Abstract
Phenotypic diversity is not evenly distributed across lineages. Here, we describe and apply a maximum-likelihood phylogenetic comparative method to test for different rates of phenotypic evolution between groups of the avian order Charadriiformes (shorebirds, gulls and alcids) to test the influence of a binary trait (offspring demand; semi-precocial or precocial) on rates of evolution of parental care, mating systems and secondary sexual traits. In semi-precocial species, chicks are reliant on the parents for feeding, but in precocial species the chicks feed themselves. Thus, where the parents are emancipated from feeding the young, we predict that there is an increased potential for brood desertion, and consequently for the divergence of mating systems. In addition, secondary sexual traits are predicted to evolve faster in groups with less demanding young. We found that precocial development not only allows rapid divergence of parental care and mating behaviours, but also promotes the rapid diversification of secondary sexual characters, most notably sexual size dimorphism (SSD) in body mass. Thus, less demanding offspring appear to facilitate rapid evolution of breeding systems and some sexually selected traits.
Keywords: behavioural ecology, macroevolution, phylogenetics, birds, phenotypic rates
1. Introduction
Why are some lineages phenotypically diverse while others are conservative? Most birds are socially monogamous with biparental care of the young (Lack 1968), and variation across species is probably constrained by the ability of a single parent to raise the brood (Lack 1968; Bennett & Owens 2002). Many polygamous and uniparental species have precocial young, indicating that the feeding ability of the young is an important constraint on the evolution of polygamy and uniparental care. Furthermore, if chick development limits the diversification of breeding behaviour, we would also expect it to influence sexually selected traits, since sexual selection is expected to be more intense in polygamous species in which intense competition for mates is an essential feature of the breeding system (Ligon 1999).
Developmental mode varies across a continuum incorporating aspects of the morphological, physiological and behavioural state of the chicks at hatching, ranging from naked and helpless (altricial; e.g. passerines) to down-covered and largely independent (super-precocial or precocial; bush-turkeys, gamebirds, many shorebirds). However, a fundamental dichotomy occurs between semi-precocial species, in which the chicks are dependent on the parents for food, and precocial species, in which the chicks feed themselves (Starck & Ricklefs 1998). We predict that this dichotomy impacts upon the evolutionary outcomes of sexual conflict over parental care (Houston et al. 2005) since precocial development emancipates the parents from food provisioning. In semi-precocial species, offspring demands are high and there are associated costs in offspring survival if one parent deserts. Thus, semi-precocial taxa are likely to be restricted to biparental care and social monogamy (Lack 1968; Orians 1969; Temrin & Tullberg 1995; Ligon 1999; Bennett & Owens 2002). In contrast, in precocial species, where the demands of the offspring are small, the amount of parental care required is likely to be reduced with low costs of offspring survival, if one parent deserts. In precocial lineages, there exists the potential for brood desertion, a wide range of parental and mating strategies, and the divergence of mating optima between the sexes (Orians 1969; Temrin & Tullberg 1995; Székely et al. 1996; Bennett & Owens 2002). The divergence of mating optima drives sexual conflict (Chapman et al. 2003), and may result in rapid diversification of secondary sexual characters. Therefore, we predict that the rate of diversification in parental care, social mating system and in traits associated with sexual conflict and sexual selection will be higher among precocial species than among semi-precocial species.
The avian order Charadriiformes (sandpipers, plovers, gulls, auks and allies; shorebirds hereafter) is among the most diverse of all birds, and makes an excellent model system to investigate the influence of developmental mode on phenotypic evolution. First, shorebirds can be divided into precocial taxa (plovers, lapwings, sandpipers, jacanas) and semi-precocial taxa (oystercatchers, stone curlews, gulls, alcids). Second, the range of parental care strategies and social mating systems is unsurpassed in birds and the degrees of sexual conflict and sexual selection are expected to vary widely across the order (Pitelka et al. 1974; Erckmann 1983; Székely & Reynolds 1995; Thomas 2004; Thomas & Székely 2005). Finally, the availability of a supertree phylogeny (Thomas et al. 2004) including all extant members of the order provides a strong phylogenetic framework for comparative studies.
Here, we apply a maximum-likelihood phylogenetic technique to compare rates of evolution in clades defined by a dichotomous trait based on a Brownian motion model of trait evolution following Pagel (1997, 1999); see also Freckleton et al. (2002). We apply this method to test for differences in the rate of phenotypic evolution of sexually selected traits between precocial and semi-precocial species of the diverse avian order Charadriiformes. Specifically, we investigate the influence of developmental mode on mating and parental care strategies, and on secondary sexual traits including sexual size dimorphism (SSD) and male display agility (Székely et al. 2004).
2. Material and methods
(a) Comparing rates of evolution
Generalized least squares (GLS) is a statistical method that can be applied to phylogenetic problems to control for non-independence among species by reference to a variance–covariance matrix of the expected similarity between species (Pagel 1997, 1999; Freckleton et al. 2002). Under a constant-rate Brownian model of character evolution, the amount of phenotypic change is expected to be proportional to time (Felsenstein 1985). Thus, the expected covariance of any two species is proportional to the sum of their shared branch lengths in the phylogeny.
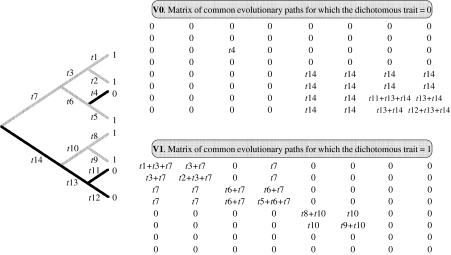
We extend Pagel's (1999) model to test the null hypothesis of no difference in evolutionary rates in two parts of the phylogeny. We consider the case of differences associated with the expression of a discrete, binary trait (states 0 and 1), although the method we describe could also be applied to the simpler case of two monophyletic or paraphyletic groups. A single variance–covariance matrix can only describe the covariance among species if we assume constant variance across the phylogeny (a single rate of trait evolution). To test for differences in rates of evolution in two parts of the phylogeny, two matrices are required; one matrix representing the expected covariance associated with the trait in state 0 (V0), and the other representing the expected covariance associated with the trait in state 1 (V1; see figure 1 for an example). This requires the reconstruction of ancestral states for the binary trait in order to partition the phylogeny. Our method is applicable to any form of ancestral state reconstruction (e.g. maximum-parsimony, maximum-likelihood, Bayesian analysis). The standard, single-rate variance–covariance matrix, V, is given by the sum of V0 and V1. If the null-model (constant Brownian variance across the phylogeny) is correct, then V0+V1 will provide the best fit to the data and phylogeny. If the variance differs in the two parts of the phylogeny, then the matrices must be scaled accordingly. We introduce the parameter θ such that V=V0+θV1. The maximum-likelihood value of θ is defined as the transformation of the phylogeny that makes the trait data best fit the Brownian motion model. The derivation and maximum-likelihood estimation of θ follow that for Pagel's (1999) λ (see Freckleton et al. (2002) for the full derivation of the likelihood model). A similar test is described by Collar et al. (2005). Their implementation differs slightly in that the method described by Collar et al. can only be applied to subtrees of a phylogeny (paraphyletic and monophyletic clades), whereas we apply our method to discrete traits distributed polyphyletically.
Figure 1.
Constructing variance–covariance matrices from a phylogeny. There are three species with a dichotomous trait in state 0 and five species in state 1. The branch lengths are shown as t1, t2, t3, … etc. The first matrix (V0) defines paths for which the trait=0 and contains all the shared path lengths for each species for which the trait=0, otherwise the entries are zero. Similarly, the second variance–covariance matrix (V1) contains all the shared path lengths for which the trait=1, otherwise the entries are zero. The usual variance–covariance matrix of the full phylogeny is the sum of these (V=V0+V1).
The estimate of θ can be tested to determine whether the data fit the constant variance model or not. If L(θ∧) is the log-likelihood at the maximum-likelihood value of θ, and L(θ′) is the log-likelihood at an alternative value of θ′, then the log-likelihood ratio
will be asymptotically χ2-distributed with one degree of freedom under the null hypothesis that θ∧=θ′. A maximum-likelihood value of θ that does not differ significantly from 1 supports the (null) constant variance model. A value of θ>1 can arise if there is greater variance in V1 than in V0 since the branch lengths (covariance) among matrix V1 must be stretched to bring the two parts of the tree into common variance. A value of θ<1 indicates the reverse.
(b) Data and phylogeny
We collated data on developmental mode, parental care, mating behaviour, body size, SSD and display agility for 203 species of shorebirds (see electronic supplementary material for sources). Developmental mode was scored according to the feeding behaviour of hatchlings. Species in which the chicks fed themselves within hours of hatching were classified as precocial, whereas species in which the chicks were reliant on the parents for feeding until fledging were classified as semi-precocial. Eight species from our sample switched from parental feeding to self-feeding within one to two weeks of hatching (i.e. prior to fledgling, these were; Cursorius coromandelicus, C. cursor, Gallinago gallinago, Philomachus pugnax, Rostratula benghalensis, Scolopax minor, S. rusticola, Xema sabini). To assess the impact of these species we carried out our analyses first with these eight taxa classified as semi-precocial, second with them classified as precocial and third with them excluded (see below). Parental care and social mating were scored independently for males and females. Male social mating system was scored towards increased frequency of polygamy by three observes blind to species names, based on descriptions in the literature: 0, monogamous; 1, rare polygyny (less than 1% or only anecdotal reports of polygyny); 2, occasional polygyny (1–5%, polygyny is known to occur but it is infrequent); 3, moderate polygyny (6–20%, polygyny is well known but is not regarded as typical of the species); 4, frequent polygyny (greater than 20%, polygyny is considered the main mating system for the species). Female social mating system was scored similarly but with respect to frequency of polyandry (from monogamy (0) to frequent polyandry (4)). The duration of care for each sex was scored following Székely & Reynolds (1995) and Reynolds & Székely (1997). Male displays are expected to reflect sexual selection, with ground displays associated with male–male competition, and acrobatic aerial displays associated with female mate choice (Székely et al. 2000, 2004). Male display agility was scored following Székely et al. (2000, 2004). SSD in wing length was calculated as log(male wing length/female wing length). SSD in body mass was calculated as log(male body mass/female body mass). Finally, we used log-transformed body mass (in grams) and wing length (in millimetres) as measures of body size. Note that we did not investigate rates of extra pair paternity since data for shorebirds are limited (n=14 species; Griffith et al. 2002).
All analyses were conducted using a dated supertree phylogeny of shorebirds (Thomas et al. 2004) since this is the only complete phylogeny available for the extant members of the order.
(c) Analyses
We reconstructed the ancestral states of developmental mode across the supertree phylogeny (Thomas et al. 2004) using maximum-parsimony implemented in MacClade 4 (Maddison & Maddison 2001). To account for the uncertainty of developmental mode classification in eight species, we reconstructed ancestral states first with these species classed as semi-precocial, second with them classed as precocial and third with them excluded. With the exception of SSD in wing length, the results are fully consistent across analyses. We report the results of the semi-precocial coding, except where the maximum-likelihood estimate of θ qualitatively differs between analyses. Full results of the alternative analyses are available in the electronic supplementary material. The parsimony reconstruction was used to partition the phylogeny into the two matrices V0 and V1 as described in figure 1. In our analyses, the matrix V0 refers to the branch lengths shared among precocial species, and V1 refers to the branch lengths shared among semi-precocial species.
The maximum-likelihood value of θ was estimated for each trait, and the likelihood ratio test was used to test if the maximum-likelihood value differed significantly from θ=1. In addition, we report the phylogenetically corrected mean and 95% confidence intervals for semi-precocial and precocial taxa for each trait. All analyses were performed with R 2.1.0 (Ihaka & Gentleman 1996) using code written by R.P.F.
3. Results
For the trees used in the analysis, we first conducted simulation analysis to verify that the θ statistic performed statistically acceptably. Under a Brownian model with θ=1, we found that in all cases the Type I error rates (i.e. rejecting the null hypothesis of θ=1) were very close to the 5% level. For values of θ≠1, we found no evidence of bias. Some of our data are ordinal data, rather than continuous. For these data we conducted an additional simulation study (see electronic supplementary material). Ordinal traits have elevated Type I error rates but note that this did not qualitatively alter any of our results.
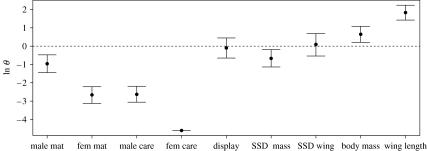
The maximum-likelihood estimate of θ was significantly less than 1 for male-mating system, female-mating system, male care, female care and SSD in body mass (see table 1 and figure 2), indicating that each of these traits has evolved at a higher rate among precocial taxa than semi-precocial taxa. The respective mean trait values for semi-precocial and precocial taxa suggest that the differences in rates are not dependent on the mean value of each trait (table 1). For instance, the mean value for SSD in body mass is higher among semi-precocial taxa, but rates are higher in precocial taxa, whereas both the mean value and rate of evolution are higher for body mass among semi-precocial.
Table 1.
Maximum-likelihood estimates of θ and comparison of phylogenetically corrected mean trait values.
| θ | χ2 | p | semi-precocial taxa mean±95% confidence intervals | precocial taxa mean±95% confidence intervals | |
|---|---|---|---|---|---|
| male mating system | 0.383 | 14.736 | <0.001 | 0.928±0.037 | −0.711±0.062 |
| female mating system | 0.070 | 108.263 | <0.001 | 0.973±0.014 | 0.592±0.053 |
| male care | 0.072 | 104.238 | <0.001 | 5.634±0.026 | 7.541±0.101 |
| female care | 0.010 | 426.746 | <0.001 | 6.331±0.007 | 4.731±0.077 |
| display agility | 0.907 | 0.123 | 0.726 | 0.631±0.036 | 0.736±0.038 |
| SSD body mass | 0.513 | 7.408 | 0.006 | 0.005±0.002 | −0.056±0.003 |
| SSD wing length | 1.101 | 0.092 | 0.762 | 0.006±0. 001 | −0.018±0.001 |
| body mass | 1.911 | 8.260 | 0.004 | 2.436 ± 0.014 | 2.144±0.010 |
| wing length | 6.253 | 66.292 | <0.001 | 2.293±0.009 | 2.202±0.004 |
Figure 2.
Ln maximum-likelihood estimates of θ with 95% confidence intervals. Note that all values are ln-transformed for graphical purposes only, hence values of θ<0 indicate rates of evolution are higher among precocial shorebirds and values of θ>0 indicate rates of evolution are higher in semi-precocial taxa.
The maximum-likelihood estimate of θ did not differ significantly from 1 for either male display agility or SSD in wing length (table 1 and figure 2). However the latter result is dependent on the coding of the eight taxa with uncertain developmental mode. When scored as precocial, the maximum-likelihood estimate of θ differs from 1 (θ=0.451, χ2=14.340, p<0.001). Removal of the eight taxa with uncertain states suggests that θ does not differ from 1 (θ=0.944, χ2=0.070, p=0.791).
The maximum-likelihood estimate of θ was significantly larger than 1 for both body mass and wing length (table 1, figure 2), indicating that rates of evolution of both traits are higher among semi-precocial taxa.
4. Discussion
(a) Developmental mode and rates of phenotypic evolution
Our phylogenetic analyses strongly suggest that developmental mode influences phenotypic evolution. First, rates of diversification of male and female mating systems, and male and female parental care, are unequivocally higher among species with precocial young than those with semi-precocial young. This indicates that semi-precocial development constrains parental and breeding behaviour and that this is because parents are tied to food provisioning in these taxa. Thus, our results are consistent both with Lack's (1968) observations and with recent comparative analyses of developmental mode (Temrin & Tullberg 1995; Thomas & Székely 2005). Developmental mode is therefore a major determinant of the costs of desertion. However, the observed pattern of parental care and breeding system is likely also to be due to the potential benefits of desertion accrued through increased mating opportunities (Bennett & Owens 2002). We suggest that this is consistent with a phylogenetic hierarchy of avian life-histories and mating systems whereby some lineages are predisposed to certain traits or behaviours by features of their life-history that evolved deep within their evolutionary history, but ecological facilitation and social interactions determine the actual expression of these traits (Owens & Bennett 1995, 1997).
Variation in the intensity of sexual conflict and sexual selection, as evidenced by variation in mating system and parental care, is expected to result in concomitant variation in secondary sexual characteristics. We investigated three traits that have previously been shown to be related to sexual selection in shorebirds (Székely et al. 2000, 2004) with equivocal results. That we found rate differences in one measure of SSD (sexual dimorphism in body mass) but equivocal results in a second measure (sexual dimorphism in wing length) is unexpected. However, this result may be explained by the large influence exerted by the small number of taxa with uncertain developmental mode. These eight species include the most extreme example of wing length dimorphism among shorebirds (the ruff Philomachus pugnax). Rates of evolution of sexual dimorphism in wing length are significantly higher among precocial taxa only when these taxa are treated as precocial, if they are treated as semi-precocial. If they are excluded, no difference is found. Coupled with the lack of any difference in the rate of evolution of male display agility, these results put into question the influence of developmental mode on the rate of evolution of secondary sexual traits. Previous studies have demonstrated that sexual selection, rather than niche differentiation, explains SSD in shorebirds, although the relationship is not straightforward since the direction and degree of SSD are also dependent on the type of male display. Ground displays are associated with male-biased dimorphism whereas female-biased dimorphism is associated with aerial acrobatic displays as well as polyandrous mating systems (Székely et al. 2000, 2004). This relationship may cloud the influence of other traits on SSD. In addition, our scoring of display was necessarily crude (limited to three character states) since detailed studies of display are limited. Nonetheless, despite these problems, the higher rates of evolution of SSD in body mass were unequivocal. Moreover, while one might predict greater diversity of SSD in clades with a wider range of mean body size by chance alone, this does not apply to our results since semi-precocial taxa, rather than precocial, have higher rates of evolution in body size for both body mass and wing length.
Greater variation in both body size measures among semi-precocial taxa compared to precocial taxa was unexpected. However, this may not necessarily be a consequence of developmental mode differences. Rather, we suggest that it may be attributed to the presence of gulls, terns and alcids in the semi-precocial sample. Many of these taxa occupy coastal–marine habitats that are not represented among our precocial sample. This major habitat shift may open up a range of ecological niches, particularly with regard to feeding, which may consequently allow body size to diversify. These predictions require formal testing.
(b) Comparative analyses of phenotypic traits
The novel method implemented in our analyses adds to a growing body of phylogenetic comparative methods that use GLS (e.g. Pagel 1997, 1999; Martins & Hansen 1997; Freckleton et al. 2002). Existing methods for comparing rates of trait evolution using independent contrasts (Garland 1992) and maximum-likelihood (Collar et al. 2005) were limited to comparing either two monophyletic groups, or a monophyletic group against a paraphyletic group. Consequently, they cannot be applied to comparisons of groups defined by a discrete trait, if the trait has more than a single independent origin across the phylogeny. This difference appears to be mainly one of implementation and parameterization; examination of the code used by Collar et al. (2005) indicates that their method could be readily adapted to estimate θ for polyphyletic traits. By incorporating ancestral states in the discrete trait, our method allows rates to be compared among such polyphyletic groups. A caveat to this is that errors in the ancestral state reconstruction will result in the incorrect partitioning of the variance covariance matrix. Such errors are analogous to errors in the phylogeny since both directly influence the expected covariance between species. Both types of error can in principle be accounted for by considering multiple phylogenetic hypotheses and ancestral reconstructions.
5. Conclusions
Taken together, by emancipating the parents from feeding the offspring, our results strongly suggest that precociality has important implications for the divergence of parental care and mating behaviours. Moreover, it promotes the rapid diversification of some secondary sexual characters, notably SSD in body mass. Hence, the demands of the young are a key component of the evolution of both sexual conflict and sexual selection in shorebirds.
Acknowledgments
We thank Fiona Sharpe for scoring the mating system variables. G.H.T was supported by a University of Bath Research Studentship. R.P.F. is a Royal Society University Research Fellow.
Supplementary Material
1. A table of all sources of data; 2. Results of simulations on theta to assess type I error; and 3. Additional results based on precocial coding and uncertain taxa removed.
References
- Bennett P.M, Owens I.P.F. Oxford University Press; Oxford, UK: 2002. Evolutionary ecology of birds: life history, mating system and extinction. [Google Scholar]
- Chapman T, Arnqvist G, Bangham J, Rowe L. Sexual conflict. Trends Ecol. Evol. 2003;18:41–47. 10.1016/S0169-5347(02)00004-6 [Google Scholar]
- Collar D.C, Near T.J, Wainright P.C. Comparative analysis of morphological diversity: does disparity accumulate at the same rate in two lineages of centrarchid fishes? Evolution. 2005;59:1783–1794. [PubMed] [Google Scholar]
- Erckmann W.J. The evolution of polyandry in shorebirds: an evaluation of hypotheses. In: Wasser S.K, editor. Social behavior of female vertebrates. Academic Press; New York, NY: 1983. pp. 113–168. [Google Scholar]
- Felsenstein J. Phylogenies and the comparative method. Am. Nat. 1985;125:1–15. doi: 10.1086/703055. 10.1086/284325 [DOI] [PubMed] [Google Scholar]
- Freckleton R.P, Harvey P.H, Pagel M. Phylogenetic analysis and comparative data: a test and review of the evidence. Am. Nat. 2002;160:712–726. doi: 10.1086/343873. 10.1086/343873 [DOI] [PubMed] [Google Scholar]
- Garland T., Jr Rate tests for phenotypic evolution using phylogenetically independent contrasts. Am. Nat. 1992;140:509–519. doi: 10.1086/285424. 10.1086/285424 [DOI] [PubMed] [Google Scholar]
- Griffith S.C, Owens I.P.F, Thuman K.A. Extra pair paternity in birds: a review of interspecific variation and adaptive function. Mol. Ecol. 2002;11:2195–2212. doi: 10.1046/j.1365-294x.2002.01613.x. 10.1046/j.1365-294X.2002.01613.x [DOI] [PubMed] [Google Scholar]
- Houston A.I, Székely T, McNamara J.M. Conflict between parents over care. Trends Ecol. Evol. 2005;20:33–38. doi: 10.1016/j.tree.2004.10.008. 10.1016/j.tree.2004.10.008 [DOI] [PubMed] [Google Scholar]
- Ihaka R, Gentleman R. A language for data analysis and graphics. J. Comput. Graph. Stat. 1996;5:299–314. [Google Scholar]
- Lack D. Methuen and Co; London, UK: 1968. Ecological adaptations for breeding in birds. [Google Scholar]
- Ligon J.D. Oxford ornithology series. Oxford University Press; Oxford, UK: 1999. The evolution of avian breeding systems. [Google Scholar]
- Maddison D.R, Maddison W.P. Sinauer Associates; Sunderland, MA: 2001. MacClade 4: analysis of phylogeny and character evolution. Version 4.03. [Google Scholar]
- Martins E.P, Hansen T.F. Phylogenies and the comparative method: a general approach to incorporating phylogenetic information into the analysis of interspecific data. Am. Nat. 1997;149:646–667. 10.1086/286013 [Google Scholar]
- Orians G.H. On the evolution of mating systems in birds and mammals. Am. Nat. 1969;103:589–603. 10.1086/282628 [Google Scholar]
- Owens I.P.F, Bennett P. Ancient ecological diversification explains life-history variation among living birds. Proc. R. Soc. B. 1995;261:227–232. [Google Scholar]
- Owens I.P.F, Bennett P. Variation in mating systems among birds: ecological basis revealed by hierarchical comparative analysis of mate desertion. Proc. R. Soc. B. 1997;264:1103–1110. 10.1098/rspb.1997.0152 [Google Scholar]
- Pagel M. Inferring evolutionary processes from phylogenies. Zool. Scripta. 1997;26:331–348. 10.1111/j.1463-6409.1997.tb00423.x [Google Scholar]
- Pagel M. Inferring the historical patterns of biological evolution. Nature. 1999;401:877–884. doi: 10.1038/44766. 10.1038/44766 [DOI] [PubMed] [Google Scholar]
- Pitelka F.A, Holmes R.T, MacLean S.F., Jr Ecology and evolution of social organization in Arctic sandpipers. Am. Zool. 1974;14:185–204. [Google Scholar]
- Reynolds J.R, Székely T. The evolution of parental care in shorebirds: life histories, ecology, and sexual selection. Behav. Ecol. 1997;8:126–134. [Google Scholar]
- Starck J.M, Ricklefs R.E. Patterns of development: the altricial–precocial spectrum. In: Starck J.M, Ricklefs R.E, editors. Avian growth and development: evolution within the altricial–precocial spectrum. Oxford University Press; Oxford, UK: 1998. pp. 3–30. [Google Scholar]
- Székely T, Reynolds J.D. Evolutionary transitions in parental care in shorebirds. Proc. R. Soc. B. 1995;262:57–64. [Google Scholar]
- Székely T, Webb J.N, Houston A.I, McNamara J.M. An evolutionary approach to offspring desertion in birds. Curr. Ornithol. 1996;13:271–330. [Google Scholar]
- Székely T, Reynolds J.D, Figuerola J. Sexual size dimorphism in shorebirds, gulls, and alcids: the influence of sexual and natural selection. Evolution. 2000;54:1404–1413. doi: 10.1111/j.0014-3820.2000.tb00572.x. [DOI] [PubMed] [Google Scholar]
- Székely T, Freckleton R.P, Reynolds J.D. Sexual selection explains Rensch's rule of size dimorphism in shorebirds. Proc. Natl Acad. Sci. USA. 2004;101:12 224–12 227. doi: 10.1073/pnas.0404503101. 10.1073/pnas.0404503101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temrin H, Tullberg B.S. A phylogenetic analysis of the evolution of avian mating systems in relation to altricial and precocial young. Behav. Ecol. 1995;6:296–307. [Google Scholar]
- Thomas, G.H. 2004 Sexual conflict, ecology and breeding systems in shorebirds: phylogenetic analyses. Unpublished Ph.D. thesis.
- Thomas G.H, Székely T. Evolutionary pathways in shorebird breeding systems: sexual conflict, parental care, and chick development. Evolution. 2005;59:2222–2230. [PubMed] [Google Scholar]
- Thomas G.H, Wills M.A, Székely T. A supertree approach to shorebird phylogeny. BMC Evol. Biol. 2004;4:28. doi: 10.1186/1471-2148-4-28. 10.1186/1471-2148-4-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
1. A table of all sources of data; 2. Results of simulations on theta to assess type I error; and 3. Additional results based on precocial coding and uncertain taxa removed.