Abstract
Eusocial organisms are characterized by cooperative brood care, generation overlap and reproductive division of labour. Traits associated with eusociality are most developed in ants, termites, paper wasps and corbiculate bees; the fossil record indicates that each of these advanced eusocial taxa evolved in the Late Cretaceous or earlier (greater than 65 Myr ago). Halictid bees also include a large and diverse number of eusocial members, but, in contrast to advanced eusocial taxa, they are characterized by substantial intra- and inter-specific variation in social behaviour, which may be indicative of more recent eusocial evolution. To test this hypothesis, we used over 2400 bp of DNA sequence data gathered from three protein-coding nuclear genes (opsin, wingless and EF-1a) to infer the phylogeny of eusocial halictid lineages and their relatives. Results from relaxed molecular clock dating techniques that utilize a combination of molecular and fossil data indicate that the three independent origins of eusociality in halictid bees occurred within a narrow time frame between approximately 20 and 22 Myr ago. This relatively recent evolution helps to explain the pronounced levels of social variation observed within these bees. The three origins of eusociality appear to be temporally correlated with a period of global warming, suggesting that climate may have had an important role in the evolution and maintenance of eusociality in these bees.
Keywords: eusociality, sweat bees, Halictidae, social insects, behavioural evolution
1. Introduction
The evolution of eusociality in numerous lineages has intrigued biologists since the time of Darwin and is generally considered to mark one of the major evolutionary transitions in the history of life (Maynard Smith & Szathmáry 1995). Eusocial animal societies are characterized by cooperative brood care, generation overlap and a division of labour in which sterile individuals work for the direct reproduction of others (Wilson 1971; Michener 1974). These societies typically display a high degree of reproductive skew where relatively few individuals monopolize reproductive output while the remaining mature individuals engage in alloparental care (Crespi & Yanega 1995; Gadagkar 1995; Sherman et al. 1995). Beginning with the seminal contribution by Hamilton (1964) on kin selection, a number of ideas have been developed that account for the evolution of eusociality by means of natural selection, including inclusive fitness theory, protected invasion, parental manipulation, assured fitness returns, head start and semisocialism/mutualism hypotheses (Crespi 1996; Dugatkin 1997). But while much attention has been given to how eusociality evolved, the implications of when eusociality evolved and the historical context of this evolution have remained largely unexamined.
Eusocial behaviour involves a complex suite of adaptations including alloparental care, reproductive division of labour, caste polymorphism and polyphenism, complex communication and cooperative activities such as foraging, nest defence and construction, and brood rearing (Wilson 1971; Michener 1974). Some have hypothesized that the longer the genetic, behavioural and morphological traits associated with eusociality exist in a lineage, the more integrated they become such that a ‘point of no return’ in social evolution is eventually reached and members of the lineage are unable to revert back to a solitary condition (Wilson 1971; Wilson & Hölldobler 2005). The most advanced eusocial organisms indeed are quite old (table 1) and have not undergone reversal to solitary life. Ants and termites can be argued to be the two most successful eusocial taxa, as measured by many criteria including total biomass, number of species, behavioural diversity and ecological interactions (Wilson 1990). Eusociality in these two groups is tightly integrated, usually resulting in extreme morphological differentiation between castes, and eusocial traits are never lost in free-living (non-parasitic) species (Thorne & Traniello 2003). The available fossil evidence indicates Mesozoic origins of eusociality for both groups: in the Middle Cretaceous for ants and Early Cretaceous or Late Jurassic for termites (table 1). Two other ecologically dominant eusocial taxa, vespid wasps and corbiculate bees, also show high levels of caste specialization and eusocial trait integration in some species (e.g. hornets and honey bees). Available fossil evidence for these groups likewise argues for origins sometime in the Cretaceous (table 1).
Table 1.
Estimated ages of origin for major lineages of eusocial insects based on the fossil record. (Only lineages with relevant known fossil evidence are included in this list.)
| lineage | age (Myr BP) | references |
|---|---|---|
| corbiculate bees | ≥65 | Michener & Grimaldi 1988; Engel 2000b, 2001a,b; Michener 2000 |
| Formicidae (ants) | 100–120 | Grimaldi & Agosti 2000; Dlussky et al. 2004; Nel et al. 2004; Engel & Grimaldi 2005 |
| Isoptera (termites) | ≥130 | Emerson 1968; Thorne et al. 2000 |
| Vespidae (wasps) | ≥65 | Wenzel 1990; Carpenter 1993 |
In contrast to these advanced eusocial groups, halictid bees (sweat bees) display immense diversity and flexibility in their expression of eusociality. The family Halictidae comprises over 3500 described species, approximately 830 of which are presumed to be eusocial (Michener 2000). Eusocial halictid bees are widespread, occurring on every continent where bees are found. Colonies range in size and complexity from small, annual colonies with a single queen and just a few workers to enormous, perennial colonies with a single queen and over 500 workers (Wilson 1971; Michener 1974). Previous phylogenetic studies indicate that eusociality evolved three times within this group, with multiple reversals back to simpler modes of eusociality or completely solitary nesting within each of the three primarily eusocial clades (Packer 1991; Wcislo & Danforth 1997; Danforth 2002). Many eusocial halictid species have non-eusocial relatives among closely related species or even within populations of the same species (Sakagami & Munakata 1972; Packer 1990; Plateaux-Quénu 1992; Wcislo 1997; Yanega 1997; Soucy & Danforth 2002; Cronin & Hirata 2003; Richards et al. 2003), a situation never found in advanced eusocial taxa.
The social diversity and flexibility found in halictid bees suggest that eusociality may have originated more recently than it has in advanced eusocial taxa. To test this hypothesis, we estimated the antiquity of eusociality in this group using a combination of molecular phylogenetic and fossil data. Our age estimates yield strong support for the recent evolution of halictid eusociality, and also suggest that the three origins of eusociality in this group occurred at approximately the same time.
2. Material and methods
(a) Data collection and taxon sampling
We combined previously published data from three nuclear genes, long-wavelength rhodopsin (opsin), wingless and the F2 copy of elongation factor-1a (EF-1a; Danforth et al. 1999, 2004; Danforth 2002), with new data gathered from nine additional species (using the same molecular protocols described in these papers). Our new taxa were selected to increase sampling of key eusocial species. We thus included a total of 66 species within the bee family Halictidae, with representatives from all subfamilies (Rophitinae, Nomiinae, Nomioidinae and Halictinae), tribes (Halictini and Augochlorini) and most genera (all taxonomy follows Michener (2000) unless otherwise noted). Locality data and GenBank accession numbers can be found in table 2 in the electronic supplementary material. Previous studies have revealed three independent origins of eusociality within Halictinae: once in the common ancestor of the monophyletic group that includes Augochlora and Augochlorella, once in the common ancestor of the genus Halictus and once in the common ancestor of the Hemihalictus series (i.e. weak-veined Lasioglossum; Danforth 2002). We sampled broadly within all three eusocial lineages, including two eusocial genera within Augochlorini (Augochlora and Augochlorella), all three subgenera of Halictus and eight representatives of the Hemihalictus series. From these predominantly eusocial clades we also included the solitary species Halictus (Halictus) quadricinctus and Lasioglossum (Hemihalictus) lustrans, as well as the socially polymorphic species Lasioglossum (Evylaeus) calceatum.
(b) Phylogenetic methods
Alignments were generated using Clustal W provided by the Lasergene DNASTAR program MegAlign and adjusted manually to correct obvious alignment errors. We used published sequences for Apis mellifera to establish reading frames and intron/exon boundaries. The two entire opsin introns and a total of 137 bp within the two EF-1a introns were unalignable and excluded from all analyses. The resulting dataset consisted of 1526 nucleotide sites from EF-1a (592 parsimony informative), 489 sites from opsin (176 parsimony informative) and 405 sites from wingless (125 parsimony informative), totalling 2420 sites (893 parsimony informative). We performed Bayesian phylogenetic analyses using the program MrBayes 3.0 (Huelsenbeck & Ronquist 2001) under a general time-reversible, site-specific rate (GTR+SSR) model with nine discrete rate categories corresponding to the three codon positions within each gene: wingless, nt1, nt2 and nt3; opsin, nt1, nt2 and nt3; EF-1a, nt1, nt2, nt3 and a 10th category for the EF-1a introns. We ran four simultaneous chains saving trees every 100 generations for 1 000 000 generations (a total of 10 000 trees). We plotted the likelihood scores against generation time to identify convergence and discarded as burnin the first 2000 trees. We computed the majority rule consensus of the remaining 8000 trees using PAUP* v. 4.0b10 (Swofford 2002). The topology from this analysis was verified using other models of molecular evolution (Kimura two-parameter+SSR, Hasegawa-Kishino-Yano+SSR, GTR+invariant sites+gamma) with essentially identical results. We also performed equal weights parsimony analyses using PAUP*. Following Danforth et al. (2003), gaps within introns were coded as a fifth state, as most were less than 5 bp in length. We conducted heuristic searches with tree bisection and reconnection (TBR) branch swapping and 100 random addition replicates. We calculated non-parametric bootstrap support values using 1000 replicates of TBR searches with 10 random sequence additions per replicate.
(c) Dating methods
A likelihood ratio test indicated violation of rate consistency among lineages (p≪0.001) and, hence, the lack of a molecular clock. Using the tree topology resulting from our Bayesian phylogenetic analysis under the GTR+SSR model, we estimated branch lengths and divergence dates using the MULTIDIVTIME Bayesian divergence dating method (Thorne & Kishino 2002). This method accommodates molecular clock violations by allowing rate variation both among lineages and among genes, accomplished by implementing a stochastic model for rate change among branches in the phylogeny. The means and variances of branch lengths were estimated separately for each gene under an F84+gamma model (model complexity is limited by the dating program) with parameter values obtained from the program PAML (Yang 1997). We used Rophitinae as the outgroup when estimating branch lengths because members of this subfamily are consistently placed as the sister group to all other halictid subfamilies in morphological (Pesenko 1999) and molecular (Danforth et al. 2004) studies. We explored several different a priori means and variances on the resulting root node (i.e. the most recent common ancestor of Nomiinae, Nomioidinae and Halictinae). The oldest date, 100±20 Myr BP, reflects the consensus that bees originated sometime in the early to mid Cretaceous, coincident with the period of rapid angiosperm diversification (Crepet 1996; Grimaldi 1999; Engel 2001a) and, therefore, represents a reasonable maximum prior age for a node nested well within the phylogeny of bees. The most recent priors, 65±20 and 65±40 Myr BP, were chosen based on the oldest fossil bee, Cretotrigona prisca, estimated to date to the Late Cretaceous (Michener 2000; Engel 2001a), as well as the available fossil evidence within Halictidae (see below). We also considered a prior age of 80±20 Myr BP, which falls between these two extremes.
Divergence times were also estimated using the penalized likelihood approach provided by the program r8s 1.7 (Sanderson 2002), another relaxed molecular clock dating technique. This method uses semiparametric rate-smoothing to optimize rate changes among branches. For this analysis, the mean branch lengths for all genes combined were estimated on the Bayesian topology under a GTR+I+G model using PAUP*. The mean values of the priors discussed above for the Bayesian analysis were instead used to fixed the root node in the r8s analysis.
In both types of dating analyses, we further constrained three internal nodes with minimum ages based on the bee fossil record. The root node of Augochlorina (a subtribe of Augochlorini; sensu Engel 2000a) was calibrated with a minimum age of 23 Myr BP based on several fossils from this group in Dominican amber (Engel 2000a). The root node of Caenohalictini (sensu Danforth et al. 2004) was calibrated with the same minimum age using the fossil Eickwortapis dominicana from Dominican amber (Michener & Poinar 1996). Finally, the root node for Halictini (sensu Danforth et al. 2004) was calibrated with a minimum age of 42 Myr BP based on the Baltic amber fossil Electrolictus antiquus (Engel 2001a). Furthermore, we conducted secondary analyses in which we removed some of these fossil constraints in order to test the influence of each calibration on the estimated ages.
3. Results
Bayesian analysis resulted in a phylogeny with virtually all nodes showing posterior probabilities of 100, including each of the three nodes representing eusocial origins (figure 1). Also monophyletic with posterior probabilities of 100 were each of the three ingroup subfamilies (Nomiinae, Nomioidinae, Halictinae), the two tribes within Halictinae and all included genera except the genus Ruizantheda, whose paraphyly relative to Pseudagapostemon is strongly supported by the data. A close relationship between these two genera has been noted previously (Michener 2000). An equal weights parsimony analysis of the same data yielded three most parsimonious trees whose strict consensus was almost completely congruent with the Bayesian tree. The two analyses differed topologically only in relationships among genera of Rophitinae (which was not used for the dating analysis) and the placement of Lasioglossum figueresi within the Hemihalictus series. Parsimony bootstrap values were generally very high throughout the tree, with only eight of 50 internal nodes receiving values below 75% (figure 3 and table 3 in the electronic supplementary material). Each of the three nodes representing eusocial origins received bootstrap values of 100.
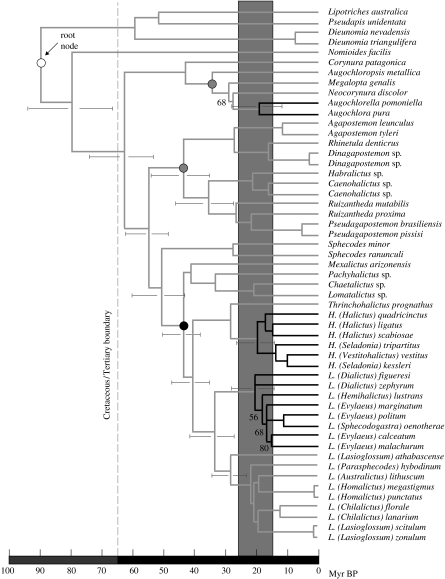
Figure 1.
Chronogram for the three eusocial lineages of halictid bees (black branches) derived from Bayesian phylogenetic and dating methods. The taxa H. quadricinctus, L. calceatum and L. lustrans have secondarily reverted to a socially polymorphic or solitary condition. All resolved nodes in the tree received 100% posterior support except the four shown with their respective values. The open circle indicates the root node set in this analysis with an a priori age of 80±20 Myr BP. Shaded circles indicate Dominican amber fossil minimum-age constraints (23 Myr BP) and the solid circle indicates Baltic amber fossil minimum-age constraint (42 Myr BP). Shaded vertical bar represents the Late Oligocene warming and Mid-Miocene climatic optimum. Error bars are 95% credibility intervals. Abbreviations: H, Halictus; L, Lasioglossum.
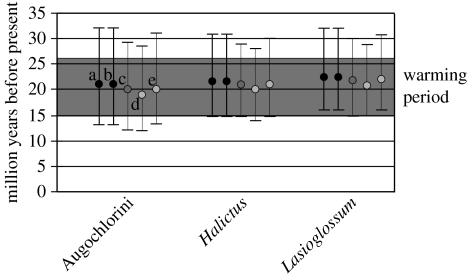
Bayesian dating analyses generated mean estimates for the antiquity of eusociality at approximately 20–22 Myr BP (figure 1). Specifically, the mean age estimates and 95% credibility intervals for these three eusocial clades were: 20 (12, 29) Myr BP for eusocial Augochlorini; 21 (15, 28) Myr BP for Halictus and 22 (15, 29) Myr BP for eusocial Lasioglossum. These estimates were robust to a range of a priori values for the age of the root node; the inferred age for each of the three eusocial lineages changed by only 1–2 Myr BP when the a priori root value was varied from 65 to 100 Myr BP (figure 2).
Figure 2.
Sensitivity analyses of the Bayesian dating method with means and 95% credibility intervals of the three eusocial lineages as inferred under the following a priori root node ages: a, 100±20 Myr BP; b, 100±20 Myr BP, including only the Baltic amber fossil calibration; c, 80±20 Myr BP; d, 65±20 Myr BP; and e, 65±40 Myr BP. Grey shaded bar, warming period.
The two Dominican fossils (23 Myr BP) provided conservative minimum-age constraints, as indicated by the estimated ages of 35 and 45 Myr BP, respectively, for their associated nodes after molecular dating (figure 1). Removal of these two calibrations, leaving only the Baltic fossil calibration remaining, generated identical age estimates (figure 2).
Results from the penalized likelihood dating analysis were consistent with those from the Bayesian dating analysis, yielding mean ages of approximately 15–25 Myr BP for all three eusocial clades, depending on the value fixed for the root node. At the lower bound of 65 Myr BP for the root node, mean estimates for the three eusocial clades ranged from 13.6 (Augochlorini) to 20.6 Myr BP (Halictus). At the upper bound of 100 Myr BP, mean estimates ranged from 18.4 (Augochlorini) to 24.6 Myr BP (Halictus).
4. Discussion
Our results demonstrate that the origins of eusociality in halictid bees were much more recent compared to other major groups of eusocial insects. Fossil evidence for corbiculate bees, vespid wasps, ants and termites shows that each of these lineages originated sometime in the Mesozoic (greater than 65 Myr ago), whereas our fossil-calibrated Bayesian dating analyses indicate that eusocial halictid lineages most likely originated approximately 20–22 Myr ago. The use of molecular dating is still in its infancy and may be subject to sources of inaccuracy including model selection, estimation of branch lengths, uncertainties in phylogenetic reconstruction and application of an incomplete fossil record (Thorne & Kishino 2005). Our results are robust to some possible sources of such error, although we recognize that, as in all molecular divergence dating studies, the precise dates may be subject to revision based upon refinement of techniques and future data such as new halictid fossils. However, it now appears quite evident that eusociality in halictids originated remarkably late compared to these other lineages of eusocial insects.
Another group of bees, the allodapines, also shows a wide range of social forms, from solitary to fully eusocial, with several types of presumed intermediate ‘subsocial’ forms as well (Wilson 1971; Michener 1974). Unfortunately, the lack of allodapine fossils precludes estimating the age of this group directly from the fossil record. A recent molecular phylogenetic study argues that eusociality evolved very early in this group and may be, in the authors' words, ‘ancient’ (Schwarz et al. 2003). It remains to be seen whether allodapine eusociality actually evolved in the Mesozoic as in the other major eusocial groups discussed above, or instead originated closer to the more recent dates for halictid bees.
The recent origins of halictid eusociality highlight the valuable role these bees can play in providing a more direct window into the origins of eusociality compared to more ancient eusocial lineages. Some characteristics of eusociality commonly found in ants, termites and other ancient eusocial taxa may not have had sufficient time to evolve or develop fully in halictid bees. Easily observable traits such as morphological caste differentiation and nest complexity appear more rudimentary in halictids. It is possible that more subtle eusocial phenomena, for example worker policing in which social cooperation is enforced by means of mutual monitoring (Ratnieks & Wenseleers 2005), may also be more transparent in eusocial halictids due to their recent origins. Furthermore, the general mechanisms behind the reproductive switch from a solitary to eusocial lifestyle are now beginning to be uncovered (Amdam et al. 2004). A partial bivoltine life cycle (two generations per year) in particular has been hypothesized to favour eusociality (Seger 1983) and is thought to be the pathway by which paper wasps evolved eusociality (Hunt & Amdam 2005). The recent origins of halictid eusociality have left these bivoltine mechanisms largely intact and functional in these bees, as revealed by the climatic sensitivity of the reproductive switch within some species (see below). Application of these models to halictids may be particularly fruitful.
As well as providing evidence for recent eusocial origins, our results indicate that eusociality evolved nearly simultaneously in three independent lineages, within the time frame of a few million years. This suggests that a common environmental factor might have triggered these evolutionary events. One such factor—climate—has already been shown to influence the expression of eusociality in extant halictid species. Some social halictid species inhabit warm, southerly regions, whereas closely related, solitary species live in cooler, temperate regions (Wcislo 1997). Furthermore, populations within some eusocial species revert to a solitary state in regions of cooler climate (Sakagami & Munakata 1972; Eickwort et al. 1996; Wcislo & Danforth 1997; Miyanaga et al. 1999; Richards 2001; Soucy 2002; Soucy & Danforth 2002; Cronin & Hirata 2003). This may occur because the growing season in colder regions is too short to support social populations that require sequential worker and reproductive broods. For example, Halictus rubicundus is distributed across Eurasia and in North America down to the southern border of the United States. In this species, eusocial behaviour is favoured in warmer areas of its range, while solitary behaviour is limited to cooler areas and higher elevations (Eickwort et al. 1996; Soucy 2002). The expression of eusociality in H. rubicundus is correlated with the number of days with snow on the ground, and evidence of genetic structuring between eusocial and solitary populations argues for an evolutionary response to these climatic influences (Soucy & Danforth 2002).
Because eusociality is both recent and labile in halictids, the same factors responsible for its maintenance may also be responsible for its origin. Our dating estimates allow us to begin to investigate the environmental factors affecting eusocial evolution over a historical timeframe. Models based on oxygen-isotope data from deep-sea cores indicate a pronounced global warming trend from approximately 26 to 15 Myr BP, during the late Oligocene warming and the Mid-Miocene climatic optimum (Mutti 2000; Zachos et al. 2001). The correlation between three independent origins of halictid eusociality and this period of global warming accords well with the contemporary influence of climate on the expression of eusociality in this group. We speculate that climatic changes may have been a critical factor in the evolution of eusociality in these bees. Any propensity in bee populations toward facultative eusociality in warmer regions of temperate areas, due to partial bivotinism or other mechanisms, could be intensified as the temperatures in those areas increased. This hypothesis applies reasonably well to Halictus and Lasioglossum, taxa inhabiting primarily Northern Hemisphere regions (Michener 2000) with substantial historical variation in climate. It seems less likely that global warming changes have affected a neotropical group such as the Augochlorini, although it is possible that temperature increases may have influenced populations at higher altitudes, where some such species now occur.
We would like to connect the historical biogeography of these bees with global climate trends, in order to determine the geographic location of these eusocial origins, but such a link remains difficult to establish based upon current knowledge. A recent biogeographic reconstruction suggests a Southern Hemisphere origin for halictid bees, with subsequent dispersal events into the Northern Hemisphere (Danforth et al. 2004). However, the alternative idea of an initial radiation in the Northern Hemisphere followed by repeated extinction events and colonizations into the Southern Hemisphere (Michener 1979) remains plausible.
Eusociality evidently did not evolve in halictid bees during other warming episodes, implying that if climate is indeed a critical factor, it must interact with other environmental and/or genetic components. The relative influence of environmental versus genetic factors on the origin of eusociality has long been a hotly debated topic (Evans 1977; Crespi 1996; Wilson & Hölldobler 2005). The multitude of factors that may favour eusocial evolution includes genetic systems and relatedness (e.g. haplodiploidy), phenotype (e.g. morphology and behaviour), ecology (e.g. nesting sites, food sources and natural enemies) and demography (e.g. survivorship and fecundity; Crespi & Choe 1997). The recent origins and extensive variability of halictid eusociality indicate that additional study of these bees may enable researchers to identify and disentangle these factors, leading to a richer understanding of the origins and evolution of social insect societies.
Acknowledgments
We thank E. Almeida, C. Michener, L. Packer, T. Schultz, P. Ward and two anonymous reviewers for helpful comments on the manuscript. This work was supported by grants from the National Science Foundation DEB-9815236 and DEB-0211701 to B.N.D, with travel funds from the Cornell International Agriculture Program. S.G.B is supported by National Science Foundation grant EF-0431330.
Footnotes
Present address: Department of Psychology, University of Connecticut, Storrs, CT, USA.
Supplementary Material
Voucher and GenBank codes, and summary of phylogenetic and dating results.
References
- Amdam G.V, Norberg K, Fondrk M.K, Page R.E., Jr Reproductive ground plan may mediate colony-level selection effects on individual foraging behavior in honey bees. Proc. Natl Acad. Sci. USA. 2004;101:11 350–11 355. doi: 10.1073/pnas.0403073101. 10.1073/pnas.0403073101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter J.C. Biogeographic patterns in the Vespidae (Hymenoptera): two views of Africa and South America. In: Goldblatt P, editor. Biological relationships between Africa and South America. Yale University Press; New Haven, CT: 1993. pp. 139–155. [Google Scholar]
- Crepet W.L. Timing in the evolution of derived floral characters: Upper Cretaceous (Turonian) taxa with tricolpate and tricolpate-derived pollen. Rev. Paleobot. Palynol. 1996;90:339–359. 10.1016/0034-6667(95)00091-7 [Google Scholar]
- Crespi B.J. Comparative analysis of the origins and losses of eusociality: causal mosaics and historical uniqueness. In: Martins E.P, editor. Phylogenies and the comparative method in animal behavior. Oxford University Press; Oxford, UK: 1996. pp. 253–287. [Google Scholar]
- Crespi B.J, Choe J.C. Explanation and evolution of social systems. In: Crespi B.J, Choe J.C, editors. Social behavior in insects and arachnids. Cambridge University Press; Cambridge, UK: 1997. pp. 499–524. [Google Scholar]
- Crespi B.J, Yanega D. The definition of eusociality. Behav. Ecol. 1995;6:109–115. [Google Scholar]
- Cronin A.L, Hirata M. Social polymorphism in the sweat bee Lasioglossum (Evylaeus) baleicum (Cockerell) (Hymenoptera, Halictidae) in Hokkaido, northern Japan. Insect. Soc. 2003;50:379–386. 10.1007/s00040-003-0693-1 [Google Scholar]
- Danforth B.N. Evolution of sociality in a primitively eusocial lineage of bees. Proc. Natl Acad. Sci. USA. 2002;99:286–290. doi: 10.1073/pnas.012387999. 10.1073/pnas.012387999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danforth B.N, Sauquet H, Packer L. Phylogeny of the bee genus Halictus (Hymenoptera: Halictidae) based on parsimony and likelihood analyses of nuclear EF-1α sequence data. Mol. Phylogenet. Evol. 1999;13:605–618. doi: 10.1006/mpev.1999.0670. 10.1006/mpev.1999.0670 [DOI] [PubMed] [Google Scholar]
- Danforth B.N, Conway L, Ji S. Phylogeny of eusocial Lasioglossum reveals multiple losses of eusociality within a primitively eusocial clade of bees (Hymenoptera: Halictidae) Syst. Biol. 2003;52:23–36. doi: 10.1080/10635150390132687. [DOI] [PubMed] [Google Scholar]
- Danforth B.N, Brady S.G, Sipes S.D, Pearson A. Single-copy nuclear genes recover Cretaceous-age divergences in bees. Syst. Biol. 2004;53:309–326. doi: 10.1080/10635150490423737. 10.1080/10635150490423737 [DOI] [PubMed] [Google Scholar]
- Dlussky G.M, Brothers D.J, Rasnitsyn A.P. The first Late Cretaceous ants (Hymenoptera: Formicidae) from southern Africa, with comments on the origin of the Myrmicinae. Insect Syst. Evol. 2004;35:1–13. [Google Scholar]
- Dugatkin L.A. Oxford University Press; Oxford, UK: 1997. Cooperation among animals: an evolutionary perspective. [Google Scholar]
- Eickwort G.C, Eickwort J.M, Gordon J, Eickwort M.A. Solitary behavior in a high-altitude population of the social sweat bee Halictus rubicundus (Hymenoptera: Halictidae) Behav. Ecol. Sociobiol. 1996;38:227–233. 10.1007/s002650050236 [Google Scholar]
- Emerson A.E. Cretaceous insects from Labrador 3. A new genus and species of termite (Isoptera: Hodotermitidae) Psyche (Camb.) 1968;74:276–289. [Google Scholar]
- Engel M.S. Classification of the bee tribe Augochlorini (Hymenoptera: Halictidae) Bull. Am. Mus. Nat. Hist. 2000a;250:1–89. 10.1206/0003-0090(2000)250%3C0001:COTBTA%3E2.0.CO;2 [Google Scholar]
- Engel M.S. A new interpretation of the oldest fossil bee (Hymenoptera: Apidae) Am. Mus. Novit. 2000b;3296:1–11. 10.1206/0003-0082(2000)3296%3C0001:ANIOTO%3E2.0.CO;2 [Google Scholar]
- Engel M.S. A monograph of the Baltic amber bees and the evolution of the Apoidea (Hymenoptera) Bull. Am. Mus. Nat. Hist. 2001a;259:1–192. 10.1206/0003-0090(2001)259%3C0001:AMOTBA%3E2.0.CO;2 [Google Scholar]
- Engel M.S. Monophyly and extensive extinction of advanced eusocial bees: insights from an unexpected Eocene diversity. Proc. Natl Acad. Sci. USA. 2001b;98:1661–1664. doi: 10.1073/pnas.041600198. 10.1073/pnas.041600198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel M.S, Grimaldi D.A. Primitive new ants in Cretaceous amber from Myanmar, New Jersey, and Canada (Hymenoptera: Formicidae) Am. Mus. Novit. 2005;3485:1–23. [Google Scholar]
- Evans H.E. Extrinsic versus intrinsic factors in the evolution of insect sociality. BioScience. 1977;27:613–617. [Google Scholar]
- Gadagkar R. Why the definition of eusociality is not helpful to understand its evolution and what we should do about it. Oikos. 1995;70:485–487. [Google Scholar]
- Grimaldi D.A. The co-radiations of insects and angiosperms in the Cretaceous. Ann. Missouri Bot. Gard. 1999;86:373–406. [Google Scholar]
- Grimaldi D.A, Agosti D. A formicine in New Jersey Cretaceous amber (Hymenoptera: Formicidae) and early evolution of the ants. Proc. Natl Acad. Sci. USA. 2000;97:13 678–13 683. doi: 10.1073/pnas.240452097. 10.1073/pnas.240452097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton W.D. The genetical evolution of social behavior [Parts I and II] J. Theor. Biol. 1964;6:1–52. doi: 10.1016/0022-5193(64)90039-6. 10.1016/0022-5193(64)90038-4 [DOI] [PubMed] [Google Scholar]
- Huelsenbeck J.P, Ronquist F. MrBayes: Bayesian inference of phylogeny. Bioinformatics. 2001;17:754–755. doi: 10.1093/bioinformatics/17.8.754. 10.1093/bioinformatics/17.8.754 [DOI] [PubMed] [Google Scholar]
- Hunt J.H, Amdam G.V. Bivoltinism as an antecedent to eusociality in the paper wasp genus Polistes. Science. 2005;308:264–267. doi: 10.1126/science.1109724. 10.1126/science.1109724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard Smith J, Szathmáry E. Oxford University Press; Oxford, UK: 1995. The major transitions in evolution. [Google Scholar]
- Michener C.D. Harvard University Press; Cambridge, UK: 1974. The social behavior of bees. [Google Scholar]
- Michener C.D. Biogeography of the bees. Ann. Missouri Bot. Gard. 1979;66:277–347. [Google Scholar]
- Michener C.D. Johns Hopkins University Press; Baltimore, MD: 2000. The bees of the world. [Google Scholar]
- Michener C.D, Grimaldi D.A. The oldest fossil bee: apoid history, evolutionary stasis, and antiquity of social behavior. Proc. Natl Acad. Sci. USA. 1988;85:6424–6426. doi: 10.1073/pnas.85.17.6424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michener C.D, Poinar G., Jr The known bee fauna of the Dominican amber. J. Kansas Entomol. Soc. 1996;69:353–361. [Google Scholar]
- Miyanaga R, Maeta Y, Sakagami S.F. Geographical variation of sociality and size-linked color patterns in Lasioglossum (Evylaeus) apristum (Vachal) in Japan (Hymenoptera, Halictidae) Insect. Soc. 1999;46:224–232. 10.1007/s000400050138 [Google Scholar]
- Mutti M. Bulk δ18O and δ13C records from Site 999, Colombian Basin, and Site 1000, Nicaraguan Rise (latest Oligocene to middle Miocene): diagenesis, link to sediment parameters, and paleoceanography. Proc. Ocean Drill. Program Sci. Results. 2000;165:275–283. [Google Scholar]
- Nel A, Perrault G, Perrichot V, Néraudeau D. The oldest ant in the Lower Cretaceous amber of Charente-Maritime (SW France) (Insecta: Hymenoptera: Formicidae) Geol. Acta. 2004;2:23–29. [Google Scholar]
- Packer L. Solitary and eusocial nests in a population of Augochlorella striata (Provancher) (Hymenoptera; Halictidae) at the northern edge of its range. Behav. Ecol. Sociobiol. 1990;27:339–344. 10.1007/BF00164004 [Google Scholar]
- Packer L. The evolution of social behavior and nest architecture in sweat bees of the subgenus Evylaeus (Hymenoptera: Halictidae): a phylogenetic approach. Behav. Ecol. Sociobiol. 1991;29:153–160. 10.1007/BF00166396 [Google Scholar]
- Pesenko Y.A. Phylogeny and classification of the family Halictidae revised (Hymenoptera: Apoidea) J. Kansas Entomol. Soc. 1999;72:104–123. [Google Scholar]
- Plateaux-Quénu C. Comparative biological data in two closely related eusocial species: Evylaeus calceatus, Scop., and Evylaeus albipes, F., (Hym., Halictinae) Insect. Soc. 1992;39:351–364. 10.1007/BF01240620 [Google Scholar]
- Ratnieks F.L.W, Wenseleers T. Policing insect societies. Science. 2005;307:54–56. doi: 10.1126/science.1106934. 10.1126/science.1106934 [DOI] [PubMed] [Google Scholar]
- Richards M.H. Nesting biology and social organization of Halictus sexcinctus (Fabricius) in southern Greece. Can. J. Zool. 2001;79:2210–2220. 10.1139/cjz-79-12-2210 [Google Scholar]
- Richards M.H, von Wettberg E.J, Rutgers A.C. A novel social polymorphism in a primitively eusocial bee. Proc. Natl Acad. Sci. USA. 2003;100:7175–7180. doi: 10.1073/pnas.1030738100. 10.1073/pnas.1030738100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakagami S.F, Munakata M. Distribution and bionomics of a transpalearctic eusocial halictine bee, Lasioglossum (Evylaeus) calceatum in northern Japan with notes on its solitary life cycle at high altitude. J. Fac. Sci. Hokkaido Univ. Ser. VI Zool. 1972;18:411–439. [Google Scholar]
- Sanderson M.J. Estimating absolute rates of molecular evolution and divergence times: a penalized likelihood approach. Mol. Biol. Evol. 2002;19:101–109. doi: 10.1093/oxfordjournals.molbev.a003974. [DOI] [PubMed] [Google Scholar]
- Schwarz M.P, Bull N.J, Cooper S.J.B. Molecular phylogenetics of allodapine bees, with implications for the evolution of sociality. Syst. Biol. 2003;52:1–14. doi: 10.1080/10635150390132632. [DOI] [PubMed] [Google Scholar]
- Seger J. Partial bivoltinism may cause alternating sex-ratio biases that favour eusociality. Nature. 1983;301:59–62. 10.1038/301059a0 [Google Scholar]
- Sherman P.W, Lacey E.A, Reeve H.K, Keller L. The eusociality continuum. Behav. Ecol. 1995;6:102–108. [Google Scholar]
- Soucy S.L. Nesting biology and socially polymorphic behavior of the sweat bee Halictus rubicundus (Hymenoptera: Halictidae) Ann. Entomol. Soc. Am. 2002;95:57–65. doi: 10.1111/j.0014-3820.2002.tb01343.x. [DOI] [PubMed] [Google Scholar]
- Soucy S.L, Danforth B.N. Phylogeography of the socially polymorphic sweat bee Halictus rubicundus (Hymenoptera: Halictidae) Evolution. 2002;56:330–341. doi: 10.1111/j.0014-3820.2002.tb01343.x. [DOI] [PubMed] [Google Scholar]
- Swofford D.L. Sinauer Associates; Sunderland, MA: 2002. PAUP*. Phylogenetic analysis using parsimony (*and other methods), v. 4. [Google Scholar]
- Thorne J.L, Kishino H. Divergence time and evolutionary rate estimation with multilocus data. Syst. Biol. 2002;51:689–702. doi: 10.1080/10635150290102456. 10.1080/10635150290102456 [DOI] [PubMed] [Google Scholar]
- Thorne J.L, Kishino H. Estimation of divergence times from molecular sequence data. In: Nielsen R, editor. Statistical methods in molecular evolution. Springer; New York, NY: 2005. pp. 233–256. [Google Scholar]
- Thorne B.L, Traniello J.F.A. Comparative social biology of basal taxa of ants and termites. Ann. Rev. Entomol. 2003;48:283–306. doi: 10.1146/annurev.ento.48.091801.112611. 10.1146/annurev.ento.48.091801.112611 [DOI] [PubMed] [Google Scholar]
- Thorne B.L, Grimaldi D.A, Krishna K. Early fossil history of the termites. In: Abe T, Bignell D.E, Higashi M, editors. Termites: evolution, sociality, symbioses, ecology. Kluwer Academic Publishing; Dordrecht, The Netherlands: 2000. pp. 77–93. [Google Scholar]
- Wcislo W.T. Behavioral environments of sweat bees (Halictinae) in relation to variability in social organization. In: Crespi B.J, Choe J.C, editors. The evolution of social behavior in insects and arachnids. Cambridge University Press; Cambridge, UK: 1997. pp. 316–332. [Google Scholar]
- Wcislo W.T, Danforth B.N. Secondarily solitary: the evolutionary loss of social behavior. Trends Ecol. Evol. 1997;12:468–474. doi: 10.1016/s0169-5347(97)01198-1. 10.1016/S0169-5347(97)01198-1 [DOI] [PubMed] [Google Scholar]
- Wenzel J.W. A social wasp's nest from the Cretaceous Period, Utah, USA, and its biogeographical significance. Psyche (Camb.) 1990;97:21–29. [Google Scholar]
- Wilson E.O. Harvard University Press; Cambridge, MA: 1971. The insect societies. [Google Scholar]
- Wilson E.O. Excellence in ecology. Ecology Institute; 2. Oldendorf/Luhe, Germany: 1990. Success and dominance in ecosystems: the case of the social insects. [Google Scholar]
- Wilson E.O, Hölldobler B. Eusociality: origin and consequences. Proc. Natl Acad. Sci. USA. 2005;102:13 367–13 371. doi: 10.1073/pnas.0505858102. 10.1073/pnas.0505858102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanega D. Demography and sociality in halictine bees (Hymenoptera: Halictidae) In: Crespi B.J, Choe J.C, editors. The evolution of social behavior in insects and arachnids. Cambridge University Press; Cambridge, UK: 1997. pp. 293–315. [Google Scholar]
- Yang Z. PAML: a program package for phylogenetic analysis by maximum likelihood. CABIOS. 1997;13:555–556. doi: 10.1093/bioinformatics/13.5.555. [DOI] [PubMed] [Google Scholar]
- Zachos J, Pagani M, Sloan L, Thomas E, Billups K. Trends, rhythms, and aberrations in global climate 65 ma to present. Science. 2001;292:686–693. doi: 10.1126/science.1059412. 10.1126/science.1059412 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Voucher and GenBank codes, and summary of phylogenetic and dating results.