Abstract
Conflict among siblings over parental investment, particularly over parental feeding, is a feature of family life in many kinds of animals. In some bird species, the size of prey items provided to juveniles has been implicated as a cause of aggressive competition among sibling chicks, because prey size determines whether dominance allows monopolization of parental offerings. Our experiment was meant to test the generality of this factor in creating intrafamilial conflict. We investigated sibling competition in relation to prey size using the carnivorous, brood-tending leech Helobdella papillornata. We equalized the total amount of food available to H. papillornata broods, but varied the size of individual prey items. Competition, measured by disparity in body size at independence, was more intense in broods provisioned with small items than in broods receiving large items, but similar between broods receiving large items and broods fed ad libitum. These patterns suggest that the intensity of conflict did not depend only on the total food amount, but was enhanced by small prey size. Our results indicate that conflict over the provision of parental resources to offspring can have a similar basis across very dissimilar organisms.
Keywords: body size inequality, Glossiphoniidae, parental care, prey-size hypothesis
1. Introduction
Parental care is a resource that may be worth fighting for, even to the detriment of one's siblings. Intrabrood competition is particularly evident in some bird species, in which nestlings display competitive begging, eviction of siblings from the nest, or aggressive pecking to the point of overt siblicide (Mock & Parker 1997). Intrabrood competition is part of a larger suite of kin-directed behaviours, whose evolutionary dynamics are governed by the logic of Hamilton's (1964) rule. Diploid parents, equally related to all their sexually reproduced offspring, should favour equal investment among brood-mates, if they are equally valuable in terms of potential fitness, while individual offspring would prefer greater investment in themselves than in siblings. However, often the potential value of offspring varies due to genetic differences or ecological circumstances, so that parents should skew their investment in favour of particular offspring, or allow competition among unequal offspring to skew the investment for them. In either case, offspring may be selected to behave selfishly and attempt to take a larger share of investment than their siblings (Trivers 1974; Macnair & Parker 1979; Parker et al. 1989). Competitive solicitation of parental care and interference competition enforced by dominance hierarchies among offspring may evolve as manifestations of the underlying evolutionary conflict of interest within a brood.
Resource-based rivalry is particularly evident in bird species in which dominance among nestlings leads to siblicide. Mock (1985) proposed that prey size affects intrabrood aggression, based on the difference in typical rates of siblicidal aggression in two related water birds (Family: Ardeidae): the great egret (Casmerodius albus) and the great blue heron (Ardea herodias). The two species are sympatric in the southern US and ecologically similar, but differ strongly in the level of conflict among nestlings. In a great egret brood, the last hatched chick frequently dies from aggressive pecking by its slightly older and larger siblings, whereas such an aggressive siblicide is rare in great blue herons (Mock 1984). Mock (1985) hypothesized that the difference in intrabrood conflict is due to the typical size of the prey items provided to the nestlings. Egret parents feed their young by regurgitating boluses of small fishes, which can be intercepted as they leave the parental bill by the dominant chick or chicks. Heron parents typically regurgitate much larger fishes onto the nest floor that cannot be monopolized by any one nestling. Cross-fostering experiments revealed some behavioural plasticity: heron chicks significantly escalated their aggression when small boluses were fed by egret foster parents, and egret broods reduced aggression, albeit not to a statistically significant degree, when large prey were fed by heron foster parents (Mock 1984).
Parental feeding is a critical determinant of growth and survival in altricial birds (Ricklefs 1968), and rapid growth may even be a mechanism of competing with siblings (Royle et al. 1999). Comparisons among many avian species suggest that direct feeding of small prey items by parents may be a necessary, but not sufficient condition for overtly aggressive competition to evolve (Mock & Parker 1997). However, explicit tests of the prey-size hypothesis have been conducted with only a few avian taxa (Drummond 2001), and never in other care-giving organisms. Inclusive fitness theory implies that intrafamilial conflict of interest should occur whenever parents provide a non-sharable resource in a spatially restricted nursery (Mock & Parker 1997), so that the prey-size hypothesis may be widely applicable in other kinds of organisms.
We tested whether prey size affects intrabrood competition in the hermaphroditic Australian brood-tending leech, Helobdella papillornata (Glossiphoniidae: Euhirudinea: Annelida), which provisions its offspring with small gastropods for several weeks after the young hatch (Govedich & Davies 1998). It is not immediately obvious that juvenile leeches would face competition for food with their siblings as strong as that experienced by altricial chicks of some avian species. Ectothermy and indeterminate growth in young H. papillornata might be expected to ameliorate the urgency of parental feeding and thus to reduce the fitness pay-off from competitive success over siblings.
Glossiphoniids are unique among leeches in providing post-hatching care to their young (Kutschera & Wirtz 1986; Sawyer 1986). Helobdella papillornata parents produce clutches of about 20–60 eggs. Newly hatched juveniles are too small to subdue prey, and they remain attached to the maternal parent's ventral surface by their posterior sucker for several weeks, when they are fed prey items obtained by the parent (figure 1). The parental provisioning behaviour potentially allows some degree of resource monopolization by a minority of juveniles in the brood. Parents hold newly killed gastropods with their anterior sucker and curl up to present the prey to the brood on their ventral surface. Juveniles insert their head and proboscis through the shell aperture to feed, but the size of the aperture appears to limit the number of juvenile leeches that can feed simultaneously. Although it is difficult, even under the microscope, to observe behaviours of the tiny, translucent juveniles, it seems likely that some individuals can gain feeding priority at the expense of their siblings, if the snail aperture is sufficiently small.
Figure 1.
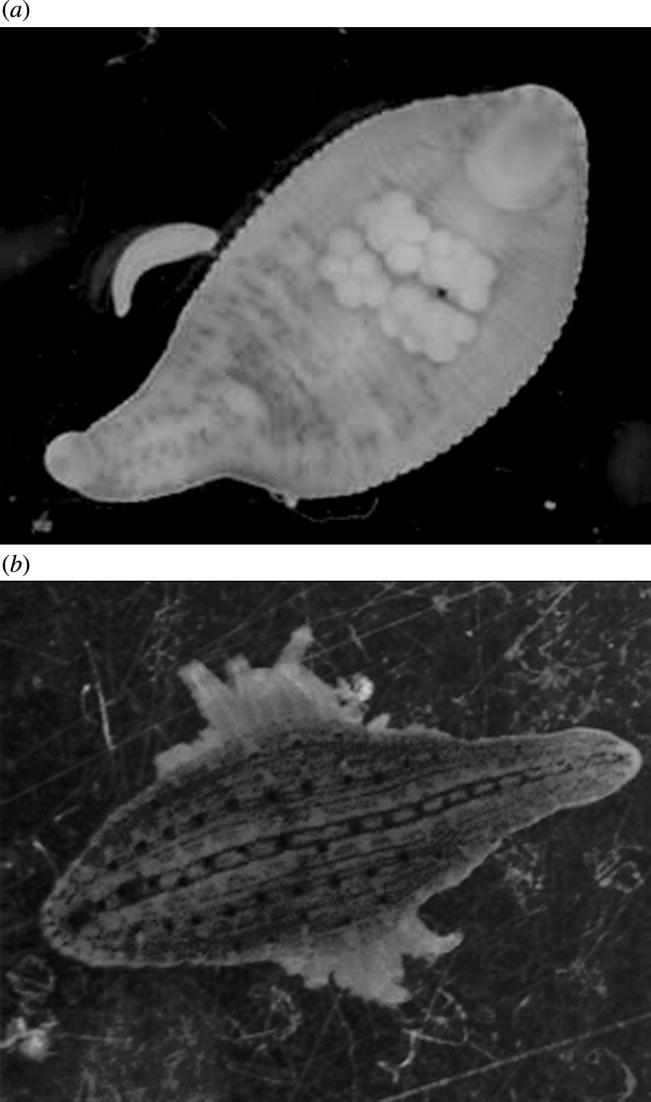
(a) Ventral view of Helobdella papillornata parent with attached egg cocoon and (b) dorsal view of parent partially covering juveniles, which are attached to its ventral surface. Parents are approximately 7 mm in total length in these views.
2. Material and methods
We randomly distributed 27 egg-bearing adults of H. papillornata among three experimental treatments. Each parent was isolated with its brood in 150 ml of a standard medium of distilled water and mineral salts that mimicked fresh stream water (Tan et al. 2004), to which an anti-fungal agent was added. Parental leeches in the first treatment received only large prey items (L treatment), those in the second had only small items (S treatment), and those in the third had randomly chosen items available ad libitum (AL treatment). Two parents in the S treatment died of apparent fungal infection despite the anti-fungal agent, and are not considered in the data analysis. Clutch sizes (and initial brood sizes, as hatching is nearly always 100% under laboratory conditions) ranged from 36 to 62 in the L treatment (mean±1 s.d.=47.9±8.3), from 34 to 51 in the S treatment (mean±1 s.d.=42.0±6.0) and from 40 to 61 in the AL treatment (mean±1 s.d.=47.0±7.2). Although these differences among the three treatments were not statistically significant (F2,22=1.42, p=0.26), sample sizes were small and the null hypothesis of equality should not be assumed. To compensate the observed variation in brood sizes, we adjusted the total food consumption rates of each brood by its size, as explained later.
Prey items were live Potamopyrgus antipodarum snails, a New Zealand species widely established in streams throughout southeastern Australia. Each parent in the L treatment received one large snail (mean aperture height 1.77 mm, range 1.74–1.82 mm) every 6 days starting from the day eggs hatched and continuing throughout the experiment. Each parent in the S treatment received one small snail (mean aperture height 1.11 mm, range 1.07–1.15 mm) every day during the experiment. We had previously determined the scaling relationship between soft tissue mass in milligrams, m, and aperture height in millimetres, h, in P. antipodarum to be m=0.027h3.83. Thus, the difference in mean aperture height of 1.77 mm versus 1.11 mm corresponded to a sixfold difference in soft body mass of the snails, so that the schedule of providing snails to parents in the L and S treatments made food available at equal average rates, but in different portions. Each parent in the AL group daily received randomly selected snails (mean aperture height 1.66 mm, range 1.04–2.58 mm) ad libitum. Snails that were killed had their soft tissues completely consumed. Captured prey items are always offered first to the brood, and it is rare for parental leeches to feed during the period of brood care.
The time needed to sift through a large pool of P. antipodarum snails to find specific and uncommon aperture sizes, especially for the S treatment, placed a practical limit on the number of broods we could include in each treatment. The resulting small sample sizes limited the statistical power in tests of differences among treatments.
Parents in all three treatments occasionally declined to hunt an offered snail, an ordinary behaviour among brood-tending adults in this species (Paez et al. 2004). When a snail was not killed, we replaced it with a new snail at the next scheduled feeding date. The occasional quiescence of parents did not disrupt the equality between the L and S treatments in mean total food intake. We calculated the mean rate of tissue consumption (milligram dry weight per day per juvenile in the initial brood) for each brood (table 1). The mean consumption rates were nearly identical between the L and S treatments (t-test, t12=0.035, p=0.97), while AL broods consumed snail tissue at a rate about six times greater (table 1).
Table 1.
Consumption rate, mortality rate and mean body size of juveniles at independence in broods of three treatment groups (AL, ad libitum; L, large prey items; S, small prey items). (Entries show means±1 s.d. for N=9 broods (AL and L) or N=7 broods (S). One-way ANOVA for each variable tests differences among the three treatment groups (consumption rates were log-transformed to homogenize variances).)
| AL | L | S | ANOVA | ||
|---|---|---|---|---|---|
| F2,22 | p | ||||
| consumption rate (mg per juvenile per day) | 0.0983±0.0024 | 0.0159±0.0027 | 0.0159±0.0031 | 296.9 | <0.001 |
| mortality rate (%) | 10±14 | 25±15 | 39±23 | 4.6 | 0.024 |
| mean body size (mm2) | 1.79±0.42 | 1.53±0.51 | 2.21±0.72 | 3.1 | 0.065 |
We monitored the hatching date of eggs (synchronous within a clutch) and the number of surviving juveniles in each brood daily throughout the experiment. On the date of independence of each juvenile that survived to independence (clearly marked by the cessation of physical attachment to the parent), we measured its length and width from calibrated digitised micrographs taken at 60× magnification, using ImageJ v. 1.30 software (National Institutes of Health, Bethesda, MD, USA). The product of length and width served as a size measure. We assessed the disparity of body size at independence within each brood by four measures: the range between the largest and the smallest surviving juvenile in the brood, the range divided by the brood mean, the variance of size within the brood and the Gini index (Sen 1973). We used four indicators to check the robustness of our results, since we had no a priori reason to suppose that any particular measure of size disparity reflected inequality in access to food more than another.
3. Results
Mean mortality rate was lowest in the AL broods, intermediate in L broods and highest in the S broods (table 1). Low mortality in AL broods corresponds to their larger total food consumption, while higher mortality in the S treatment than in the L treatment accords with the monopolization of small prey by a few offspring. However, only the mortality difference between AL and S broods was significant (Tukey post hoc contrast, p=0.02), while the difference between L and S broods was not significant (Tukey post hoc contrast, p=0.32). Mean body size within broods did not differ significantly among treatments, although the ANOVA F-test (p=0.065) approached the traditional significance level. The observed power of the ANOVA (i.e. power to detect the observed effect size of among- and within-treatment mean squares as significant at 0.05 probability, given the sample sizes used) was 0.59, a value that is only moderate, due to the small number of broods in each treatment. Allowing an interpretation of possible biological effect, despite the test falling marginally outside conventional significance, we note that mean body size was largest in the S broods with restricted food access and smallest in the L broods with less restricted access (table 1), implying that competition-induced mortality in the S broods allowed the fewer surviving juveniles to attain larger average size.
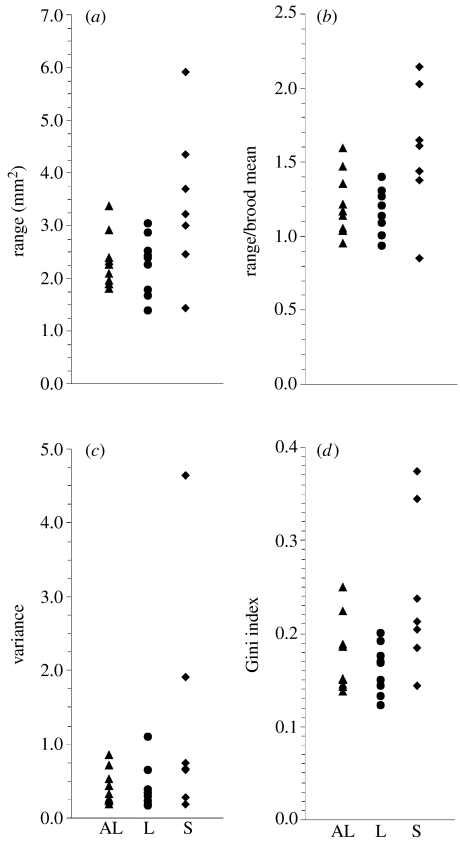
Body size disparity within broods provides a stronger test for prey-size effect than does difference among brood means, because the putative competition over food shares occurs within, not between, broods. We obtained consistent evidence of enhanced intrabrood competition in the small prey-size treatment. All four measures of intrabrood inequality show greater disparity within S broods than within L and AL broods (figure 2). The difference among treatments was statistically significant (p<0.05) for three of the four measures, and was near this level of significance (p=0.08) for the fourth measure, variance (table 2).
Figure 2.
Four indices of intrabrood disparity. (a) Range; (b) range/brood mean; (c) variance; (d) Gini index. Each symbol represents the measure of a single brood (triangles, ad libitum treatment; circles, large prey treatment; diamonds, small prey treatment).
Table 2.
Intrabrood disparity in juvenile size (measured in mm2). (ANOVA compares treatment means; Tukey contrasts present significance of pair-wise differences.)
| ANOVA | Tukey post hoc contrast | ||||
|---|---|---|---|---|---|
| F2,22 | p | AL versus L | AL versus S | L versus S | |
| range | 6.47 | 0.006 | 0.872 | 0.021 | 0.007 |
| range/brood mean | 12.30 | <0.001 | 0.473 | 0.003 | <0.001 |
| variance | 2.82 | 0.081 | — | — | — |
| Gini index | 5.19 | 0.014 | 0.854 | 0.046 | 0.015 |
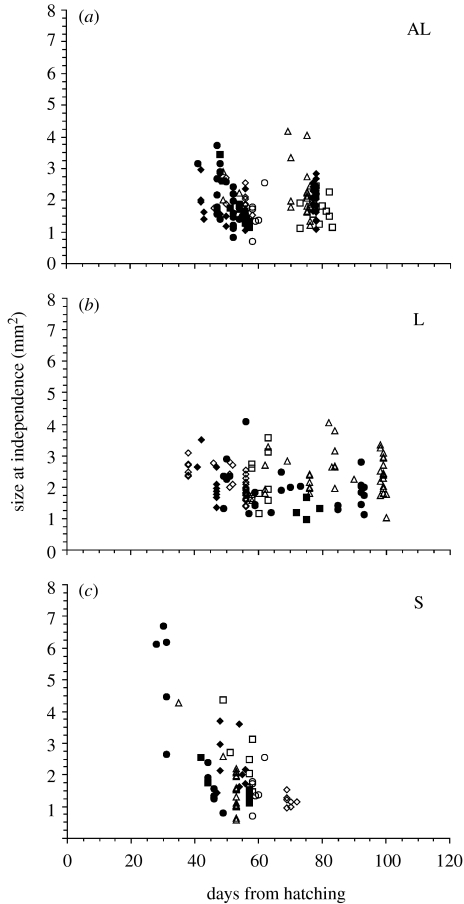
The first juveniles in the AL and L treatments left their parents about 40 days after hatching (figure 3). Departures continued for another 40–60 days, but the duration of parental care bore no relation to size at independence (AL: r=0.03, p=0.69; L: r=0.025, p=0.78). The first juveniles to achieve independence in the S treatment left their parents 28–35 days after hatching (figure 3), earlier than in the other treatments, and tended to be larger than juveniles from the AL or L treatments. There was a significant negative correlation between size and age at independence in the S treatment (r=−0.66, p<0.0001) in contrast to the other treatments. Sibling competition and monopolization of parental provision is further implicated in the S treatment by noting that the earliest departures at the largest sizes occurred in the S brood with the highest mortality rate.
Figure 3.
Size at independence in relation to duration of attachment to parent. Treatments: (a) AL, ad libitum; (b) L, large prey; (c) S, small prey. Different symbols represent different broods within treatments.
4. Discussion
Prey size is strongly implicated as a factor in sibling competition by our results. If the intensity of competition within H. papillornata broods depended only on the absolute amount of food available, we would expect the consequences of competition to be similar between L and S broods, which ate at the same mean rate, and less severe in AL broods, which enjoyed a more abundant supply of food. Instead, we found that intrabrood disparity in final body size tended to be high in S broods, but similar between L and AL broods (figure 2; table 2). This result does not rule out a role of food amount in modulating competition in leech broods (cf. Drummond 2001), but it also implicates prey size as a factor that exacerbates competition at a given level of total food supply.
Mock (1984, 1985) proposed the prey-size hypothesis to explain siblicidal aggression within broods. The prey-size effect we observed could be produced by interference competition without active fighting and aggression among young leeches, and in this sense, we did not test the specific competitive mechanism of Mock's (1985) hypothesis. Nonetheless, the logic of the original hypothesis can be generalized to other forms of sibling competition for parental provisioning. Our experiment tests the effect of prey size in this more general sense.
Intrabrood behaviours are difficult to study in H. papillornata because of the tiny size and translucency of juveniles, and because the mother shields the brood with her body (figure 1). Nonetheless, our casual observations indicate that larger juveniles are often attached to more anterior positions on the ventral surface of the maternal leech, a position that should allow them first access to the food as the parent curls up to present prey. If their larger size allows them to exclude siblings from this favourable position, sibling competition in H. papillornata may resemble competition among European starling (Sturnus vulgaris) chicks for positions nearest to the nest entrance, where the probability of receiving a meal from a returning parent is highest (Kacelnik et al. 1995), or aggressive competition among suckling piglets for access to the anterior teats of their mother (Fraser & Thompson 1991). Even without such position effects, it seems likely that the largest contestants would have the greatest ability to interfere with their brood-mates' access to an offered snail.
Do successful competitors increase their fitness? In the S treatment, with the most intense competition, the first juveniles to attain independence left their parents after shorter periods of care, and often at larger size, than occurred in the other treatments (figure 3). Large leeches produce more and larger eggs (Tan et al. 2004), and body size provides advantages during mate choice that translate to greater spermatophore donation (Walton et al. in press).
The behavioural mechanisms that offspring use to compete and that parents use to distribute their investment will affect the evolutionary outcome of intrafamilial conflict of interests (Godfray 1995; Parker et al. 2002a). For example, whether begging by bird nestlings is primarily a solicitation of parental care based on degree of need, or a competition with siblings to exploit parental response to the loudest caller, depends on whether and how parents control the allocation of provisioning (Parker et al. 2002b). We know little about how H. papillornata parents assess or respond to offspring's demand for feeding, and about the mechanisms that offspring may use to gain advantage over their siblings. We have not observed obvious solicitation of parental care, although tactile or chemical cues that signal a desire for feeding could easily be overlooked in such small and little studied organisms. It also seems unlikely to us that a parental leech could preferentially direct food towards particular juveniles, given the manner in which they present prey items to their brood as a whole. Thus, we expect that parental leeches are passive providers that allow competitive interactions within the brood to determine resource distribution. Bonabeau et al. (1998) have shown that this can be a fitness-maximizing parental strategy under conditions of initial overproduction of offspring and unpredictable resource supply. Juvenile mortality occurred even in some of our AL broods with access to abundant food, suggesting that H. papillornata parents might normally produce clutch sizes above their provisioning capacity, even in a benign laboratory environment.
Compared to the abundant and detailed data on sibling interactions in birds (Mock & Parker 1997), this investigation provides only a broad outline of how one factor affects intrabrood competition in leeches. It remains to be demonstrated that feeding priority within broods is related to body size or specific behaviours of some individuals, and that small body size at independence (or mortality) results from an early disadvantage in food access that creates a ‘downward spiral’ of competitive weakness. Microscopic observations of feeding interactions within broods can provide some insight into these questions, provided the very tiny juveniles can be individually marked. It would also be helpful to know the relation between food ingestion and time spent feeding, and whether this relation changes as the snail tissue is consumed. Obtaining such information would be a considerable technical challenge. Finally, an ideal experiment would have involved snails that had different aperture sizes but the same amount of soft tissue, so that the costs of parental hunting and juvenile competition could be equalized between the L and S treatments by equalizing the frequency of provisioning events. The scaling relationship between available food and aperture size in P. antipodarum does not allow such treatments with natural snails. In a preliminary experiment, we created artificial prey from mollusc tissue (commercially available clams) inserted in glass capillary tubes of different diameters, but these were handled in obviously unnatural ways by parental and juvenile leeches. For example, parents often extracted the tissue from the capillary tubes and presented the naked food to their young ones, something we have never observed with snails. Manipulations of prey size are difficult to achieve experimentally even with large birds in laboratory settings (Mock et al. 1987), and our use of natural variation among P. antipodarum snails seems to offer substantial, if imperfect, insight into prey-size effects. We conclude that, although the competitive behaviours may differ, prey size can exacerbate intrabrood competition in similar ways in organisms such as birds and glossiphoniid leeches.
Acknowledgments
We thank Belinda Lees for tending our stock populations of Helobdella and collecting Potamopyrgus from the field, and Lauren Carrington for supplying the photograph for figure 1b. Comments from Hugh Drummond, Alan Lill, Doug Mock, Aldo Poiani and an anonymous referee helped us to improve the presentation of ideas. This work was supported by a Discovery-Projects grant from the Australian Research Council.
Footnotes
Present address: Department of Biological Sciences, Northern Arizona University, Flagstaff, AZ 86011-5640, USA.
References
- Bonabeau E, Denoubourg J.-L, Theraulaz G. Within-brood competition and the optimal partitioning of parental investment. Am. Nat. 1998;152:419–427. doi: 10.1086/286179. doi:10.1086/286179 [DOI] [PubMed] [Google Scholar]
- Drummond H. A reevaluation of the role of food in broodmate aggression. Anim. Behav. 2001;61:517–527. doi:10.1006/anbe.2000.1641 [Google Scholar]
- Fraser D, Thompson B.K. Armed sibling rivalry among piglets. Behav. Ecol. Sociobiol. 1991;29:9–15. doi:10.1007/BF00164289 [Google Scholar]
- Godfray H.C.J. Signalling of need between parents and young: parent–offspring conflict and sibling rivalry. Am. Nat. 1995;146:1–24. doi:10.1086/285784 [Google Scholar]
- Govedich F.R, Davies R.W. The first record of the genus Helobdella (Hirudinoidea: Glossiphoniidae) from Australia, with a description of a new species, Helobdella papillornata. Hydrobiologia. 1998;389:45–49. doi:10.1023/A:1003543314841 [Google Scholar]
- Hamilton W.D. The genetical evolution of social behaviour. J. Theor. Biol. 1964;7:1–16. doi: 10.1016/0022-5193(64)90038-4. doi:10.1016/0022-5193(64)90038-4 [DOI] [PubMed] [Google Scholar]
- Kacelnik A, Cotton P.A, Stirling L, Wright J. Food allocation among nestling starlings: sibling competition and the scope of parental choice. Proc. R. Soc. B. 1995;259:259–263. [Google Scholar]
- Kutschera U, Wirtz R. A leech that feeds its young. Anim. Behav. 1986;34:941–942. doi:10.1016/S0003-3472(86)80087-2 [Google Scholar]
- Macnair M.R, Parker G.A. Models of parent-offspring conflict. III. Intra-brood conflict. Anim. Behav. 1979;27:1202–1209. doi:10.1016/0003-3472(79)90067-8 [Google Scholar]
- Mock D.W. Siblicidal aggression and resource monopolization in birds. Science. 1984;225:731–733. doi: 10.1126/science.225.4663.731. [DOI] [PubMed] [Google Scholar]
- Mock D.W. Siblicidal brood reduction: the prey size hypothesis. Am. Nat. 1985;125:327–343. doi:10.1086/284346 [Google Scholar]
- Mock D.W, Parker G.A. Oxford University Press; Oxford, UK: 1997. The evolution of sibling rivalry. [Google Scholar]
- Mock D.W, Lamey T.C, Williams C.F, Ploger B.J. Proximate and ultimate roles of food amount in regulating egret sibling aggression. Ecology. 1987;68:1760–1772. doi: 10.2307/1939867. doi:10.2307/1939867 [DOI] [PubMed] [Google Scholar]
- Paez D, Govedich F.R, Bain B.A, Kellett M, Burd M. Effects of parental care on hunting behaviour of Helobdella papillornata (Euhirudinea: Glossiphoniidae) Hydrobiologia. 2004;519:185–188. doi:10.1023/B:HYDR.0000026504.39761.b3 [Google Scholar]
- Parker G.A, Mock D.W, Lamey T.C. How selfish should stronger sibs be? Am. Nat. 1989;133:846–868. doi:10.1086/284956 [Google Scholar]
- Parker G.A, Royle N.J, Hartley I.R. Intrafamilial conflict and parental investment: a synthesis. Phil. Trans. R. Soc. B. 2002a;357:295–307. doi: 10.1098/rstb.2001.0950. doi:10.1098/rstb.2001.0950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker G.A, Royle N.J, Hartley I.R. Begging scrambles with unequal chicks: interactions between need and competitive ability. Ecol. Lett. 2002b;5:206–215. doi:10.1046/j.1461-0248.2002.00301.x [Google Scholar]
- Ricklefs R.E. On the limitation of brood size in passerine birds by the ability of adults to nourish their young. Proc. Natl Acad. Sci. USA. 1968;61:847–851. doi: 10.1073/pnas.61.3.847. doi:10.1073/pnas.61.3.847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royle N.J, Hartley I.R, Owens I.P.F, Parker G.A. Sibling competition and the evolution of growth rates in birds. Proc. R. Soc. B. 1999;266:923–932. doi:10.1098/rspb.1999.0725 [Google Scholar]
- Sawyer R.T. Oxford University Press; New York, NY: 1986. Leech biology and behaviour, II. Feeding biology, ecology and systematics. [Google Scholar]
- Sen A.K. Clarendon Press; Oxford, UK: 1973. On economic inequality. [Google Scholar]
- Tan G.N, Govedich F.R, Burd M. Social group size, potential sperm competition and reproductive investment in a hermaphroditic leech, Helobdella papillornata (Euhirudinea: Glossiphoniidae) J. Evol. Biol. 2004;17:574–580. doi: 10.1111/j.1420-9101.2004.00692.x. doi:10.1111/j.1420-9101.2004.00692.x [DOI] [PubMed] [Google Scholar]
- Trivers R.L. Parent-offspring conflict. Am. Zool. 1974;14:249–264. [Google Scholar]
- Walton, E., Govedich, F. R., Bain, B. A. & Burd, M. In press. Mate choice associated with body size in a simultaneous hermaphrodite, Helobdella papillornata (Euhirudinea: Glossiphoniidae). Invert. Reprod. Dev