of how maternal immunization may prime the offspring immune system.
Acknowledgments
Supported by NSF grant 0206435, NSF graduate research fellowship, Society for Integrative and Comparative Biology, Center for the Integrative Study of Animal Behavior at Indiana University, Department of Biology at Indiana University (to J. L. G.); the Swedish Research Council (to H. G. S., M. Sa. and J. Å. N.); the Swedish Research Council for Environment, Agricultural Sciences & Spatial Planning, Carl Tryggers Stiftelse, Crafoordska Stiftelsen (to D. H.). We thank O. Hellgren for field assistance, D. Sejberg for lab assistance and E.D. Ketterson, B.J. Heidinger and K. Lessells for comments that significantly improved the quality of the manuscript. Research was approved by the Indiana University—Bloomington Institutional Animal Care and Use Committee and the ethical committee for animal research, Malmö/Lund, Sweden.
Supplementary Material
Detailed description of methods used to quantify humoral immunity in females and their offspring, plus associated references. Both total antibody levels and LPS-specific antibody responses were measured.
References
- Agrawal A.A, Laforsch C, Tollrian R. Transgenerational induction of defences in animals and plants. Nature. 1999;401:60–63. doi:10.1038/43425 [Google Scholar]
- Anderson R.W. On the maternal transmission of immunity—a molecular attention hypothesis. Biosystems. 1995;34:87–105. doi: 10.1016/0303-2647(94)01444-c. doi:10.1016/0303-2647(94)01444-C [DOI] [PubMed] [Google Scholar]
- Apanius V. Ontogeny of immune function. In: Starck J.M, Ricklefs R.E, editors. Avian growth and development: evolution within the altricial–precocial spectrum. Oxford University Press; New York, NY: 1998. pp. 203–222. [Google Scholar]
- Bauchau V, Seinen I. Clutch desertion and re-nesting in pied flycatchers: an experiment with progressive clutch removal. Anim. Behav. 1997;54:153–161. doi: 10.1006/anbe.1996.0460. doi:10.1006/anbe.1996.0460 [DOI] [PubMed] [Google Scholar]
- Biozzi, G., Mouton, D., Heumannand, A. & Bouthillier, Y. 1982 Genetic regulation of immunoresponsiveness in relation to resistance against infectious diseases. In Proc. Second World Congress on Genetic Applications to Livestock Production 5, 150.
- Blount J.D, Surai P.F, Nager R.G, Houston D.C, Møller A.P, Trewby M.L, Kennedy M.W. Carotenoids and egg quality in the lesser black-backed gull Larus fuscus: a supplemental feeding study of maternal effects. Proc. R. Soc. B. 2002;269:29–36. doi: 10.1098/rspb.2001.1840. doi:10.1098/rspb.2001.1840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boa-Amponsem K, Dunnington E.A, Siegel P.B. Antibody transmitting ability of hens from lines of chickens differing in response to SRBC antigen. Br. Poult. Sci. 1997;38:480–484. doi: 10.1080/00071669708418025. [DOI] [PubMed] [Google Scholar]
- Bonneaud C, Mazuc J, Gonzalez G, Haussy C, Chastel O, Faivre B, Sorci G. Assessing the cost of mounting an immune response. Am. Nat. 2003;161:367–379. doi: 10.1086/346134. doi:10.1086/346134 [DOI] [PubMed] [Google Scholar]
- Brambell F.W.R. Frontiers of biology. North Holland Publishing Company; Amsterdam, The Netherlands: 1970. The transmission of passive immunity from mother to young. [Google Scholar]
- Buechler K, Fitze P.S, Gottstein B, Jacot A, Richner H. Parasite-induced maternal response in a natural bird population. J. Anim. Ecol. 2002;71:247–252. doi:10.1046/j.1365-2656.2002.00591.x [Google Scholar]
- Carlier Y, Truyens C. Influence of maternal infection on offspring resistance towards parasites. Parasitol. Today. 1995;11:94–99. doi: 10.1016/0169-4758(95)80165-0. doi:10.1016/0169-4758(95)80165-0 [DOI] [PubMed] [Google Scholar]
- Christe P, Møller A.P, Lope F.d. Immunocompetence and nestling survival in the house martin: the tasty chick hypothesis. Oikos. 1998;83:175–179. [Google Scholar]
- Freitas A.A, Viale A.C, Sundblad A, Heusser C, Coutinho A. Normal serum immunoglobulins participate in the selection of peripheral B-cell repertoires. Proc. Natl Acad. Sci. USA. 1991;88:5640–5644. doi: 10.1073/pnas.88.13.5640. doi:10.1073/pnas.88.13.5640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost S.D.W. The immune system as an inducible defense. In: Tollrian R, Harvell C.D, editors. The ecology and evolution of inducible defenses. Princeton University Press; Princeton, NJ: 1999. pp. 104–126. [Google Scholar]
- Gonzalez G, Sorci G, Møller A.P, Ninni P, Haussy C, De Lope F. Immunocompetence and condition-dependent sexual advertisement in male house sparrows (Passer domesticus) J. Anim. Ecol. 1999;68:1225–1234. doi:10.1046/j.1365-2656.1999.00364.x [Google Scholar]
- Grindstaff J.L, Brodie E.D, Ketterson E.D. Immune function across generations: integrating mechanism and evolutionary process in maternal antibody transmission. Proc. R. Soc. B. 2003;270:2309–2319. doi: 10.1098/rspb.2003.2485. doi:10.1098/rspb.2003.2485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeb P, Werner I, Kölliker M, Richner H. Benefits of induced host responses against an ectoparasite. Proc. R. Soc. B. 1998;265:51–56. doi:10.1098/rspb.1998.0263 [Google Scholar]
- Heller E.D, Leitner G, Drabkin N, Melamed D. Passive immunization of chicks against Escherichia coli. Avian Pathol. 1990;19:345–354. doi: 10.1080/03079459008418685. [DOI] [PubMed] [Google Scholar]
- Janeway C.A, Travers P. Current Biology Limited; London, UK: 1996. Immunobiology: the immune system in health and disease. [Google Scholar]
- Koutsos E.A, Klasing K.C. The acute phase response in Japanese quail (Coturnix coturnix japonica) Comp. Biochem. Physiol. 2001;128:255–263. doi: 10.1016/s1532-0456(00)00199-x. doi:10.1016/S1096-4959(00)00324-9 [DOI] [PubMed] [Google Scholar]
- Kristan D.M. Maternal and direct effects of the intestinal nematode Heligmosomoides polygyrus on offspring growth and susceptibility to infection. J. Exp. Biol. 2002;205:3967–3977. doi: 10.1242/jeb.205.24.3967. [DOI] [PubMed] [Google Scholar]
- Lawrence E.C, Arnaud-Battandier F, Grayson J, Koski I.R, Dooley N.J, Muchmore A.V, Blaese R.M. Ontogeny of humoral immune function in normal chickens: a comparison of immunoglobulin-secreting cells in bone marrow, spleen, lungs and intestine. Clin. Exp. Immunol. 1981;43:450–457. [PMC free article] [PubMed] [Google Scholar]
- Leitner G, Melamed D, Drabkin N, Heller E.D. An enzyme-linked immunosorbent assay for detection of antibodies against Escherichia coli: association between indirect hemagglutination test and survival. Avian Dis. 1990;34:58–62. doi:10.2307/1591334 [PubMed] [Google Scholar]
- Lemke H, Hansen H, Lange H. Non-genetic inheritable potential of maternal antibodies. Vaccine. 2003;21:3428–3431. doi: 10.1016/s0264-410x(03)00394-3. doi:10.1016/S0264-410X(03)00394-3 [DOI] [PubMed] [Google Scholar]
- Littell R.C, Milliken G.A, Stroup W.W, Wolfinger R.D. SAS Institute Inc; Cary, NC: 1996. SAS system for mixed models. [Google Scholar]
- Little T.J, O'Connor B, Colegrave N, Watt K, Read A.F. Maternal transfer of strain-specific immunity in an invertebrate. Curr. Biol. 2003;13:489–492. doi: 10.1016/s0960-9822(03)00163-5. doi:10.1016/S0960-9822(03)00163-5 [DOI] [PubMed] [Google Scholar]
- Lochmiller R.L, Deerenberg C. Trade-offs in evolutionary immunology: just what is the cost of immunity? Oikos. 2000;88:87–98. doi:10.1034/j.1600-0706.2000.880110.x [Google Scholar]
- Lundberg A, Alatalo R.V. T. & A. D. Poyser; London, UK: 1992. The pied flycatcher. [Google Scholar]
- Martin A, Dunnington E.A, Gross W.B, Briles W.E, Briles R.W, Siegel P.B. Production traits and alloantigen systems in lines of chickens selected for high or low antibody-responses to sheep erythrocytes. Poult. Sci. 1990;69:871–878. doi: 10.3382/ps.0690871. [DOI] [PubMed] [Google Scholar]
- Mitsuyama M, Ohara R, Amako K, Nomoto K, Yokokura T. Ontogeny of macrophage function to release superoxide anion in conventional and germ-free-mice. Infect. Immun. 1986;52:236–239. doi: 10.1128/iai.52.1.236-239.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moret Y, Schmid-Hempel P. Immune defence in bumble-bee offspring. Nature. 2001;414:506. doi: 10.1038/35107138. doi:10.1038/35107138 [DOI] [PubMed] [Google Scholar]
- Mousseau T.A, Fox C.W. Oxford University Press; New York, NY: 1998. Maternal effects as adaptations. [Google Scholar]
- Okamoto Y, Tsutsumi H, Kumar N.S, Ogra P.L. Effect of breast feeding on the development of anti-idiotype antibody response to F glycoprotein of respiratory syncytial virus in infant mice after post-partum maternal immunization. J. Immunol. 1989;142:2507–2512. [PubMed] [Google Scholar]
- Parmentier H.K, Walraven M, Nieuwland M.G.B. Antibody responses and body weights of chicken lines selected for high and low humoral responsiveness to sheep red blood cells 1. Effect of Escherichia coli lipopolysaccharide. Poult. Sci. 1998;77:248–255. doi: 10.1093/ps/77.2.248. [DOI] [PubMed] [Google Scholar]
- Roitt I, Brostoff J, Male D. Mosby; London, UK: 1998. Immunology. [Google Scholar]
- Schmid-Hempel P. Variation in immune defence as a question of evolutionary ecology. Proc. R. Soc. B. 2003;270:357–366. doi: 10.1098/rspb.2002.2265. doi:10.1098/rspb.2002.2265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith N.C, Wallach M, Petracca M, Braun R, Eckert J. Maternal transfer of antibodies induced by infection with Eimeria maxima partially protects chickens against challenge with Eimeria tenella. Parasitology. 1994;109:551–557. doi: 10.1017/s0031182000076423. [DOI] [PubMed] [Google Scholar]
- Solomon J.B. Frontiers of biology. North Holland Publishing Company; Amsterdam, The Netherlands: 1971. Foetal and neonatal immunology. [Google Scholar]
- Tollrian R, Harvell C.D. The evolution of inducible defenses: current ideas. In: Tollrian R, Harvell C.D, editors. The ecology and evolution of inducible defenses. Princeton University Press; Princeton, NJ: 1999. pp. 306–321. [Google Scholar]
- Tollrian R, Von Elert E. Enrichment and purification of Chaoborus Kairomone from water—further steps toward its chemical characterization. Limnol. Oceanogr. 1994;39:788–796. [Google Scholar]
- Ubosi C, Gross W, Siegel P. Divergent selection of chickens for antibody production to sheep erythrocytes: age effect in parental lines and their crosses. Avian Dis. 1985;29:150. doi:10.2307/1590704 [PubMed] [Google Scholar]
- Watanabe S, Nagayama F. Studies on the serum IgG level in Japanese quail. Jpn. Poult. Sci. 1979;16:59–64. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Detailed description of methods used to quantify humoral immunity in females and their offspring, plus associated references. Both total antibody levels and LPS-specific antibody responses were measured.