Abstract
Mating signals are often directed at numerous senses and provide information about species identity, gender, receptiveness, individual identity and mate quality. Given the diversity of colourful body patterns in invertebrates, surprisingly few studies have examined the role of these visual signals in mate recognition. Here, we demonstrate the use of claw coloration as a species recognition signal in a fiddler crab (Uca mjoebergi). Furthermore, we show that distinct carapace colour patterns in Uca capricornis enable males to discriminate between their female neighbours and unfamiliar females. This is the first empirical evidence of the social importance of colour markings in fiddler crabs and the first example of visually mediated species and neighbour recognition in invertebrates other than insects.
Keywords: fiddler crabs, colour pattern, species recognition, neighbour recognition, mate recognition
1. Introduction
Successful sexual reproduction requires finding an individual of the correct species and sex that is suitably attractive and then maintaining access to that individual long enough to mate and produce offspring. Mating signals are often directed at numerous senses and provide information about species identity, gender, receptiveness, individual identity and mate quality (Marler 1961; Candolin 2003). There is tremendous diversity in animal coloration and patterning, which is involved in signalling a wide variety of information, particularly in vertebrates (Hailman 1977; Rowland 1979; Andersson 1994). Relatively little is known, however, about the role of these visual signals in mate recognition on a species and individual level (Savalli 1995; Ptacek 2000), especially in invertebrates.
At the broadest level, mate recognition involves identifying members of the correct species. Distinguishing between conspecifics and heterospecifics is the most important step in finding a mate, reducing the costs associated with courting an incompatible mate and producing infertile offspring (Ryan et al. 2001). Body coloration has long been suggested as a reproductive isolating mechanism (Wallace 1889). However, direct empirical evidence is rare, even in the relatively well-studied vertebrates (Savalli 1995; Ptacek 2000). Among invertebrates, several insects have been shown to use visual patterns to identify conspecific mates (Waage 1975; Rutowski 1977; Silberglied & Taylor 1978; Wiernasz & Kingsolver 1992; Jiggins et al. 2001; Fordyce et al. 2002). Colour is believed to be a potential isolating mechanism in some brilliantly coloured crustaceans such as mantis shrimps (Caldwell & Dingle 1975) and fiddler crabs (Salmon et al. 1978; Zucker & Denny 1979), but this has yet to be demonstrated experimentally.
The ability to recognize mates on an individual level, as opposed to the species level, may help to stabilize pair bonds and allow individuals to identify and ward off potential competitors (Balshine-Earn & Lotem 1998; Muller et al. 2003). Individual mate recognition, more accurately known as mate discrimination (Sherman et al. 1997), appears to be predominantly chemically mediated in invertebrates (Seibt 1973; Johnson 1977; Caldwell 1992; Muller et al. 2003). The only visually mediated individual recognition systems described in invertebrates are the distinct facial and abdominal markings involved in establishing dominance hierarchies in wasps (Tibbetts 2002) and possibly some undefined visual cues used by crabs (Vannini & Gherardi 1981) and crayfish (Crook et al. 2004) to recognize previous opponents.
Here, we examine the social significance of body colours in fiddler crabs, which are extremely colourful inter-tidal crustaceans. Their conspicuous colours have been linked to the reproductive and physiological state of the individual (Crane 1944; Brown et al. 1953; Thurman 1988; Zeil & Hofmann 2001), and are likely to incur a predation cost (Koga et al. 2001). However, as with most invertebrates, there have been no empirical tests of the social significance of colour markings in fiddler crabs.
Uca mjoebergi have mottled brown bodies with occasional traces of yellow or red. The male's enlarged claw is uniformly yellow (figure 1a). They live sympatrically with a number of other species, including Uca signata, which are similar in size and appearance except for their blue carapace markings and differently shaped red and white claws (figure 1a). U. mjoebergi females leave their burrows and wander through the colony in search of a male before mating within his burrow. As in many other species, males wave their single enlarged claw at any moving, crab-sized object (Land & Layne 1995), and it has long been believed that females use the species-specific movement of the males' waving displays and/or their coloration to recognize conspecifics (Salmon et al. 1978; Zucker & Denny 1979; Pope 2005). In our first experiment, we therefore examined whether U. mjoebergi females use the males' colour markings to identify conspecifics, independently of their waving display.
Figure 1.
Species recognition based on claw coloration in Uca mjoebergi. Proportion of females approaching: (a) conspecific or heterospecific males (p=0.006, n=14); (b) conspecifics painted yellow or red and white (p=0.004, n=15); (c) unpainted conspecifics or those painted yellow (p=0.004, n=15); (d) heterospecifics and conspecifics both painted yellow (p=0.3, n=15); (e) heterospecifics painted yellow or conspecifics painted red and white (p=0.0005, n=15).
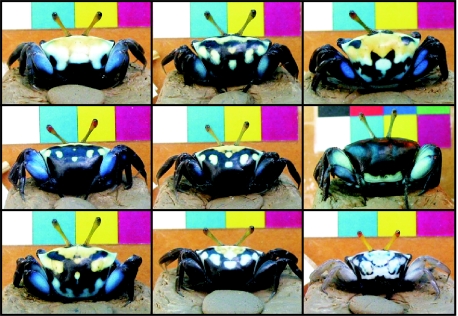
If species recognition is the sole function of body coloration in fiddler crabs, individual variation would only serve to make recognition more difficult. Yet in numerous species, individuals exhibit extreme variations in colour and pattern, particularly on their carapace (Crane 1975; Shih et al. 1999; Zeil & Hoffman 2001). While species recognition requires a signal that is relatively uniform across the population, recognition of kin, neighbours or individuals requires distinct signals on an increasingly fine scale (Beecher 1989). In Uca capricornis both sexes display variable patterns of blue, yellow, orange and white patches on their black carapace. The size, colour and number of patches vary between individuals (figure 2). In contrast to U. mjoebergi, U. capricornis males court neighbouring females during spring tide and mate at the entrance of her burrow. Individuals often feed near each other and do not immediately return to their burrows if a neighbour gets too close, which is normally an automatic response to intruding crabs (Hemmi & Zeil 2003). In Uca polita, this behaviour was used by von Hagen (1993) to suggest that this species forms pairs or ‘resident breeding units’. Clearly, males in such pairs would benefit from being able to recognize their female neighbours. Murai et al. (2002) for instance have shown that males of Uca paradussumieri are able to keep track of a female's reproductive state, but the mechanism that allows them to do so has not been identified. In our second experiment, we thus asked whether males were capable of discriminating between familiar and unfamiliar females and whether they use their unique carapace markings to do so.
Figure 2.
Variation in carapace coloration in Uca capricornis females.
2. Material and methods
Our study sites were in the vicinity of the mangrove boardwalk in the East Point Reserve, Darwin, Australia (12°24′35″ S, 130°50′00″ E). Fieldwork was conducted from September to January in 2003 and 2004.
(a) Species recognition in Uca mjoebergi
During neap tide when U. mjoebergi females actively sample males, we tethered two size-matched males 15 cm on either side of a point where we subsequently released females. The males were tethered with 1 cm of cotton super-glued to their carapace and tied to a nail stuck in the ground. While tethered animals were able to move in a restricted circle, they were never observed to wave. Wandering females were caught and placed under a container at the release point between the males. Females were released by raising the container remotely and we recorded which male she touched or approached to within approximately 2 cm. Females that left the area without approaching either male were considered not to have made a choice.
To test whether females are capable of species recognition, they were given a choice between size-matched (within 1 mm claw length) and handedness-matched U. signata and U. mjoebergi males. To examine the role of claw colour in species recognition, we painted the claw of a U. signata male yellow and caught three size- and handedness-matched U. mjoebergi males. One U. mjoebergi male was painted red and white to resemble U. signata, another was painted yellow and one was left unpainted. Paints were oil-based gloss enamel from the Dulux Tinytin range. Due to the individual variation in claw colour in both species and the absence of any knowledge of their spectral sensitivities it was impossible to choose a paint that exactly matched the claw colour as perceived by the animals. However, our results suggest that the colours we chose were within the range of natural variation in the colours of the crabs. Females were given a choice between: (i) the yellow-painted U. mjoebergi and the red and white painted U. mjoebergi, (ii) the unpainted U. mjoebergi and the yellow-painted U. mjoebergi, (iii) the yellow-painted U. mjoebergi and the yellow-painted U. signata and (iv) the red and white painted U. mjoebergi and the yellow-painted U. signata. The males were then tethered randomly on either side of the female. New females were tested on each combination of males until one made a choice. On average we had to test two females to get a choice. We used 15 sets of different males and although some males were used in more than one comparison, no males were used in more than one role.
(b) Neighbour recognition in Uca capricornis
Between neap and spring tide, we located male and female U. capricornis that were closest neighbours, living 10–30 cm apart and with no obstacles obscuring their view of each other. We caught the female and randomly assigned her to one of seven treatments: (i) unaltered control; (ii) replacing her with an unfamiliar female of a similar size; (iii) dramatically altering her appearance by painting her carapace black; applying a 2 mm spot of black paint to: (iv) a black part of her carapace or (v) on her abdomen as paint controls; (vi) painting her carapace with clear nail polish or (vii) replacing her with a freshly painted black wooden bead (1 cm diameter). We then tethered the female half way between the male and female burrows (5–15 cm from the male).
Like a number of other fiddler crab species (Crane 1944; Zeil & Hofmann 2001), U. capricornis are capable of rapidly muting their carapace colours, for instance, in response to handling, although their specific patterns generally remain discernible (unpublished data). However, unlike many species in which dramatic colour changes occur in association with the diurnal or tidal cycle (Crane 1944; Brown et al. 1953; Thurman 1988), changes in carapace patterns and their colours in U. capricornis otherwise appear to be long term, possibly associated with moulting (personal observations). Our results confirm that our experiments were not hampered by short-term colour changes.
From 1 to 2 m away, we observed the pair from the moment the male emerged from his burrow. The experiment ended once the male touched the tethered female, which was considered an approach, or after 5 min had passed with no approach. We observed both aggressive and courtship behaviour directed at the females, but it was often difficult to classify the behaviour as courtship could turn into aggression, probably due to the female's lack of response. Consequently, we simply classified the behaviour as ‘approach’ or ‘ignore’. We returned the female to her burrow at the end of the experiment and found a new pair.
To examine the effect of position on recognition (e.g. Bee & Gerhardt 2002), unaltered neighbours and unfamiliar females were tethered 10 cm from the male's burrow in the same direction as the female's burrow or in the opposite direction. All treatments were conducted on the same male in a random order. When the unfamiliar female was presented first, the neighbouring female was sealed in her burrow with a shell during the trial and was given 10 min to recover before being tested.
We examined crab residency by drawing the individual colour patterns of 61 randomly chosen crabs (26 females and 35 males) and marking their burrows. We returned the following day and noted how many crabs were in the same burrows. We regarded crabs whose burrows were sealed the next day as ‘present’.
(c) Statistics
In the two-choice trials, we tested whether U. mjoebergi females' choices differed significantly from random with exact binomial tests.
The ability of male U. capricornis to distinguish between unaltered neighbouring females and unfamiliar females was first tested with a G-test of the difference in the likelihood of approach. The likelihood of approaching the unaltered neighbour was also compared to the likelihood of approaching the other treatments. Treatments that differed significantly from the unaltered neighbour in the rate of approach were then compared to the likelihood of approaching unfamiliar females. Unless otherwise stated, p values less than 0.05 are significant after sequential Bonferroni corrections. Finally, the males' response to neighbouring females in their expected position and strangers or neighbours in an unexpected position was tested with a G-test of the difference in the likelihood of approach.
3. Results and discussion
(a) Species recognition in Uca mjoebergi
Attracting wandering females to the male's burrow is the first step towards successful reproduction in many fiddler crab species, including U. mjoebergi. The species-specific waving display has long been considered an important feature in mate recognition and attraction (Crane 1975; Salmon et al. 1978; Zucker & Denny 1979; Pope 2000), but has never been examined in isolation. While the waving display probably plays a role in discriminating between conspecific males based on male condition or other correlates of fitness (Hyatt 1977; Backwell & Passmore 1996; Jennions & Backwell 1998), our results show that it is not necessary for species recognition. Females were able to discriminate between non-waving conspecific and heterospecific males based solely on their morphological features, and preferred their own species (p=0.006, n=14; figure 1a).
Claw colour, or the associated luminance pattern, is clearly the dominant morphological feature in identifying conspecific mates in U. mjoebergi. Females were more likely to approach U. mjoebergi males painted yellow than those painted red and white to resemble U. signata (p=0.004, n=15; figure 1b). This is not because they were attracted to the yellow paint itself, as females preferred unpainted U. mjoebergi males over those painted yellow (p=0.004, n=15; figure 1c). They also failed to distinguish between conspecific and heterospecific males whose claws were painted the same colour (p=0.3, n=15; figure 1d). Furthermore, U. mjoebergi females actually preferred U. signata males whose claws were painted to resemble conspecifics over their own males painted to resemble U. signata (p=0.0005, n=15; figure 1e). This suggests that claw coloration is sufficient for species recognition in U. mjoebergi and that it can override other species-specific aspects of male morphology such as claw shape and carapace coloration, at least from a distance.
Mate choice is notoriously complex, so the characteristic coloration of fiddler crab claws may be responsible for signalling more than species identity. Differences in the shade of yellow may also act as an intraspecific signal of male quality directed at potential mates (Hill 1991) or competitors (Mateos & Carranza 1997). There is a certain amount of natural variation in the specific shade of yellow of U. mjoebergi's claw (personal observation). As seen in the test for paint preference, females appear to show a preference for males based on the specific shade of yellow of their claw, preferring the natural colour to that of the yellow paint (figure 1c).
Although the waving display does not appear to be necessary for successful species recognition, the species-specific movements may provide additional cues or may simply draw attention to the male and his specific coloration.
(b) Individual mate recognition in Uca capricornis
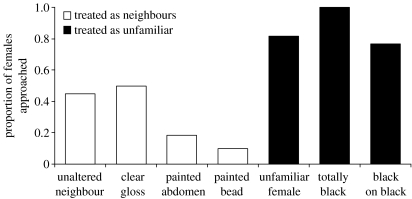
Male U. capricornis are able to discriminate between familiar neighbours and unfamiliar females (χ12=9, p=0.003). They tended to approach unfamiliar females tethered nearby, whereas they were more likely to ignore their unaltered neighbours tethered in the same position (figure 3).
Figure 3.
Mate recognition mediated by carapace coloration in Uca capricornis. Males were more likely to approach an unfamiliar female (n=22) than their neighbour (n=48) (χ12=9, p=0.003). Males were equally likely to approach unaltered neighbours, those with painted abdomens (n=11) and those with clear gloss on their carapace (n=16) (χ22=3.4, p=0.2). They were also as likely to approach a painted bead (n=11) as their unaltered neighbour (χ12=5.7, p=0.02; n.s. after sequential Bonferroni correction). Neighbours painted entirely black (n=9) and neighbours with black on a black part of their carapace (n=30) were approached as unfamiliar females (χ22=4.1, p=0.1).
While species recognition requires relatively uniform signals across the population, recognition on finer scales requires correspondingly more distinct signals (Beecher 1989). The unique colour patterns of U. capricornis allow males to discriminate between their neighbouring females and unfamiliar females (statistics presented in figure 3). Altering the neighbour's appearance by painting her black had the same effect as replacing her with an unfamiliar female (figure 3). As black females are within the range of natural colour variation, males should still recognize painted females as conspecifics, but not necessarily as the same individual.
Males are not responding to chemical cues, as they did not react to paint hidden on their neighbour's abdomen and they almost completely ignored a painted bead so they are not inherently attracted to the paint itself (figure 3). They are clearly responding to a change in the female's appearance and on a very fine scale. Neighbours whose appearance was altered slightly with a spot of black paint on a black part of her carapace were treated as strangers (figure 3). Although we cannot be sure in what way the black paint changed the reflectance of the carapace the male's lack of response to clear gloss (figure 3) seems to rule out differences in specular reflectance as a major factor. Males are most likely responding to a change in the colour or luminance pattern of the female's carapace.
Male U. capricornis treated displaced neighbours in the same way as unfamiliar females (figure 4). Males are thus not responding differently to neighbours and unfamiliar females because their colour patterns signal something about their state, but because they recognize them, at least when they are within their familiar location. Although such a response to displaced neighbours is often considered proof of individual recognition (Falls & Brooks 1975; Myrberg & Riggio 1985), it is also possible that males are unable to recognize their neighbours independent of location, that is out of the context of their normal territory (Bee & Gerhardt 2002; Husak & Fox 2003). Our data do not allow us to distinguish between the two cases as it is not clear whether the males approached displaced females because they detected a familiar female in an unfamiliar location, which might require the males' attention, or because they simply did not recognize the female. For successful mate guarding and territorial defence, males need to be able to recognize neighbouring females as distinct from other, unfamiliar females. They do not necessarily need to be able to distinguish each individual from every other in the population, as required by ‘true’ individual recognition (Beecher 1989).
Figure 4.
Recognition based on position in Uca capricornis. Males treated neighbours in an unexpected position the same as strangers in either position (χ22=1.4, p=0.5); they were more likely to approach these females than neighbours in their correct position (χ32=18.4, p=0.0004; n=20 for all trials).
What makes the recognition capability of U. capricornis particularly intriguing is the apparently short time-scale over which it occurs. U. capricornis neighbourhoods are relatively unstable. Only 38% of 61 individuals were found in the same burrow after 1 day, although males and females living in ‘pairs’ may be more stable than the general population. Males may habituate to the presence and visual appearance of specific females in specific locations (e.g. Bee & Berhardt 2001), but they would also clearly benefit from being able to identify their neighbours and thus be able to approach unfamiliar females to determine their receptivity. Murai et al. (2002) found that male U. paradussumieri track the reproductive state of neighbouring females, allowing them to predict when the females were receptive. The mechanism males use to keep track of their neighbours in this species is unknown but being able to recognize neighbours and their positions would certainly be useful (see also Zeil & Layne 2002).
4. Conclusion
This study presents the first empirical evidence of the social importance of the spectacular coloration exhibited by many fiddler crabs. We found that female U. mjoebergi use the coloration of the males' enlarged claws, independently of their waving display, to identify them as conspecifics. Male U. capricornis were also found to recognize neighbouring females based on the distinct colour patterns on their carapace.
Although fiddler crabs may have the potential for colour vision (Horch et al. 2002), we cannot conclude at this stage whether their discrimination abilities are based on colour, pattern discrimination or a combination of both. However, the strong preference female U. mjoebergi had for unpainted males over those painted yellow (figure 1c) argues against pattern discrimination, as both claws were uniformly yellow though of different hues.
Acknowledgments
This work was supported by an ANU PhD Scholarship with an additional contribution by the Centre for Visual Sciences (to T.D.), a Swiss National Science Foundation postdoctoral fellowship (to J.M.H.) and an Australian Research Council grant (to P.R.Y.B.). J.Z. acknowledges support from the ARC Centre of Excellence in Vision Science. We thank M. Jennions for discussions and statistical advice and anonymous reviewers for insistent and constructive comments on the manuscript.
References
- Andersson M. Princeton University Press; Princeton, NJ: 1994. Sexual selection. [Google Scholar]
- Backwell P, Passmore N.I. Time constraints and multiple choice criteria in the sampling behavior and mate choice of the fiddler crab, Uca annulipes. Behav. Ecol. Sociobiol. 1996;38:407–416. 10.1007/s002650050258 [Google Scholar]
- Balshine-Earn S, Lotem A. Individual recognition in a cooperatively breeding cichlid: evidence from video playback experiments. Behaviour. 1998;135:369–386. [Google Scholar]
- Bee M.A, Gerhardt H.C. Neighbour–stranger discrimination by territorial male bullfrogs (Rana catesbeiana): II. Perceptual basis. Anim. Behav. 2001;62:1141–1150. 10.1006/anbe.2001.1852 [Google Scholar]
- Bee M.A, Gerhardt H.C. Individual voice recognition in a territorial frog (Rana catesbeiana) Proc. R. Soc. B. 2002;269:1443–1448. doi: 10.1098/rspb.2002.2041. 10.1098/rspb.2002.2041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beecher M.D. Signalling systems for individual recognition: an information theory approach. Anim. Behav. 1989;38:248–261. [Google Scholar]
- Brown F.A, Fingerman M, Sandeen M.I, Weeb H.M. Persistent diurnal and tidal rhythm of colour change in the fiddler crab Uca pugilator. J. Exp. Zool. 1953;123:29–42. 10.1002/jez.1401230103 [Google Scholar]
- Caldwell R.L. Recognition, signalling and reduced aggression between former mates in a stomatopod. Anim. Behav. 1992;44:11–19. [Google Scholar]
- Caldwell R.L, Dingle H. Ecology and evolution of agonistic behaviour in stomatopods. Naturwiss. 1975;62:214–222. 10.1007/BF00603166 [Google Scholar]
- Candolin U. The use of multiple cues in mate choice. Biol. Rev. 2003;78:575–595. doi: 10.1017/s1464793103006158. 10.1017/S1464793103006158 [DOI] [PubMed] [Google Scholar]
- Crane J. On the color changes of fiddler crabs in the field. Zoologica. 1944;29:161–168. [Google Scholar]
- Crane J. Princeton University Press; Princeton, NJ: 1975. Fiddler crabs of the world. Ocypodidae: genus Uca. [Google Scholar]
- Crook R, Patullo B.W, Macmillan D.L. Multimodal individual recognition in the crayfish Cherax destructor. Mar. Freshwater Behav. Physiol. 2004;37:271–285. 10.1080/10236240400016595 [Google Scholar]
- Falls J.B, Brooks R.J. Individual recognition by song in white-throated sparrows. II. Effects of location. Can. J. Zool. 1975;53:1412–1420. [Google Scholar]
- Fordyce J.A, Nice C.C, Forister M.L, Shapiro A.M. The significance of wing pattern diversity in the Lycaenidae: mate discrimination by two recently diverged species. J. Evol. Biol. 2002;15:871–879. 10.1046/j.1420-9101.2002.00432.x [Google Scholar]
- von Hagen H.O. Waving display in females of Uca polita and of other Australian fiddler crabs. Ethology. 1993;93:3–20. [Google Scholar]
- Hailman J.P. Indiana University Press; Bloomington: 1977. Optical signals—animal communication and light. [Google Scholar]
- Hemmi J.M, Zeil J. Burrow surveillance in fiddler crabs. I. Description of behaviour. J. Exp. Biol. 2003;206:3935–3950. doi: 10.1242/jeb.00632. 10.1242/jeb.00632 [DOI] [PubMed] [Google Scholar]
- Hill G.E. Plumage colouration is a sexually selected indicator of male quality. Nature. 1991;350:337–339. 10.1038/350337a0 [Google Scholar]
- Horch K, Salmon M, Forward R. Evidence for a two pigment visual system in the fiddler crab, Uca thayeri. J. Comp. Physiol. A. 2002;188:493–499. doi: 10.1007/s00359-002-0325-7. 10.1007/s00359-002-0325-7 [DOI] [PubMed] [Google Scholar]
- Husak J.F, Fox S.F. Adult male collared lizards, Crotaphytus collaris, increase aggression towards displaced neighbours. Anim. Behav. 2003;65:391–396. 10.1006/anbe.2003.2058 [Google Scholar]
- Hyatt G.W. Field studies of size-dependent changes in waving display and other behaviour in the fiddler crab, Uca pugilator (Brachyura, Ocypodidae) Mar. Behav. Physiol. 1977;4:283–292. [Google Scholar]
- Jennions M.D, Backwell P.R.Y. Variation in courtship rate in the fiddler crab Uca annulipes: is it related to male attractiveness? Behav. Ecol. 1998;9:605–611. 10.1093/beheco/9.6.605 [Google Scholar]
- Jiggins C.D, Naisbit R.E, Coe R.L, Mallet J. Reproductive isolation caused by colour pattern mimicry. Nature. 2001;411:302–304. doi: 10.1038/35077075. 10.1038/35077075 [DOI] [PubMed] [Google Scholar]
- Johnson V.R.J. Individual recognition in the banded shrimp Stenopus hispidus (Olivier) Anim. Behav. 1977;25:418–428. 10.1016/0003-3472(77)90017-3 [Google Scholar]
- Koga T, Backwell P.R.Y, Christy J.H, Murai M, Kasuya E. Male-biased predation of a fiddler crab. Anim. Behav. 2001;62:201–207. 10.1006/anbe.2001.1740 [Google Scholar]
- Land M.F, Layne J. The visual control of behaviour in fiddler crabs. I. Resolution, thresholds and the role of the horizon. J. Comp. Physiol. A. 1995;177:81–90. [Google Scholar]
- Marler P. The evolution of visual communication. In: Blair W.F, editor. Vertebrate speciation. University of Texas Press; Austin, TX: 1961. pp. 96–121. [Google Scholar]
- Mateos C, Carranza J. The role of bright plumage in male-male interactions in the ring-necked pheasant. Anim. Behav. 1997;54:1205–1214. doi: 10.1006/anbe.1997.0516. 10.1006/anbe.1997.0516 [DOI] [PubMed] [Google Scholar]
- Muller J.K, Eggert A.K, Elsner T. Nestmate recognition in burying beetles: the “breeder's badge” as a cue used by females to distinguish their mates from male intruders. Behav. Ecol. 2003;14:212–220. 10.1093/beheco/14.2.212 [Google Scholar]
- Murai M, Koga T, Yong H.-S. The assessment of female reproductive state during courtship and scramble competition in the fiddler crab, Uca paradussumieri. Behav. Ecol. Sociobiol. 2002;52:137–142. 10.1007/s00265-002-0483-1 [Google Scholar]
- Myrberg A.A, Jr, Riggio R.J. Acoustically mediated individual recognition by a coral reef fish (Pomacentrus partitius) Anim. Behav. 1985;33:411–416. [Google Scholar]
- Pope D.S. Testing function of fiddler crab claw waving by manipulating social context. Behav. Ecol. Sociobiol. 2000;47:432–437. 10.1007/s002650050687 [Google Scholar]
- Pope D.S. Waving in a crowd: fiddler crabs signal in networks. In: McGregor P.K, editor. Animal communication networks. Cambridge University Press; Cambridge, UK: 2005. pp. 252–276. [Google Scholar]
- Ptacek M.B. The role of mating preferences in shaping interspecific divergence in mating signals in vertebrates. Behav. Process. 2000;51:111–134. doi: 10.1016/s0376-6357(00)00123-6. 10.1016/S0376-6357(00)00123-6 [DOI] [PubMed] [Google Scholar]
- Rowland W.J. The use of color in intraspecific communication. In: Burtt J.E.H, editor. The behavioral significance of color. Garland STPM Press; New York, NY: 1979. pp. 379–421. [Google Scholar]
- Rutowski R.L. The use of visual cues in sexual and species discrimination by males of the small sulphur butterfly Eurema lisa. J. Comp. Physiol. A. 1977;115:61–74. 10.1007/BF00667785 [Google Scholar]
- Ryan M.J, Phelps S.M, Rand A.S. How evolutionary history shapes recognition mechanisms. Trends Cogn. Sci. 2001;5:143–148. doi: 10.1016/s1364-6613(00)01616-8. 10.1016/S1364-6613(00)01616-8 [DOI] [PubMed] [Google Scholar]
- Salmon M, Hyatt G, McCarthy K, Costlow F.D., Jr Display specificity and reproductive isolation in the fiddler crabs Uca panacea and U. pugilator. Z. Tierpsychol. 1978;48:251–276. [Google Scholar]
- Savalli U.M. The evolution of bird coloration and plumage elaboration: a review of hypotheses. In: Power D.M, editor. Current ornithology. vol. 12. Plenum; New York, NY: 1995. pp. 141–190. [Google Scholar]
- Seibt U. Sense of smell and pair-bond in Hymenocera picta Dana. Micronesica. 1973;9:231–236. [Google Scholar]
- Sherman P.W, Reeve H.K, Pfennig D.W. Recognition systems. In: Krebs J.R, Davies N.B, editors. Behavioural ecology: an evolutionary approach. Blackwell Scientific; Oxford, UK: 1997. pp. 69–96. [Google Scholar]
- Shih H.-T, Mok H.-K, Chang H.-W, Lee S.-C. Morphology of Uca formosensis Rathburn, 1921 (Crustacea: Decapoda: Ocypodidae), an endemic fiddler crab from Taiwan, with notes on its ecology. Zool. Stud. 1999;38:164–177. [Google Scholar]
- Silberglied R.E, Taylor O.R. Ultraviolet reflection and its behavioural role in the courtship of the sulphur butterflies Colias erythema and C. philodice. Behav. Ecol. Sociobiol. 1978;3:203–243. 10.1007/BF00296311 [Google Scholar]
- Thurman C.L. Rhythmic physiological color change in crustacea: a review. Comp. Biochem. Physiol. 1988;91C:171–185. [Google Scholar]
- Tibbetts E.A. Visual signals of individual identity in the wasp Polistes fuscatus. Proc. R. Soc. B. 2002;269:1423–1428. doi: 10.1098/rspb.2002.2031. 10.1098/rspb.2002.2031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannini M, Gherardi F. Dominance and individual recognition in Potamon fluviatile (Decapoda, Brachyura): possible role of visual cues. Mar. Behav. Physiol. 1981;8:13–20. [Google Scholar]
- Waage J.K. Reproductive isolation and the potential for character displacement in the damselflies, Calopteryx maculata and C. aequabilis (Odonata: Calopterygidae) Syst. Zool. 1975;24:24–36. [Google Scholar]
- Wallace A.R. Macmillan; London, UK: 1889. Darwinism. [Google Scholar]
- Wiernasz D.C, Kingsolver J.G. Wing melanin pattern mediates species recognition in Pieris occidentalis. Anim. Behav. 1992;43:89–94. [Google Scholar]
- Zeil J, Hofmann M. Signals from ‘crabworld’: Cuticular reflections in a fiddler crab colony. J. Exp. Biol. 2001;204:2561–2569. doi: 10.1242/jeb.204.14.2561. [DOI] [PubMed] [Google Scholar]
- Zeil J, Layne J. Path integration in fiddler crabs and its relation to habitat and social life. In: Wiese K, editor. Crustacean experimental systems in neurobiology. Springer; Heidelberg, Germany: 2002. pp. 227–247. [Google Scholar]
- Zucker N, Denny R. Interspecific communication in fiddler crabs: preliminary report of a female rejection display directed toward courting heterospecific males. Z. Tierpsychol. 1979;50:9–17. [Google Scholar]