Abstract
In polygynous species with biparental care, the amount of paternal support often varies considerably. In the pied flycatcher (Ficedula hypoleuca), females mated with monogamous males receive more male assistance during the nestling phase than females mated with bigynous males, as the latter have to share their mates with another female. Bigynous males, however, give more support to their primary broods than to their secondary broods. Using a long-term dataset (31 years), the present study revealed that direct reproductive success, i.e. number of fledglings, was lower in females that mated with bigynous males, especially in secondary broods without male assistance, than in females that mated with monogamous males. Secondary broods with male assistance were more affected than primary broods. Female survival was independent of mating status. In primary broods, a delayed compensation for inferior direct reproductive success was found in terms of the number of grandoffspring, a phenomenon that did not occur in secondary broods. Delayed compensation in primary broods refers to indirect effects, i.e. good genes. According to the sexy son hypothesis, genetically superior (i.e. sexy) males may have sons with a higher number of broods belonging to a polygynous breeding status than do sons from broods with a monogamous father. This was indeed the case for sons descending from primary broods, but not for sons descending from secondary broods.
Keywords: polygyny, grandoffspring, sexy sons, fitness, genetic quality, Ficedula hypoleuca
1. Introduction
Polygyny is a common phenomenon in avian species. Data from 122 well-studied European passerines, for instance, demonstrate that 20% of these are regular polygynous (Møller 1986). In order to understand why polygynous mating systems are upheld, the female's perspective is very important (Searcy & Yasukawa 1989; Bensch 1997). In biparental species, polygyny has been demonstrated to be costly for females if male assistance is reduced (e.g. Johnson et al. 1994; Lubjuhn et al. 2000; Pribil & Searcy 2001; Moreno et al. 2002), while polygynous males are normally able to increase their fitness by attracting additional females (Arnqvist & Rowe 2005 and references therein). A basic question is, therefore, why do females choose to mate with an already-mated male?
The polygyny-threshold model (Orians 1969) proposes that the cost of sharing a male should be compensated by a superior territory quality. An experimental field test with red-winged blackbirds, Agelaius phoeniceus, indeed demonstrated that females trade mating status against territorial quality, i.e. nest predation probability (Pribil & Searcy 2001). In the case of avian mating systems, however, most studies indicate that current reproductive success, i.e. number of fledglings, is not compensated for in partially male-deserted secondary broods (see Ligon (1999) for a review). The deception hypothesis was proposed by Alatalo et al. (1981) for the pied flycatcher Ficedula hypoleuca, a polyterritorial hole nesting passerine. This hypothesis suggests that females may be manipulated into breeding with an already-mated male, and assumes that males try to hide their actual breeding status. Arnqvist & Rowe (2005) point out that the deception hypothesis has recently received some support in the avian literature.
In order to estimate a male's value to a female, direct as well as indirect benefits have to be considered (Kokko et al. 2003; Neff & Pitcher 2005). While direct benefits take the form of paternal care or territory advantages such as food or shelter, indirect benefits ensue from the genes provided by the male. These indirect benefits can result from good genes, i.e. intrinsic effects of paternal genes, or from compatible genes, i.e. interactions between the maternal and paternal genomes (Neff & Pitcher 2005). Recent studies in the avian literature support the assumption that both direct and indirect effects have an impact, as females in several bird species do use secondary sexual characteristics (e.g. plumage colour) as indicators of phenotypic condition and/or genetic quality of the males (e.g. Palokangas et al. 1994; Sheldon et al. 1999; Keyser & Hill 2000).
According to the ‘sexy son’ hypothesis (Weatherhead & Robertson 1979), secondary females can compensate any direct reproductive loss by the enhanced fitness of their sons. The hypothesis proposes that mated males father attractive, prolific sons, with the result that secondary females obtain as many grandoffspring as do females mated with monogamous males. In the case of the pied flycatcher, however, the sexy son hypothesis was heavily criticized (e.g. Alatalo & Lundberg 1986), although there are indications that sexual selection, the crucial basis for this hypothesis, does play a role in this species: male attractiveness (plumage characteristics) is correlated with male parental quality (Lifjeld & Slagsvold 1988; Siitari & Huhta 2002) and female pied flycatchers trade between a male's mating status and its secondary sexual character (Slagsvold & Drevon 1999).
Most studies have estimated fitness consequences of a female's mating status only by reproductive parameters for incubation and nestling period (e.g. Askenmo 1984; Alatalo & Lundberg 1990; Smith & Sandell 1998). These fitness approximations, however, might be insufficient since they do not take into account the survival of adults and offspring to the next breeding season, although both parameters are important for lifetime reproductive success (Both 2002; Hunt et al. 2004; Neff & Pitcher 2005).
The general view of an inferior breeding success in secondary females has been questioned by more recent studies taking future consequences into account (Both 2002; Garamszegi et al. 2004). Both (2002) could not find a significant reduction in offspring recruitment in secondary female pied flycatchers when they were partly supported by their mates. Garamszegi et al. (2004) estimated survival and lifetime reproductive success in the closely related collared flycatcher, F. albicollis. Using a relatively time-limited dataset (7 years), they argue that primary and secondary females enjoyed higher survival and similar future reproductive success compared to females mated with monogamous males.
In the present study, we investigate in the pied flycatcher, a model species in avian evolutionary ecology, the fitness consequences of polygyny for females of different mating status. Rather than restricting our fitness measures to current reproductive success during the nestling period, we also followed closely female survival and offspring recruitment. Moreover, an individual's fitness should ideally be measured by the lifetime reproductive success of its sons and daughters, i.e. the number of grandoffspring produced (Hunt et al. 2004). We therefore examined the success of pied flycatcher broods in terms of the lifetime reproductive success of their descendants, as proposed by Kokko et al. (2003). In order to focus on the indirect effect of good genes, i.e. the sexy son phenomenon, we analysed whether the chance of becoming polygynous and lifetime reproductive success differed between males descending from broods with monogamous and those with polygynous fathers. Arnqvist & Rowe (2005) highlighted that there were as yet no relevant long-term studies that simultaneously measured direct costs and indirect benefits.
2. Material and methods
The pied flycatcher is a well-studied cavity-nesting passerine bird that breeds in many forest habitats of the Palearctic region and winters in tropical West Africa (e.g. Lundberg & Alatalo 1992). F. hypoleuca is a facultative polygynous species and polygynously mated males are mostly polyterritorial (e.g. Winkel 1994).
(a) Study area and study population
Data were collected in a long-term study (1974–2003; 2004 considered only for recruiting birds) of a nest-box-breeding population of pied flycatchers in a mixed coniferous forest near Lingen/Emsland, Lower Saxony, Germany (52°27′ N, 7°15′ E). For further details on the study plot see Altenkirch & Winkel (1991). The 325 ha study area contains about 560 nest-boxes in which up to 150 pied flycatcher pairs breed per year (Winkel & Winkel 1998; W. Winkel and D. Winkel 2005, unpublished data). In this area, pied flycatchers are almost exclusively restricted to nest-boxes as breeding sites. This is due to the fact that nest-boxes are put up in excess and also this species strongly prefers artificial breeding sites over natural cavities (Lundberg & Alatalo 1992). Therefore, we were able to trap all adults of the local pied flycatcher population.
(b) Fieldwork
The nest-boxes were checked during the breeding season (mid-April until end of June) at least weekly, and data on reproductive performance were collected using standard methods (see Both 2002). Clutch size, number of hatchlings, number of fledglings, number of recruits, number of grandoffspring fledged during the recruits' lifetime and female survival to the next breeding season served as measures of female fitness. For each fitness parameter, sample size was adjusted to ensure that only undisturbed broods and uncensored data were included.
In each year, all adults were captured in the nest-boxes either during the incubation period (only females—picked up by hand) or while feeding young (both sexes—by means of traps placed inside the nest-boxes). All the nestlings were banded with uniquely numbered aluminium rings from the ‘Vogelwarte Helgoland’; adults were identified by their rings or were also banded. Survival of adult females and recruitment of juveniles were determined by the local recapture of ringed birds in any of the subsequent years.
Recapture probability and local survival rate could in theory be affected by dispersal differences in relation to mating status. In particular, it is conceivable that females with unsuccessful broods might leave the study plot, whereas females with successful broods might breed in the same area in the following years. Therefore, we investigated whether a brood without fledglings (equivalent to brood failure) during a female's first brood resulted in different lifespan (calculated as the last year of recapture minus the year of hatching) between the four female categories (see §2c below for a definition of these categories). Any effect of brood failure on the calculated lifespan would have been an indicator for differences in dispersal to other breeding sites. This was, however, not the case since the interaction term ‘brood failure×brood category’ on calculated lifespan was not significant (GLM with log-link, , p=0.956). Hence, our data, in line with other studies (Pärt & Gustafsson 1989; Slagsvold & Dale 1996; Both 2002; Garamszegi et al. 2004), found no indication for different dispersal patterns between female flycatchers of different mating status. A correction on return rates was therefore not necessary.
(c) Female brood categories
In most broods both parents feed the young. Bigynous males, however, seem to give priority to their first (equivalent to primary) females. In secondary nests, their contribution to feeding young is normally reduced or even non-existent (Lundberg & Alatalo 1992). With respect to support received from males during the nestling phase and to male mating status, four different brood categories could be distinguished for females.
A ‘primary brood’ is a brood of a bigynous male in which nestlings hatched first. Females of these broods are called ‘primary females’ in the following.
A ‘secondary brood’ is the second brood of a bigynous male that hatched later than the primary one. In the following, females of these broods are called ‘secondary females with male assistance’, due to the fact that it was possible to catch the male during the nestling phase, showing that it supported the female at least to some extent.
In several cases, no male could be caught or observed during the whole nestling period. In our pied flycatcher population, female mortality during the nestling period was extremely low (0.5% of all broods). Until now we have had no indication of a sex-biased adult mortality in this species. The ‘no-male broods’ were therefore, as a rule, secondary broods of bigynous males in which the male did not assist the female in feeding the young (see also Winkel & Winkel 1984). This explanation was recently revealed in a random sample by paternity analyses using molecular genetic tools: females not supported by a partner during the nestling phase were inseminated by bigynous males that were simultaneously feeding nestlings in another nest-box (Lubjuhn 2005, personal communication). We termed females of such ‘no-male broods’ ‘secondary females without male assistance’.
Broods of females that were supported during the nestling phase by monogamous males (males caught in one nest-box only) were called ‘monogamous broods’. However, it has to be taken into account that about 15% of such broods were in fact obscured primary broods. In such cases, a seemingly ‘monogamous brood’ was supported by a bigynous male that had been caught in one nest-box only since the secondary female was not assisted by her mate (equivalent to ‘no-male brood’, see above). This discrepancy, as a rule, remains undetected, thus reducing possible differences in fitness correlates between monogamous broods and primary/secondary broods.
In another study (Both 2002), secondary broods with male assistance were called ‘narrow sense secondary females’. In addition, Both (2002) compared a pooled sample consisting of secondary broods with male assistance and no-male broods. He called this pooled sample ‘broad sense secondary females’. In the present study, however, we decided to consider fitness estimates of secondary broods with and without male assistance separately. The application of ‘broad sense secondary females’ makes it impossible to distinguish differences in fitness consequences of secondary broods that are at least partly assisted by a male (secondary broods with male assistance) from broods without any paternal support (no-male broods).
In most passerine species, investigated females also engage in copulations with males other than their social mate (e.g. Griffith et al. 2002). It is important to note that extra-pair paternity is fairly low in the pied flycatcher population observed (Brün et al. 1996; Lubjuhn et al. 2000). Hence, although polygynous males were more often cuckolded than were monogamous males (Lubjuhn et al. 2000), genes are normally provided by the female's mate.
(d) Statistical analyses
Data were analysed using GLM techniques (Crawley 1993) with Poisson errors and a log link function for count data (clutch size, number of hatchlings, number of fledglings, number of recruits, number of grandoffspring) and with binomial error and a logit link for proportional data, i.e. female survival. In case of over-dispersion, scale correction with Pearson χ2 was done and changes in deviance were tested against the F-distribution (Crawley 1993). Differences between years as well as timing of broods (laying date) were taken into account in all analyses as covariates, since reproductive success varies between years and depends strongly on laying date (Berndt & Winkel 1967; Lundberg & Alatalo 1992). In order to account for leap years, laying date was expressed as the number of days within the year, e.g. 9 May 2000=130 days, 9 May 2001=129 days. Estimates (equivalent to a Wald test) were performed to compare fitness estimates of monogamous broods with each of the other three categories (primary, secondary, no-male broods). All significance tests are two-tailed. GLMStat 6.0 was used for analyses (Beath 2005).
3. Results
(a) Fitness estimates of different brood categories
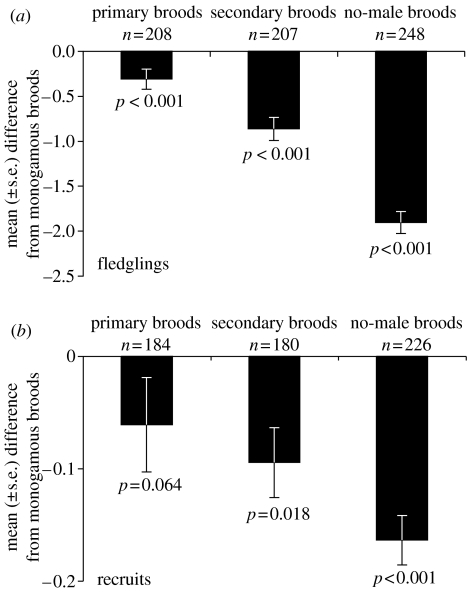
Descriptive data on breeding performance of females mated with monogamous males (i.e. monogamous broods) are given in table 1. Clutch size and number of hatchlings did not differ significantly between the four female categories (table 2). In contrast, the numbers of fledglings and recruits were lower in broods of females mated with bigynous males than in monogamous broods (table 2, figure 1). The effect size, i.e. degree of inferior breeding success, was different in primary and secondary females (see figure 1): secondary broods without male assistance, i.e. no-male broods, were more affected (41.3% fewer fledglings and 64.7% fewer recruits than monogamous broods of the same laying date) than secondary broods with male assistance (18.3% fewer fledglings and 34.8% fewer recruits). Primary broods, on the other hand, showed the least pronounced differences (5.8% fewer fledglings and 20.1% fewer recruits) than monogamous broods (figure 1).
Table 1.
Breeding performance and female survival in monogamous broods.
| clutch size | number of hatchlings | number of fledglings | number of recruits | female survival probability | number of grandoffspring | |
|---|---|---|---|---|---|---|
| sample period (breeding attempts) | 1974–2003 | 1974–2003 | 1974–2003 | 1974–2001a | 1974–2001a | 1974–1999a |
| n | 1703 | 1703 | 1676 | 1478 | 1478 | 1275 |
| mean | 5.95 | 5.69 | 5.00 | 0.30 | 0.30 | 2.29 |
| s.e. | 0.02 | 0.03 | 0.04 | 0.02 | 0.01 | 0.16 |
Additionally, data from subsequent years (up to 2004) were considered for female survival, number of recruits and for lifetime reproductive success of recruiting birds fledging from 1974 to 1999.
Table 2.
Statistical analysis of annual breeding performance and female survival in monogamous, primary and secondary (with and without male assistance) broods. The GLM was performed with Poisson errors for clutch size, number of hatchlings, number of fledglings, number of recruits and number of grandoffspring fledged, and with binomial errors for female survival (i.e. the probability of recapturing in subsequent years). Differences between brood categories were controlled for differences between years as well as laying date (GLM-model: dependent variable=year+laying date+brood category+error).
| clutch size | number of hatchlings | number of fledglings | number of recruits | number of grandoffspring | female survival | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| d.f. | χ2 | p | d.f. | χ2 | p | d.f. | F | p | d.f. | χ2 | p | d.f. | F | p | d.f. | χ2 | p | |
| year | 29 | 30.63 | 0.38 | 29 | 46.02 | 0.023 | 29 | 9.45 | <0.001 | 27 | 93.42 | <0.001 | 25 | 2.77 | <0.001 | 27 | 46.86 | 0.010 |
| laying date | 1 | 56.12 | <0.001 | 1 | 59.83 | <0.001 | 1 | 344.7 | <0.001 | 1 | 63.16 | <0.001 | 1 | 27.64 | <0.001 | 1 | 14.54 | <0.001 |
| brood category | 3 | 1.04 | 0.79 | 3 | 1.32 | 0.72 | 3 | 107.8 | <0.001 | 3 | 30.02 | <0.001 | 3 | 4.66 | 0.003 | 3 | 0.49 | 0.92 |
| mean (±s.e.) estimate of laying date | −0.011(±0.002) | −0.012(±0.002) | −0.081(±0.006) | −0.061(±0.009) | −0.102(±0.025) | −0.031(±0.009) | ||||||||||||
Figure 1.
Breeding success of primary and secondary (with and without male assistance) female pied flycatchers. Columns depict the mean (±s.e.) difference in (a) number of fledglings, and (b) number of recruits as compared to simultaneous monogamous broods, i.e. broods with the same laying date. The p-values are given for each brood category as a comparison to the breeding performance of monogamous broods (see table 2).
Female survival to the next breeding season, i.e. presence in the study area in subsequent years, did not differ significantly between the four female categories (table 2).
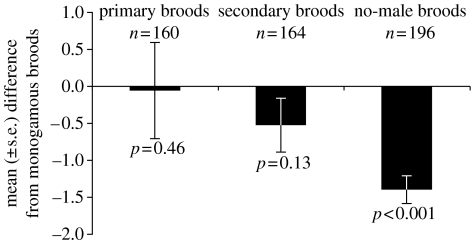
Subsequently, we analysed the difference between females belonging to different brood categories with respect to lifetime reproductive success of their descendants in the F1 generation. Secondary broods with and without male assistance produced fewer grandoffspring than monogamous broods (table 2, figure 2). This difference was more pronounced in no-male broods, i.e. secondary broods without male assistance (68.4% fewer grandoffspring than monogamous broods of the same laying date), than in secondary broods with male assistance (only 25.9% fewer grandoffspring). In contrast, primary broods did not differ significantly in their number of grandoffspring from monogamous broods (table 1, figure 2).
Figure 2.
Breeding success of primary and secondary (with and without male assistance) female pied flycatchers as measured by the number of grandoffspring fledged during the lifetime of their recruits. Columns depict the mean (±s.e.) difference in number of grandoffspring as compared to simultaneous monogamous broods, i.e. broods with the same laying date. p-values are given for each brood category as a comparison to the breeding performance of monogamous broods (see table 2).
(b) Investigation of the ‘sexy son hypothesis’
In addition to direct effects, e.g. number of fledglings (see figure 1), indirect effects might also be responsible for delayed compensation regarding the number of grandoffspring in primary broods. In order to investigate this, we compared three lifetime parameters of male recruits descending from monogamous, primary and secondary broods, respectively (see table 3). Males descending from secondary broods with and without male assistance were combined in these analyses, as both brood categories showed inferior fitness estimates (see above). Primary broods, on the other hand, revealed a delayed compensation, i.e. no significant differences in number of grandoffspring as compared to monogamous broods.
Table 3.
Statistical analysis of three lifetime parameters of male recruits descending from monogamous, primary and secondary broods. Differences between these brood categories were controlled for differences between year of birth as well as laying date of the descending brood (GLM-model with Poisson errors and log-link function: dependent variable=year+laying date+brood category+error).
| number of polygynous broods | number of fledglings | age | |||||
|---|---|---|---|---|---|---|---|
| d.f. | χ2 | p | F | p | χ2 | p | |
| year | 25 | 41.3 | 0.021 | 0.79 | 0.75 | 25.4 | 0.43 |
| laying date | 1 | < 0.01 | 0.98 | 0.07 | 0.79 | < 0.01 | 0.99 |
| brood category | 2 | 6.16 | 0.046 | 2.73 | 0.067 | 1.47 | 0.48 |
| residual | 203 | ||||||
In order to avoid censoring of the recruits' lifetime, only sons hatching between the years 1974 and 1999 were included. Recapture data and breeding performance from 1975 to 2004 were used to calculate lifetime reproductive success of these birds. In 2004, none of these males could be recaptured.
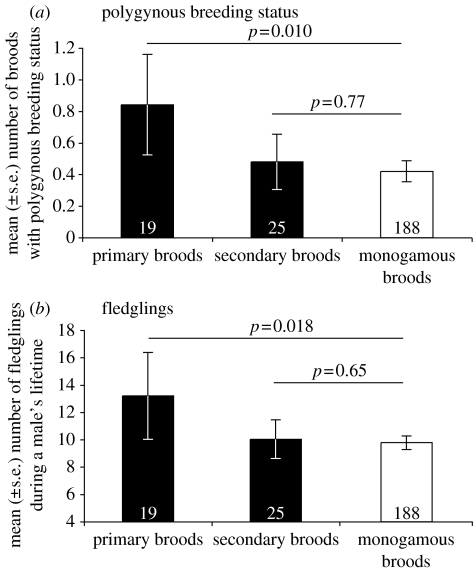
Males recruiting from primary broods had a higher number of polygamous breeding attempts than males recruiting from monogamous broods, while males recruiting from secondary broods did not differ in this respect (table 3, figure 3). These results are not confounded by differences in lifespan as males descending from different brood categories did not differ in this respect (table 3).
Figure 3.
Lifetime reproductive parameter of male pied flycatchers descending from monogamous broods, primary broods and secondary broods with/without male assistance (sample sizes are given in the columns). (a) Columns depict the mean (±s.e.) number of breeding attempts with a polygynous mating status during lifetime. (b) Columns depict the mean (±s.e.) number of fledglings produced during lifetime. p-values are given for each brood category as a comparison to the breeding performance of monogamous broods (see table 3).
The difference in number of polygynous broods corresponds with a difference in lifetime reproductive success, i.e. number of fledglings produced during a male's lifetime: males descending from primary broods produced significantly more fledglings than males descending from monogamous broods (figure 3). Males descending from secondary broods did not produce significantly more fledglings than males descending from monogamous broods (figure 3).
4. Discussion
Several studies have observed that female pied flycatchers paired with already-mated males suffer considerable reductions in terms of number of fledglings produced when compared with females mated with monogamous males (e.g. Lundberg & Alatalo 1992). Our long-term investigation confirms these studies, as we were able to demonstrate a reduced direct reproductive success in secondary females. This negative impact was more pronounced in secondary broods without male assistance than in secondary broods with male assistance. Both (2002) found a similar pattern as he observed less pronounced effects when only secondary females with male assistance were considered as compared to a pooled sample of secondary females with and without male assistance. However, data on comparing fitness estimates of primary and monogamous broods are scarce. Using a relatively time-limited dataset, Garamszegi et al. (2004) detected a reduced number of fledglings in primary broods as compared to monogamous broods of the closely related collared flycatcher. Our study revealed that in the pied flycatcher, primary females suffered a significant reduction in terms of number of fledglings as well as a trend towards a decreased number of recruits as compared with females mated with monogamous males.
It has to be stressed that detailed information on offspring dispersal is lacking for the pied flycatcher. As in other pied flycatcher studies, it might therefore be possible that offspring dispersal probability may differ between brood categories, thus altering fitness estimates. We have, however, no evidence for this assumption. The lower number of recruits of secondary females in particular is most plausibly explained by other facts, which are discussed below.
A lower reproductive success of secondary females might be either the result of poorer quality females in secondary broods (see Grønstøl et al. 2003) or the consequence of costs of sharing a male (e.g. Slagsvold & Lifjeld 1994). Our data do not support the assumption that secondary females are of lower quality, since survival probability, clutch size and body size (as measured by wing length in a sub-sample: F3,1435=0.5841, p=0.626) did not differ between secondary females and females mated with monogamous males. In the pied flycatcher, primary females normally receive more male assistance than secondary females (Lifjeld & Slagsvold 1989; Lundberg & Alatalo 1992). Our results therefore support the assumption that the reduced paternal care during the nestling stage is a decisive factor in the inferior reproductive success. To sum up, the polygyny-threshold model, i.e. compensation of reproductive success by territorial quality (Orians 1969), does not apply in our study.
It has been proposed that offspring of high genetic quality might outweigh any immediate decline in offspring production (Weatherhead & Robertson 1979; Kokko et al. 2003). This might be the case in our pied flycatcher population for primary females but is not so for secondary females. In spite of the lower direct reproductive success of primary females (as measured by the number of fledglings), their fitness—measured in terms of the number of grandoffspring fledged—did not differ significantly from females mated with monogamous males, while secondary females, on the other hand, had fewer grandoffspring. Thus, only primary females were able to compensate the immediate decline in offspring production, i.e. number of fledglings and recruits. For this reason, the delayed compensation has to be the result of increased lifetime reproductive success of birds recruiting from primary broods. Our data do indeed support this assumption. In primary broods with at least one recruit, the number of grandoffspring produced during the lifetime of their recruits was significantly higher than in monogamous broods with at least one recruit (F1,314=4.182, p=0.042; monogamous broods: M=10.4, s.e.=0.5; primary broods: M=13.7, s.e.=2.4).
Weatherhead & Robertson (1979) originally conceived the ‘sexy son hypothesis’ in order to explain instances where females chose to mate polygynously when they could have mated monogamously and produced more young. Genetically superior (i.e. sexy) males may have sons that become more often polygynous themselves than sons of less attractive (i.e. monogamous) males. Our data support this assumption with respect to sons of primary broods, and confirm that this higher number of polygynous broods is transferred to a higher lifetime reproductive success, i.e. number of fledglings during a son's lifetime. This, however, was not the case in sons descending from secondary broods. Therefore, non-genetic paternal effects, i.e. the reduced amount of male assistance in secondary broods (see Lundberg & Alatalo (1992) and references therein), also seem to be important. This result was not confounded by age, i.e. more polygynous attempts could not be explained by differences in lifetime between sons descending from monogamous and primary broods.
Alatalo & Lundberg (1986) state that nestling weight and tarsus length are reduced in broods of secondary females (see also Alatalo et al. 1982). Therefore, poor phenotypic quality most probably alters the sexual attractiveness of males descending from secondary broods. As a consequence, these males are not able to attract more females than are males descending from broods with a monogamous father. Paternal care, on the other hand, is less reduced in primary broods. Hence, phenotypic quality of males descending from primary broods can be assumed to be less affected, thus having a lower detrimental impact on sexual attractiveness. Therefore, males descending from primary broods benefit from the superior genes of their polygynous, i.e. sexually attractive father.
According to the ‘sexy son hypothesis’, one might expect broods of polygynous males to have a skewed offspring sex ratio, i.e. more sons than daughters, if their ‘attractive’ male descendants are more prolific. Alteration of sex ratio, i.e. an increased number of sons, would therefore be favourable in primary broods as it increases a female's fitness in the long-term, i.e. increases the number of grandoffspring. In contrast to the ‘sexy son hypothesis’ prediction, secondary broods should have female-sex-biased offspring, assuming that being of lower quality has stronger negative effects on the future reproductive success of males than on that of females (Albrecht & Johnson 2002). The proposed association between breeding condition and offspring sex ratio has been observed in different bird species (e.g. Westerdahl et al. 2000; Albrecht & Johnson 2002). Our data do not, however, support these assumptions as the proportion of males recruiting from monogamous, primary and secondary broods did not differ significantly (GLM with logit link, , p=0.91).
To sum up, the ‘sexy son hypothesis’ (Weatherhead & Robertson 1979) does not apply in the pied flycatcher, as only primary females benefit from superior genes of their respective mate. Fitness performance in secondary females, on the other hand, suffers from substantially reduced male support during the nestling stage that cannot be compensated by their recruits (see above). Therefore, it seems not to be advantageous to pair with an already-mated male, even if this male is attractive and this attractiveness is heritable.
Different hypotheses have been established to explain why females mate with already-mated males (Lundberg & Alatalo 1992). According to the deception hypothesis, males hide their mating status and deceive females into polygyny in the polyterritorial pied flycatcher (Alatalo et al. 1981; Alatalo & Lundberg 1984). Slagsvold & Dale (1994) criticized this hypothesis, stating that females may settle with mated males as a result of mate competition and costs of searching. These search costs occur as a consequence of the short breeding period (Winkel & Hudde 1993), which restricts mate sampling and may limit a female's chance of finding an optimal mate (Slagsvold et al. 1988; Stenmark et al. 1988). Slagsvold & Drevon (1999) provided evidence for a trade-off in mate choice. They argued that females may compromise by choosing a mated male of high quality that can provide favourable genes for the offspring, or by choosing an unmated male of lower quality that can provide a greater degree of parental care. Our results highlight the fact that good genes can increase offspring fitness due to the increased reproductive value of their ‘sexy sons’, but only if mate assistance is not reduced considerably (i.e. in primary broods). Choosing an already-mated male of high quality cannot compensate the inferior impact of reduced paternal care in our pied flycatcher population, especially if the mate abandons the female during the nestling period.
Acknowledgments
We would like to thank Doris Winkel for her continuous and valuable support in the field. We would also like to thank Jens Rolff and two anonymous referees for helpful comments on an earlier version of the manuscript. Linda Froome-Döring improved the English language of the final version.
References
- Alatalo R.V, Lundberg A. Polyterritorial polygyny in the pied flycatcher—evidence for the deception hypothesis. Ann. Zool. Fenn. 1984;21:217–228. [Google Scholar]
- Alatalo R.V, Lundberg A. The sexy son hypothesis: data from the pied flycatcher Ficedula hypoleuca. Anim. Behav. 1986;34:1454–1462. [Google Scholar]
- Alatalo R.V, Lundberg A. Polyterritorial polygyny in the pied flycatcher. In: Slater P.J.B, Rosenblatt J.S, Beer L, editors. Advances in study behaviour. vol. 19. Academic Press; San Diego, CA: 1990. pp. 1–27. [Google Scholar]
- Alatalo R.V, Carlson A, Lundberg S. The conflict between male polygamy and male monogamy: the case of the pied flycatcher Ficedula hypoleuca. Am. Nat. 1981;117:738–753. 10.1086/283756 [Google Scholar]
- Alatalo R.V, Lundberg A, Stahlbrandt K. Why do pied flycatcher females mate with already-mated males? Anim. Behav. 1982;30:585–593. [Google Scholar]
- Albrecht D.J, Johnson L.S. Manipulation of offspring sex ratio by second-mated female house wrens. Proc. R. Soc. B. 2002;269:461–465. doi: 10.1098/rspb.2001.1914. 10.1098/rspb.2001.1914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altenkirch W, Winkel W. Versuche zur Bekämpfung der Lärchenminiermotte (Coleophora laricella) mit Hilfe insektenfressender Vögel. Waldhygiene. 1991;18:233–255. [Google Scholar]
- Arnqvist G, Rowe L. Princeton University Press; Princeton, NJ: 2005. Sexual conflict. [Google Scholar]
- Askenmo C.E.H. Polygyny and nest site selection in the pied flycatcher. Anim. Behav. 1984;32:972–980. [Google Scholar]
- Beath K.J. 2005. GLMStat—generalized linear modelling. URL http://www.glmstat.com. [Google Scholar]
- Bensch S. The cost of polygyny: definitions and applications. J. Avian Biol. 1997;28:345–352. [Google Scholar]
- Berndt R, Winkel W. Die Gelegegröße des Trauerschnäppers (Ficedula hypoleuca) in Beziehung zu Ort, Zeit, Biotop und Alter. Vogelwelt. 1967;88:97–136. [Google Scholar]
- Both C. Fitness costs of polygyny in female pied flycatchers Ficedula hypoleuca. Ardea. 2002;90:129–138. [Google Scholar]
- Brün J, Winkel W, Epplen J.T, Lubjuhn T. Elternschaftsnachweise bei Trauerschnäppern Ficedula hypoleuca am Westrand ihres mitteleuropäischen Verbreitungsareals. J. Ornithol. 1996;137:435–446. [Google Scholar]
- Crawley M.J. Blackwell; Oxford, UK: 1993. GLIM for ecologists. [Google Scholar]
- Garamszegi L.Z, Török J, Michl G, Møller A.P. Female survival, lifetime reproductive success and mating status in a passerine bird. Oecologia. 2004;138:48–56. doi: 10.1007/s00442-003-1408-z. 10.1007/s00442-003-1408-z [DOI] [PubMed] [Google Scholar]
- Griffith S.C, Owens I.P.F, Thuman K.A. Extra-pair paternity in birds: a review of interspecific variation and adaptive function. Mol. Ecol. 2002;11:2195–2212. doi: 10.1046/j.1365-294x.2002.01613.x. 10.1046/j.1365-294X.2002.01613.x [DOI] [PubMed] [Google Scholar]
- Grønstøl G.B, Byrkjedal I, Fiksen Ø. Predicting polygynous settlement while incorporating varying female competitive strength. Behav. Ecol. 2003;14:257–267. 10.1093/beheco/14.2.257 [Google Scholar]
- Hunt J, Bussière L.F, Jennions M.D, Brooks R. What is genetic quality? Trends Ecol. Evol. 2004;19:329–333. doi: 10.1016/j.tree.2004.03.035. 10.1016/j.tree.2004.03.035 [DOI] [PubMed] [Google Scholar]
- Johnson I.S, Kermott L.H, Lein M.R. Territorial polygyny in house wrens—are females sufficiently compensated for the cost of mate sharing? Behav. Ecol. 1994;5:98–104. [Google Scholar]
- Keyser A.J, Hill G.E. Structurally based plumage coloration is an honest signal of quality in male blue grosbeaks. Behav. Ecol. 2000;11:202–209. 10.1093/beheco/11.2.202 [Google Scholar]
- Kokko H, Brooks R, Jennions M.D, Morley J. The evolution of mate choice and mating biases. Proc. R. Soc. B. 2003;270:653–664. doi: 10.1098/rspb.2002.2235. 10.1098/rspb.2002.2235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lifjeld J.T, Slagsvold T. Mate fidelity of renesting pied flycatchers Ficedula hypoleuca in relation to characteristics of the pair mates. Behav. Ecol. Sociobiol. 1988;22:117–123. 10.1007/BF00303546 [Google Scholar]
- Lifjeld J.T, Slagsvold T. Allocation of parental investment by polygynous pied flycatcher males. Ornis Fenn. 1989;66:3–14. [Google Scholar]
- Ligon J.D. Oxford ornithology series. Oxford University Press; New York, NY: 1999. The evolution of avian breeding systems. [Google Scholar]
- Lubjuhn T, Winkel W, Epplen J.T, Brün J. Reproductive success of monogamous and polygynous pied flycatchers (Ficedula hypoleuca) Behav. Ecol. Sociobiol. 2000;48:12–17. 10.1007/s002650000208 [Google Scholar]
- Lundberg A, Alatalo R.V. T & AD Poyser; London, UK: 1992. The pied flycatcher. [Google Scholar]
- Møller A.P. Mating systems among European passerines: a review. Ibis. 1986;128:234–250. [Google Scholar]
- Moreno J, Veiga J.P, Romasanta M, Sánchez S. Effects of maternal quality and mating status on female reproductive success in the polygynous spotless starling. Anim. Behav. 2002;64:197–206. [Google Scholar]
- Neff B.D, Pitcher T.E. Genetic quality and sexual selection: an integrated framework for good genes and compatible genes. Mol. Ecol. 2005;14:19–38. doi: 10.1111/j.1365-294X.2004.02395.x. 10.1111/j.1365-294X.2004.02395.x [DOI] [PubMed] [Google Scholar]
- Orians G.H. On the evolution of mating systems in birds and mammals. Am. Nat. 1969;103:589–602. 10.1086/282628 [Google Scholar]
- Palokangas P, Korpimäki E, Hakkarainen H, Huhta E, Tolonen P, Alatalo R.V. Female kestrels gain reproductive success by choosing brightly ornamented males. Anim. Behav. 1994;47:443–448. 10.1006/anbe.1994.1058 [Google Scholar]
- Pribil S, Searcy W.A. Experimental confirmation of the polygyny threshold model for red-winged blackbirds. Proc. R. Soc. B. 2001;268:1643–1646. doi: 10.1098/rspb.2001.1720. 10.1098/rspb.2001.1720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pärt T, Gustafsson L. Breeding dispersal in the collared flycatcher (Ficedula albicollis): possible causes and reproductive consequences. J. Anim. Ecol. 1989;58:305–320. [Google Scholar]
- Searcy W.A, Yasukawa K. Alternative models of territorial polygyny in birds. Am. Nat. 1989;134:323–343. 10.1086/284984 [Google Scholar]
- Sheldon B.C, Andersson S, Griffith S.C, Örnberg J, Sendecka J. Ultraviolet colour variation influences blue tit sex ratios. Nature. 1999;402:874–877. 10.1038/47239 [Google Scholar]
- Siitari H, Huhta E. Individual color variation and male quality in pied flycatchers (Ficedula hypoleuca): a role of ultraviolet reflectance. Behav. Ecol. 2002;13:737–741. 10.1093/beheco/13.6.737 [Google Scholar]
- Slagsvold T, Dale S. Why do female pied flycatchers mate with already mated males—deception or restricted mate sampling? Behav. Ecol. Sociobiol. 1994;34:239–250. 10.1007/s002650050039 [Google Scholar]
- Slagsvold T, Dale S. Disappearance of female pied flycatchers in relation to breeding stage and experimentally induced molt. Ecology. 1996;77:461–471. [Google Scholar]
- Slagsvold T, Drevon T. Female pied flycatchers trade between male quality and mating status in mate choice. Proc. R. Soc. B. 1999;266:917–921. 10.1098/rspb.1999.0724 [Google Scholar]
- Slagsvold T, Lifjeld J.T. Polygyny in birds: the role of competition between females for male parental care. Am. Nat. 1994;143:59–94. 10.1086/285596 [Google Scholar]
- Slagsvold T, Lifjeld J.T, Stenmark G, Breiehagen T. On the cost of searching for a mate in female pied flycatchers Ficedula hypoleuca. Anim. Behav. 1988;36:433–442. [Google Scholar]
- Smith H.G, Sandell M.I. Intersexual competition in a polygynous mating system. Oikos. 1998;83:484–495. [Google Scholar]
- Stenmark G, Slagsvold T, Lifjeld J.T. Polygyny in the pied flycatcher Ficedula hypoleuca: a test of the deception hypothesis. Anim. Behav. 1988;36:1646–1657. [Google Scholar]
- Weatherhead P.J, Robertson R.J. Offspring quality and the polygyny threshold: ‘the sexy son hypothesis’. Am. Nat. 1979;113:201–208. 10.1086/283379 [Google Scholar]
- Westerdahl H, Bensch S, Hansson B, Hasselquist D, von Schantz T. Brood sex ratios, female harem status and resources for nestling provisioning in the great reed warbler (Acrocephalus arundinaceus) Behav. Ecol. Sociobiol. 2000;47:312–318. 10.1007/s002650050671 [Google Scholar]
- Winkel W. Polygynie des Trauerschnäppers (Ficedula hypoleuca) im Braunschweiger Raum. Vogelwarte. 1994;37:199–205. [Google Scholar]
- Winkel W, Hudde H. Ficedula hypoleuca (Pallas 1764)—Trauerfliegenschnäpper, Trauerschnäpper. In: Glutz von Blotzheim U.G, editor. Handbuch der Vögel Mitteleuropas. vol. 13. Aula; Wiesbaden, Germany: 1993. pp. 165–263. [Google Scholar]
- Winkel W, Winkel D. Polygynie des Trauerschnäppers (Ficedula hypoleuca) am Westrand seines Areals in Mitteleuropa. J. Ornithol. 1984;125:1–14. 10.1007/BF01652934 [Google Scholar]
- Winkel W, Winkel D. Bestandszunahme des Trauerschnäppers (Ficedula hypoleuca) am Westrand seines mitteleuropäischen Verbreitungsgebietes. Vogelwarte. 1998;39:222–224. [Google Scholar]