Abstract
In seabirds a broad variety of morphologies, flight styles and feeding methods exist as an adaptation to optimal foraging in contrasted marine environments for a wide variety of prey types. Because of the low productivity of tropical waters it is expected that specific flight and foraging techniques have been selected there, but very few data are available. By using five different types of high-precision miniaturized logger (global positioning systems, accelerometers, time depth recorders, activity recorders, altimeters) we studied the way a seabird is foraging over tropical waters. Red-footed boobies are foraging in the day, never foraging at night, probably as a result of predation risks. They make extensive use of wind conditions, flying preferentially with crosswinds at median speed of 38 km h−1, reaching highest speeds with tail winds. They spent 66% of the foraging trip in flight, using a flap–glide flight, and gliding 68% of the flight. Travelling at low costs was regularly interrupted by extremely active foraging periods where birds are very frequently touching water for landing, plunge diving or surface diving (30 landings h−1). Dives were shallow (maximum 2.4 m) but frequent (4.5 dives h−1), most being plunge dives. While chasing for very mobile prey like flying fishes, boobies have adopted a very active and specific hunting behaviour, but the use of wind allows them to reduce travelling cost by their extensive use of gliding. During the foraging and travelling phases birds climb regularly to altitudes of 20–50 m to spot prey or congeners. During the final phase of the flight, they climb to high altitudes, up to 500 m, probably to avoid attacks by frigatebirds along the coasts. This study demonstrates the use by boobies of a series of very specific flight and activity patterns that have probably been selected as adaptations to the conditions of tropical waters.
Keywords: tracking, global positioning system, accelerometers, altitude, wind, prey capture
1. Introduction
Locomotion is the behaviour that most dictates the morphology and physiology of animals (Dickinson et al. 2000). The variety of morphologies and flight types observed in birds provides a good example from which to determine structure–function relationships. In seabirds that forage over the open ocean in search of food, a broad variety of feeding methods exist (Ashmole 1971), reflected in a corresponding great variety of morphologies and flight styles (Pennycuick 1987). When they are breeding, seabirds can forage at extensive distances from the colonies, and this is made possible in the most pelagic species such as albatrosses or petrels by specific types of flight such as dynamic soaring, which allows them to forage at low costs (Pennycuick 1982). Wind has a major influence on the movement of these species at small as well as at large scales (Weimerskirch et al. 2000). Thus foraging seabirds have to adjust their movements according to the hierarchical distribution of food resources, but also to the wind conditions to optimize their search pattern (Fauchald 1999; Fritz et al. 2003).
In tropical waters where food resources are scarce (Longhurst & Pauly 1987), seabirds have probably more difficulties to search for food than in colder waters, and it is generally assumed that the energetic cost of locomotion is a strong force selecting for proficient flight (Ballance 1995; Ballance & Pitman 1999). For example frigatebirds have a very specific type of flight that allows them to reduce flight costs at the expense of flight speed and provisioning rates (Weimerskirch et al. 2003). The other typical tropical seabirds are terns, boobies and tropicbirds, and in the first two species energetic costs of flight appear to be lower than would be predicted based on size alone (Flint & Nagy 1984; Ballance 1995). Boobies represent a paradox because compared with the other typical tropical seabirds, they return every day to the colonies, indicating that they are able to find scattered food at a limited range from the colonies. The way they forage is unknown, compared with their congeners from the temperate waters such as gannets (Garthe et al. 1999, 2000; Hamer et al. 2001; Grémillet et al. 2004; Ropert-Coudert et al. 2004a,b), or other pelagic species such as albatrosses, petrels or penguins. Red-footed boobies, Sula sula, are probably the most pelagic boobies (Nelson 1978; Schreiber et al. 1996) and a study measuring energy expenditure during foraging indicate that costs are much lower than would be predicted by allometric equations (Ballance 1995), and much lower than that of the closely related temperate gannet (Birt-Friesen et al. 1989), suggesting that they might have some specialized mode of locomotion.
Studying movements of animals in their environment in general, and of seabirds in the marine environment in particular, is not easy, but has been made possible in the past decade by the development of miniaturized electronic devices that allow the study of their movement and behaviour remotely (Boyd et al. 2004). Until now, most studies have investigated the two-dimensional foraging strategy of flying seabirds, or the three-dimensional movements of diving species like penguins, or plunge-diving flying species like gannets (Ropert-Coudert et al. 2004a) or alcids (Kato et al. 2003). Here we study for the first time, to our knowledge, the movement of a foraging flying seabird in three dimensions, i.e. by reconstructing the two-dimensional movement with conventional telemetry (global positioning systems (GPS) and Argos transmitters), the diving behaviour (time depth recorders) and flight characteristics (accelerometers), but also the altitudinal movement (altimeters). By combining the use of these loggers, our aim was to describe precisely the foraging behaviour of red-footed boobies and to examine whether a seabird foraging in tropical waters uses a specialized flight strategy to search for food compared with other species in temperate or polar waters. More specifically, we expect these seabirds to have flight patterns that reduce foraging costs, and feeding techniques that are specific to the capture of elusive prey such as flying fishes that constitute the main prey of red-footed boobies.
2. Methods
The study was carried out on Europa Island (22.3° S, 40.3° E), in the Mozambique Channel between 18 August and 30 September 2003. On each nest of our study colony one bird within the pair was marked from a distance using a sprayer with a yellow patch of picric acid on the white tail. This yellow patch allowed rapid identification of birds at a distance. The study colony was monitored three to four times a day (at dawn and dusk, i.e. at 06.00 and 17.45, and once or twice times in the middle of the day) to control for the presence of adults on the nest and thus to infer the duration of foraging trips and departure and return of birds fitted with loggers. Birds were captured by hand for those accessible from the ground, or with a 6 m telescopic fishing pole fitted with a nylon noose for the birds nesting higher in the trees.
To study the horizontal movements of boobies, 16 individuals were fitted with a GPS receiver with integrated antenna and a 1 Mbyte flash memory operated by a rechargeable battery (Newbehavior; Zürich, Switzerland (Steiner et al. 2000)) recording at 10 s intervals. The loggers were sealed into small polyethylene bags; the overall weight of the device and its waterproof package was 32 g. In addition 16 birds were fitted for two to five successive foraging trips (46 foraging trips in total) with platform terminal transmitters (PTT) 100 satellite transmitters (Microwave telemetry, Columbia, MA) weighing 20 g, to study the flight directions from the colonies to the foraging areas. The locations obtained by the Argos system were filtered following Weimerskirch et al. (2000), with a maximum flight speed of 95 km h−1 for red-footed boobies, based on the recording of GPS (see § 3).
To study the activity patterns of boobies, we equipped 22 birds for one to five successive foraging trips with 6 g activity recorders (IMV2, Immersion Monitor, v. 2; Ferguson Manufacturing, Winston Salem, NC) that record the presence of seawater across its electrodes to infer the time the device was immersed in seawater and the time it was dry. Fitted on a metal band with tape, it allowed us to measure precisely the timing of landing or diving on water and of take-offs, and thus the duration of bouts spent on the water, or in flight.
To study the flight behaviour and dive depths we deployed cylindrical accelerometers: time depth four-channel data loggers (M190-D2GT, 12-bit resolution, 60×15 mm, 20 g; Little Leonardo, Tokyo, Japan) on 12 birds for one to two trips. The devices simultaneously monitored depth (every 1 s), temperature (every 1 min) and acceleration (16 Hz) along two axes. The units contain a tilt sensor capable of measuring both dynamic (e.g. vibration) and static acceleration (e.g. gravity). Loggers were attached to the birds’ tails so that acceleration was measured along the following two axes: the surging acceleration measured along the longitudinal body axis of the birds and the heaving acceleration measured dorso-ventrally (Watanuki et al. 2003; Ropert-Coudert et al. 2004a). The absolute accuracy for the depth sensor was 0.1 m. Acceleration data were analysed using Igor Pro, v. 4.01 (Wavemetrics Inc. 2000, Lake Oswego, OR, USA).
To study the vertical movements of birds we used 30 g Suunto X6 altimeters (Suunto Oy, Vantaa, Finland) recording altitude every 10 s over 10 complete foraging trips on eight individuals. Data on altimeters were corrected for shift in atmospheric pressure through recordings on a fixed-location altimeter in the colony (Weimerskirch et al. 2003, 2004). Altimeters also allowed us to monitor diving events. To estimate the depths recorded by the altimeter, we calibrated the logger in a tank, and obtained a linear relationship between altitude and depth.
PTTs, GPS, accelerometers and altimeters were taped under the three central tail feathers using Tesa tape and activity recorders were placed on the tarsus, taped on a metal band. Because red-footed boobies are relatively small seabirds (mass 780–1050 g), only one type of logger was fitted on each bird (representing a maximum—for GPS—of 3–4% of the bird body mass for the GPS).
Wind strength and direction were obtained from the meteorological station of Europa Island three times a day and from ocean surface winds derived from the SeaWinds Scatterometer available on http://manati.wwb.noaa.gov/quikscat/. To compare expected and observed flight directions we used five categories, from tail to head winds: under the null hypothesis that flight direction relative to wind is not related to wind direction, the proportion of birds flying in each direction would be 3:4:5:4:3 (Spear & Ainley 1997). For each foraging trip we estimated the destination bearing as the bearing between the colony and the most distant point of a trip. The sinuosity of flight tracks measured by GPS was calculated as the ratio of the actual distance covered to the straight-line distance between every sixth fix, i.e. every 60 s. A value of one indicates a straight line.
Statistical analyses were performed using STATISTICA, v. 6.0. Average values are given ±1 s.d. Because the individuals were tracked for several successive trips, to avoid pseudoreplication problems we analysed the data on the characteristics of the trips using mixed-model ANOVAs with categories entered as fixed factors and individuals as a random factor.
3. Results
The foraging trips of red-footed boobies consisted of travelling phases where birds moved rapidly at a constant high speed and constant general headings, although their route is often zig-zagging, and in foraging phases where birds were alternating periods on the water, diving and in flight, with continuous changes in flight directions and speeds (figures 1 and 2).
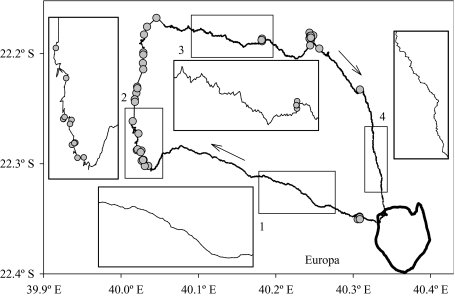
Figure 1.
Typical foraging trip of a red-footed booby from Europa Island on 23 September 2003 studied with GPS. Arrows indicate the movement direction; grey dots, foraging sections. Grey boxes indicate four sectors enlarged on the four adjacent boxes where sinuosity of the track section varied (box 1, almost straight line; box 3, zigzag route). The wind was blowing from 160° (south-southeast) at 15 knots (1 knot=1 nautical mile h−1) during the entire foraging trip.
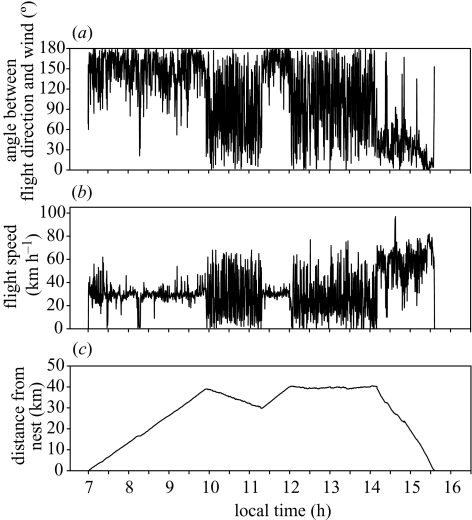
Figure 2.
Recording of the changes (a) in flight direction with regard to the wind direction (the wind was blowing from the same direction at constant speeds (20 knots) throughout the foraging trip); (b) in flight speed; and (c) in distance from the colony during a foraging trip. Periods of travelling from and to the colony are indicated by fairly constant speeds and flight directions, and increase or decrease of distance from or to the colony, whereas periods of foraging show varying speeds and continuous flight direction.
3.1 Flight speed, flight heading and influence of wind
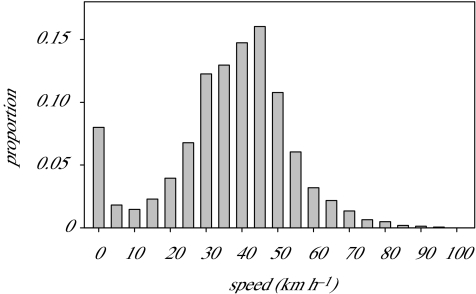
GPS recordings indicate that the distribution of instantaneous speeds (speeds measured between two successive GPS locations with a 10 s interval) was bi-modal, with speeds lower than 10 km h−1 corresponding to birds sitting on water (0 km h−1), drifting or mixing sitting on water and flight or dive (10>speeds>0 km h−1; figure 3). Higher speeds corresponded to birds in flight. The median flight speed was 38 km h−1. During travelling phases flight speed was strongly influenced by the position of the bird with regard to the wind direction, birds flying at a higher speed with a tail wind compared with head winds (figure 4).
Figure 3.
Frequency distribution of 14878 speeds recorded by GPS between two locations at 10 s intervals.
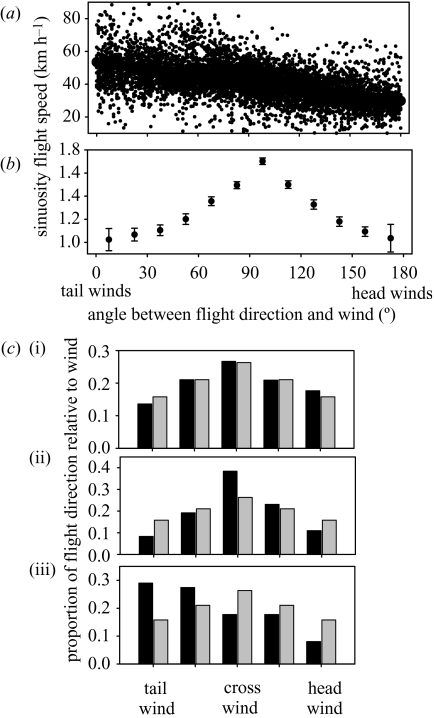
Figure 4.
(a) Instantaneous (between two successive GPS locations) flight speeds and (b) average sinuosity of flight tracks according to the angle between the flight track and the wind direction at 1 min intervals. (c) Expected and observed flight direction relative to wind direction at various time-scales. (i) Instantaneous (10 s intervals between two GPS locations); (ii) route (considering the route between every six fixes, i.e. 1 min); and (iii) overall flight heading between the colony and the first foraging zone. Black bars, observed; grey bars, expected.
When travelling there was no detectable response in flight direction at 10 s intervals (instantaneous) to wind direction (figure 4). However, at a larger scale, birds avoided head and tail winds and preferred routes (flight direction between two locations at 1 min intervals) with side winds (; figure 4). At this scale the sinuosity of flight track was highest with side winds, and the route was straight with tail and head winds (figure 4). During the study period, the wind was blowing mainly from the south (56% of days) and the east (31%), and never from the west. General headings of trips taken at departure were oriented to the west (37%), north and south (29 and 27%, respectively) and rarely east. When leaving the colony, birds headed to foraging areas preferably with tail winds (figure 4).
3.2 Flight pattern
When in flight and travelling, birds alternated regularly flapping and gliding bouts (figure 5), flapping on average 31.4±9.4% and 44.6±12.6% of flight time during travelling and foraging phases (Wilcoxon test for paired samples: Z=2.5 p=0.012), respectively. Flapping frequency was very constant throughout the foraging trip, being on average 3.7±0.2 flapss−1 (range 3.2–4.6). The duration of flapping bouts was 1.7±0.44 s during travelling and 2.55±0.97 s during foraging (Z=2.5 p=0.012), whereas glide duration was not different (3.8±1.39 versus 3.36±1.20 Z=1.4 p=0.162).
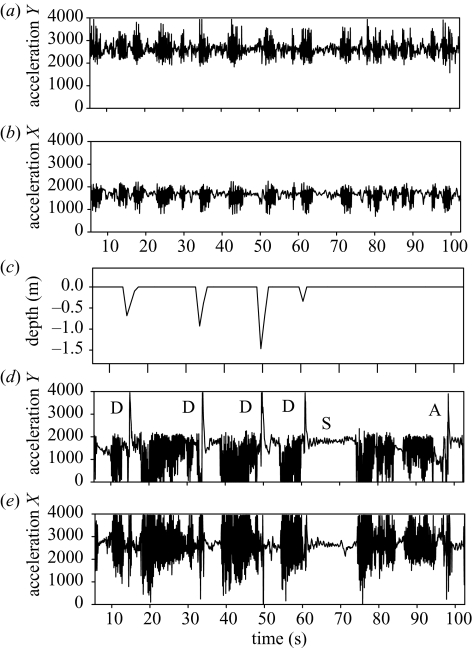
Figure 5.
(a) and (b) Two-way accelerations during a travelling phase showing the succession of flapping bouts and gliding bouts. (c) Depth, and (d) and (e) two-way accelerations during a foraging phase where the bird was alternatively in flapping phases in flight, plunge diving at various depths (D), aerial diving (A) and sitting on the sea surface (S).
3.3 Activity
Birds never spent the night at sea. When they returned after dusk, GPS recordings show that birds did not always land on the colony where they nest, but often landed in nearby colonies or in roosts on fig trees located in the centre of the island. When they landed at night in nearby sites, they returned to their nest at dawn.
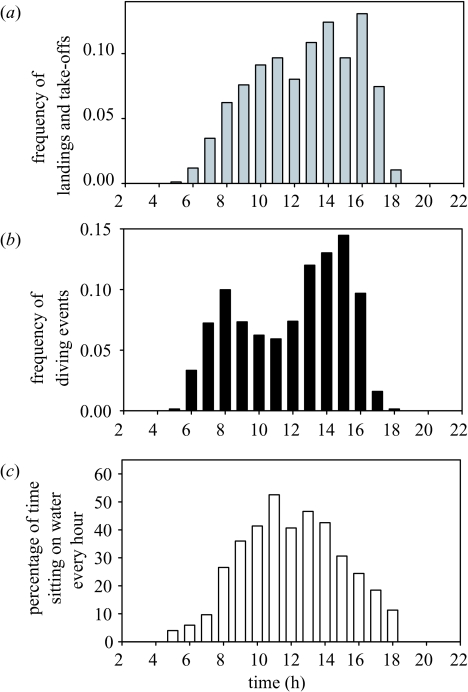
Activity recorders indicated that from the time they left the colony to when they returned to land, birds spent 66.7±14.9% n=31 trips, range 36.0–94.8) of the foraging time in flight, the rest sitting on the water or diving. Accelerometers gave similar results (64.4±16.8% n=14, range 27.2–89.3). The duration of bouts on water (time elapsed between landing and next take-off) lasted between 1 s and 2 h (average 1.6±1.0 min); bouts in flights were longer, ranging from 1 s to 6 h (average 3.3±2.2 min). Birds spent most of the foraging time in flight in the morning and evening, but almost half of the time sitting on the water or diving in the middle of the day (figure 6). They landed on the sea (or dived) on average 29.6±12.2 times per hour (range 8–65) and landings and take-offs peaked in the afternoon (figure 6).
Figure 6.
Frequency distribution of (a) take-off and landing, (b) dives according to the time of the day and (c) percentage of time spent on the water every hour.
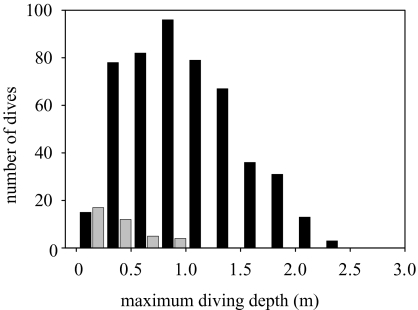
Red-footed boobies are shallow divers, with a maximum recorded diving depth of 2.4 m, and an average diving depth of 0.87±0.52 m (figure 7). The duration of dives ranged between 1 and 8 s (average 1.67±0.84 s), and the number of dives per hour was 4.4±3.7 (range 0.7–14.5, n=15). Dives are mainly (93%) plunge-dives, birds plunging from a certain altitude into the water, the rest being surface dives (the bird dives when sitting on the surface). The maximum depth attained during plunge diving was deeper than that attained during surface dives (0.98±0.47 m versus 0.42±0.22 m respectively; mixed ANOVA: F19=87.6 p=0.003). After a dive, birds remained on the water for brief periods before taking off (average 26.0±88.6 s, range 0–1051 s). Red-footed boobies also per formed dives to the surface that had a typical dive pattern when recorded by the accelerometer, but the bird did not enter the water column (see figure 5). These ‘aerial dives’ were less frequent than real underwater dives (1.5±0.8, range 0.3–3.6 surface divesh−1), but still represented 30.3±19.3% (range 9.1–66.7) of the diving-like behaviour.
Figure 7.
Frequency distribution of the depths attained during plunge dives (black bars) and surface dives (grey bars).
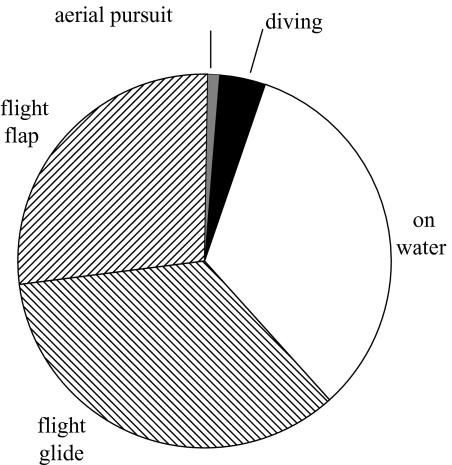
The overall activity budget derived from accelerometers indicates that birds spent more than half of the foraging trip in flight, and 33% sitting on the water, and the rest in dives (figure 8).
Figure 8.
Time budget of red-footed boobies as a percentage of the time spent in various activities during foraging trips.
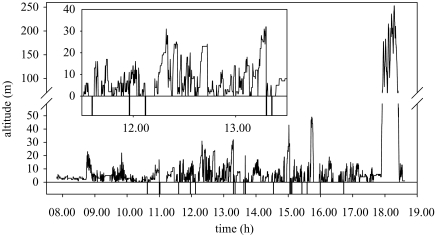
3.4 Flight altitude
When foraging, red-footed boobies stay generally at low altitudes between 1 and 30–40 m, climbing regularly and descending to the sea level either to dive or to sit on the sea surface (figure 9). In six out of 10 trips birds climbed to high altitudes, up to 520 m, before returning to the colony. The final climb and descent phase lasted 24–42 min (average 33 min) and birds climbed at a rate of 0.3 m s−1 (maximum 2.1 m s−1) to maximum altitudes ranging between 120 and 520 m (average 290±163 m; figure 9). Accelerometers indicate that, during the climbs, birds flapped on average 72% of the time. Descent rates were particularly fast, attaining 3.6 m s−1, and were on average 1.3 m s−1. This fast descent rate is probably the reason for the high speeds observed during the final part of the trip as recorded by some birds equipped with GPS.
Figure 9.
Changes in altitude of a red-footed booby fitted with an altimeter. Negative values indicate dives. Inset shows altitude changes between 11.30 and 13.30.
The average flight altitude, excluding the final climb, was 9.0±3.5 m (range 5.4–17 m). When foraging or travelling, birds regularly climb to altitudes of higher than 50 m, in addition to the final climb (see, for example, figure 9). In 4 out of 10 trips, they briefly reached altitudes ranging between 110 and 220 m. When plunge diving; the average altitude 10 s before the dive was 9.2±7.4 m (range 2–32 m).
4. Discussion
4.1 Timing of foraging
Previous studies have suggested that red-footed boobies are the only booby to spend the night at sea (Nelson 1978; Schreiber et al. 1996) but our study demonstrates that they are exclusively diurnal foragers, leaving early in the morning, and returning to the colony before or after dusk (see also Verner 1965). Upon their return to the island, birds did not return systematically to their nests, but often joined roosts far from the colonies, which probably explains why red-footed boobies were thought to spend the night at sea. Our study shows that birds never spent the night sitting on the sea surface, while they often did so during the day. This suggests that there is a high selective pressure not to stay on the sea surface at night, as in other tropical seabird species such as frigatebirds or sooty terns, which spend the whole night at sea in flight (Schreiber et al. 2002; Weimerskirch et al. 2003, 2004a). Predation, notably by sharks, may be the major reason for such behaviour but the inability to forage at night may also be involved. This is in sharp contrast with pelagic temperate and polar seabirds that generally spend the night on the water: albatrosses and petrels (Weimerskirch et al. 1997; Weimerskirch & Guionnet 2002), including the closely related gannets (Garthe et al. 1999, 2003; Ropert-Coudert et al. 2004a). However, gannets do not feed during the night (Garthe et al. 1999; Grémillet et al. 2004; Ropert-Coudert et al. 2004a,b); they only rest, whereas albatrosses or petrels can feed during the night (Weimerskirch & Wilson 1992; Weimerskirch et al. 1997; Croxall & Prince 1994).
4.2 Activity budget and foraging technique
Red-footed boobies are considered long-range foragers compared with other boobies and are able to forage at maximum distances of 150 km from the colonies during trips lasting a maximum of 12 h, as a result of the duration of daytime (this study; Weimerskirch et al. 2005). On average red-footed boobies spend two-thirds of their foraging time in flight, the rest sitting on the water or diving. This value is much higher than those observed in seabirds from temperate or polar waters such as gannets (Garthe et al. 1999, 2003; Ropert-Coudert et al. 2004b), alcids (Kato et al. 2003) or albatrosses (Weimerskirch & Guionnet 2002). Our study also shows that boobies are extremely active when foraging, and that active foraging does not take place throughout the foraging trip, but only in some areas, often located at the extremity of the trip, similar to Northern and Cape gannets (Garthe et al. 1999; Grémillet et al. 2004). When actively foraging, birds are continuously landing, or diving, at a rate of 30 landings or dives per hour, including 4.5 dives per hour under the sea surface. This rate of landing on the water is much higher than that of temperate gannets (Garthe et al. 2003; Ropert-Coudert et al. 2004b).
Red-footed boobies are shallow divers, diving to maximum depths of 2.4 m. These shallow dives are associated with a typical plunge-diving behaviour similar to that of gannets where birds plunge from a certain height to reach depths (Ropert-Coudert et al. 2004a). However, dives are much shallower than those of gannets, which can reach depths of 6–10 m (Garthe et al. 2000; Grémillet et al. 2004), possibly because boobies plunge from lower altitudes (4–9 m (Schreiber et al. 1996; this study)) than gannets (more than 30 m (Lee & Redish 1981)), gannets may also use wing propulsion underwater (Ropert-Coudert et al. 2004a). Also, in addition to plunge-diving, red-footed boobies have a significant number (1.5 h−1) of their dive-like behaviour that is not associated with underwater diving, but probably with dive movements to catch prey at the surface. These aerial dives detected by accelerometers, corresponding to vertical or oblique surface dives, in addition to the very short bouts on the water that represent the majority of the sea landings, probably correspond to their specific prey capture technique. They allow red-footed boobies to pursue and capture mobile prey such as flying fishes and squids at or above the surface (Diamond 1974; Hertel & Ballance 1999). This technique may be specific to this species because it has been suggested that other boobies plunge-dive only (Hertel & Ballance 1999), but further studies on the other species are needed to confirm this.
On Europa Island, boobies feed mainly on flying fishes and flying squids (M. Le Corre and H. Weimerskirch, unpublished data). These prey are therefore captured at shallow depths or just at—or above—the surface, and made available for boobies by sub-surface predators that push such prey to the surface (Au & Pitman 1986; Ballance et al. 1997). The particular technique of hunting that we have described here could be related to the way boobies catch prey just at the surface, in association with sub-surface predators. Timing of prey capture, as indicated by the frequency of dives, peaked in the morning and in the evening, i.e. at a similar time to frigatebirds in the same area (Weimerskirch et al. 2004). These peaks of activity could be related to associations with tunas because the feeding activity of skipjack tunas, the major tuna species with whom tropical seabirds like red-footed boobies associate, takes place similarly in the early morning and late afternoon (Froese & Pauly 2003).
4.3 Foraging costs and influence of wind
Red-footed boobies have an overall low cost of foraging (Ballance 1995). However, when they are actively foraging, the proportion of flapping is high and the numbers of take-offs and landings per unit of time are extremely high. The very high frequency of landings and take-offs suggests a high cost of foraging (Weimerskirch et al. 2000; Shaffer et al. 2001). In addition, diving is almost exclusively made of successive plunge-dives that require the bird to take off for the next dive, surface dives being rare compared with gannets (1.4% of the dives in Cape gannets (Ropert-Coudert et al. 2004a)). How can the potential high costs of foraging be reconciled with the relatively low cost of the overall foraging trip measured in this species (Ballance 1995)? When travelling, red-footed boobies use a flap–glide flight, where they alternate on average 3.5 flaps lasting on average 1.7 s, with a glide lasting 3.8 s, spending 69% of the flight gliding, i.e. a very important proportion of the flight, indicating that costs of flight are probably much reduced this way. Their relatively low wing loading (see Pennycuick 1987) compared with other boobies (Hertel & Ballance 1999) also suggests that they are efficient gliders, even compared with other gannets (Cape gannets glide 22% of the flight time (Ropert-Coudert et al. 2004b)). The low aspect ratio of red-footed boobies is more difficult to explain, but may be advantageous for a species requiring high skills for aerial pursuit predatory behaviour (Hertel & Ballance 1999). As a consequence of the high gliding rate, foraging costs are much lower in red-footed boobies than in gannets (Birt-Friesen et al. 1989; Ballance 1995). Similarly within petrels, despite similar masses giant petrels have higher flight costs, and higher wing loading and a lower aspect ratio than albatrosses (Obst & Nagy 1992).
Red-footed boobies breed in the trade-wind zone of the tropics. On Europa Island, strong winds blow throughout the year, mainly from the south, and constitute a predictable source of energy for the flight of seabirds. The ability of boobies to glide during an extended proportion of the foraging time is probably made possible by the use of wind (Ballance 1995; Spear & Ainley 1997). In that respect red-footed boobies present some striking convergences in their flight behaviour with albatrosses by using the wind and gliding extensively. They look like miniature albatrosses, their morphology being in several respects similar, with long narrow wings. However, because albatrosses use dynamic soaring, and red-footed boobies a flap–glide flight, the way they use the wind is different. Red-footed boobies fly primarily with side winds (see also Spear & Ainley 1997) and avoid head as well as tail winds. Whereas avoiding a head wind is logical, avoiding tail winds is more surprising because flying downwind is believed to be energetically more efficient (Pennycuick 1989). The avoidance of tail wind has also been noted from observation at sea of flap–gliding species, which suggests that avoiding tail winds must be advantageous in terms of prey detection—a too high speed does not allow optimal prey detection—or in terms of efficient position of the wing with respect to wind (Spear & Ainley 1997). The sinuosity of flight track was highest with side winds, and the route was straight with tail and head winds. This is different from albatrosses, which tackle head wind and thus have a high sinuosity with head winds (Alerstam et al. 1993; Weimerskirch et al. 2000, 2002; H. Weimerskirch, unpublished data). The straight-line route of boobies in head winds appears paradoxical but is probably related to the particular morphology of boobies, which use flapping to a large extent and thus can flap against head wind, whereas albatrosses do not use flapping flight.
4.4 Altitude
The use of altimeters indicates that red-footed boobies do not fly as high in the sky as, for example, frigatebirds do (Weimerskirch et al. 2003, 2004). Instead, they are moving at a few metres above the sea surface. However, they can reach high altitudes in two circumstances. During the travelling phases of their trip, birds regularly climb to altitudes of 20–50 m, which may be related to the use of wind but is more likely to reflect prey searching. Indeed, seabirds such as red-footed boobies are searching over vast expanses of ocean for scattered prey patches. Locating ephemeral prey is probably favoured by taking some altitude to spot prey movements at the sea surface, but also to spot the movements of other congeners or other seabird species such as sooty terns that are feeding. This technique of monitoring the actions of other individuals is known as local enhancement (Haney et al. 1992) and is common in seabirds (see review by Nevitt & Veit 1999).
During their return flight most birds finish their trip by climbing to high altitudes, up to 500 m, before descending rapidly to the island. This strategy has an associated cost because climbing to high altitude involves an increased proportion of flapping during the climb, 0.72 compared to the average value of 0.44 during other travelling sections of the trip. This behaviour could enhance the spotting of the colony and reaching it using the shortest route, as well as avoiding the attacks of frigatebirds that patrol along the seashore and at sea in the vicinity of the island (Le Corre & Jouventin 1997) to parasitize seabirds returning from the sea with full stomachs. Birds descending at high speeds from an altitude taken further offshore are not attacked by frigatebirds, because of their speed and ability to rush rapidly into the forest.
4.5 Optimization of flight track
When foraging, the movement of seabirds is optimized so that it minimizes the time spent to reach food resources whose distribution is often predictable in some specific oceanic sectors (Hunt et al. 1999), and minimizes the energy expenditure that is often influenced by wind conditions (Furness & Bryant 1996; Weimerskirch et al. 2000). Whereas they show no significant response to wind direction at a small scale (every 10 s), red-footed boobies favour side winds at a medium scale (route during 1 min). The advantage of flying with side winds is probably related to the optimal use of wind by boobies, to reduce flight costs. At a larger scale, another significant pattern appears: when leaving the colonies for foraging grounds birds appear to have an overall heading with tail winds, suggesting a possible selection of overall flight routes relative to wind direction. However, because wind was blowing primarily from the south and east, flight directions at departure may rather be dictated by the presence of food patches in the north or west. The differential response of birds in flight direction to wind direction at different scales illustrates the importance of considering the behaviour of foraging seabirds in a hierarchical spatial system (Fauchald 1999; Nevitt & Veit 1999). Like albatrosses (Fritz et al. 2003) the path of red-footed boobies is probably adjusted at different scales according to prey distribution and wind conditions. At a very small scale, the zigzagging movement is aimed at adjusting flight according to wind condition to take advantage of the wind to perform the flap glide flight. At a larger scale, birds try to keep crosswinds, and probably adjust their route according to the searching of prey patches that are patchily distributed, because active foraging takes place in some scattered specific sectors. At the same time birds regularly take altitude to increase the ability to spot prey patches, or other seabirds feeding. At a larger scale the general heading taken from the colony is influenced possibly by wind, but also by previous knowledge of productive zones. Such a strategy is close to that observed in wandering albatrosses (Fritz et al. 2003), a species whose reliance on wind is well known.
5. Conclusions
Our study demonstrated the use by red-footed boobies of a series of very specific flight and activity patterns that are not observed in temperate and polar seabirds and have probably been selected as adaptations to the conditions of tropical waters. They include a very active foraging technique with numerous landings at the sea surface, and shallow dives associated with the capture of fast-moving prey such as flying fishes. The extensive use of the flap–glide technique, with long periods of gliding, appears as an adaptation to the windy conditions of the trade wind zone that results in low foraging costs. Another specific technique is the climb at medium to high altitude to spot prey patches or favour local enhancement, and when returning to the colony to avoid klepto-parasitism by frigatebirds. Biologists have long been attracted to the study of locomotor extremes because they provide clear examples from which to determine structure–function relationships (Dickinson et al. 2000). Tropical seabirds are good examples because they are facing extreme conditions by foraging in an impoverished environment, where extreme adaptations in morphologies and foraging techniques are expected. The spectacular foraging strategy of frigatebirds (Weimerskirch et al. 2003, 2004) and the particular flight behaviour of red-footed boobies are good examples of the relationships between the abiotic (wind) and biotic environment and the evolution of morphologies, energetic and behavioural.
6. Uncited references
Please cite Hamer et al. (2001); Hedd et al. (1997); Ropert-Coudert et al. (2001).
Acknowledgments
We thank the Direction Régionale de l’Environnement (DIREN) of Île de la Réunion for financial support, the Préfet de la Réunion and the Direction of Météo France for authorizations and help to work on Europa, the Forces Armées de la Zone Sud de l’Océan Indien (FAZSOI) for transport to and logistical support on Europa. We thank Sébastien Jaquemet for help with the field study and Kaori Kobayashi for her extensive help with the analysis of the accelerometer data.
References
- Alerstam T., Gudmundsson G.M., Larsson B. Flight tracks and speeds of Antarctic and Atlantic seabirds, radar and optical measurements. Phil. Trans. R. Soc. Lond. B. 1993;340:55–67. [Google Scholar]
- Ashmole N.P. Seabird ecology and the marine environment. In: Farner D.S., King J.R., editors. Avian biology. vol. 1. Academic; New York: 1971. pp. 223–286. [Google Scholar]
- Au D.W.K., Pitman R.L. Seabird interactions with dolphins and tuna in the eastern tropical Pacific. Condor. 1986;88:304–317. [Google Scholar]
- Ballance L.T. Flight energetics of free-ranging red-footed boobies (Sula sula) Physiol. Zool. 1995;68:887–914. [Google Scholar]
- Ballance L.T., Pitman R.L. Foraging ecology of tropical seabirds. In: Adams N.J., Slotow R.H., editors. Proc. 22nd Int. Ornithol. Congr., Durban. Birdlife South Africa; Johannesburg: 1999. pp. 2057–2071. [Google Scholar]
- Ballance L.T., Pitman R.L., Reilly S.B. Seabird community structure along a productivity gradient, importance of competition and energetic constraint. Ecology. 1997;78:1502–1518. [Google Scholar]
- Birt-Friesen V.L., Montevecchi W.A., Cairns D.K., Macko S.A. Activity-specific metabolic rates of free-living northern gannets and other seabirds. Ecology. 1989;70:357–367. [Google Scholar]
- Boyd I.L., Kato A., Ropert-Coudert Y. Bio-logging science: sensing beyond the boundaries. Mem. Natl Inst. Polar Res. 2004;58:1–14. (Special Issue) [Google Scholar]
- Croxall J.P., Prince P.A. Dead or alive, night or day, how do albatrosses catch squid? Ant. Sci. 1994;6:155–162. [Google Scholar]
- Diamond A.W. The red-footed booby on Aldabra Atoll, Indian ocean. Ardea. 1974;62:196–218. [Google Scholar]
- Dickinson M.D., Farley C.T., Full R.J., Koehl M.A.R., Kram R., Lehman S. How animals move, an integrative view. Science. 2000;288:100–106. doi: 10.1126/science.288.5463.100. [DOI] [PubMed] [Google Scholar]
- Fauchald P. Foraging in a hierarchical patch system. Am. Nat. 1999;153:603–613. [Google Scholar]
- Flint E.N., Nagy K.A. Flight energetics of free living sooty terns. Auk. 1984;101:288–294. [Google Scholar]
- Fritz H., Said S., Weimerskirch H. Scale-dependent hierarchical adjustments of movement patterns in a long range foraging seabird. Proc. R. Soc. B. 2003;270:1143–1148. doi: 10.1098/rspb.2003.2350. doi:10.1098/rspb.2003.2350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froese, R. & Pauly, D. 2003 Fishbase. World Wide Web electronic publication, www.fishbase.org.
- Furness R.W., Bryant D.M. Effect of wind on field metabolic rates on Northern fulmars. Ecology. 1996;77:1181–1188. [Google Scholar]
- Garthe S., Grémillet D., Furness R.W. At sea activity and foraging efficiency in chick rearing northern gannets Sula bassana, a case study in Shetland. Mar. Ecol. Prog. Ser. 1999;185:93–99. [Google Scholar]
- Garthe S., Benvenuti S., Montevecchi W.A. Pursuit plunging by Northern gannets (Sula bassana) feeding on Capelin (Mallotus villosus) Proc. R. Soc. B. 2000;267:1717–1722. doi: 10.1098/rspb.2000.1200. doi:10.1098/rspb.2000.1200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garthe S., Benvenuti S., Montevecchi W.A. Temporal patterns of foraging activities of northern gannets, Sula bassanus, in the northwest Atlantic Ocean. Can. J. Zool. 2003;81:453–461. [Google Scholar]
- Grémillet D., Dell’Omo G., Ryan P.G., Peters G., Ropert-Coudert Y., Weeks S.J. Offshore diplomacy, or how seabirds mitigate intra-specific competition: a case study based on GPS tracking of cape gannets from neighbouring colonies. Mar. Ecol. Prog. Ser. 2004;268:265–279. [Google Scholar]
- Hamer K.C., Phillips R.A., Hill J.K., Wanless S., Wood A.G. Contrasting foraging strategies of gannets (Morus bassana) at two North Atlantic colonies, foraging trip duration and foraging area fidelity. Mar. Ecol. Prog. Ser. 2001;224:283–290. [Google Scholar]
- Haney J.C., Fristrup K.M., Lee D.S. Geometry of visual recruitment by seabirds to ephemeral foraging flocks. Ornis Scand. 1992;23:49–62. [Google Scholar]
- Hedd A., Gales R., Brothers N., Robertson G. Diving behaviour of the shy albatross Diomedea cauta in Tasmania, initial findings and dive recorder assessment. Ibis. 1997;139:452–460. [Google Scholar]
- Hertel F., Ballance L.T. Wing ecomorphology of seabirds from Johnston Atoll. Condor. 1999;101:549–556. [Google Scholar]
- Hunt G.L., Mehlum F., Russell R.W., Irons D., Decker M.B., Becker P.H. Physical processes, prey abundance, and the foraging ecology of seabirds. In: Adams N.J., Slotow R.H., editors. Proc. 22 Int. Ornithol. Congr., Durban. BirdLife South Africa; Johannesburg: 1999. pp. 2040–2056. [Google Scholar]
- Kato A., Watanuki Y., Naito Y. Foraging behaviour of chick-rearing Rhinoceros Auklets at Teuri Island, Japan, determined by acceleration-depth recording micro data loggers. J. Avian Biol. 2003;34:282–287. [Google Scholar]
- Le Corre M., Jouventin P. Kleptoparasitism in tropical seabirds, vulnerability and avoidance responses of a host species, the red-footed booby. Condor. 1997;99:162–168. [Google Scholar]
- Lee D.N., Redish P.E. Plummeting gannets: a paradigm of ecological optics. Nature. 1981;293:293–294. [Google Scholar]
- Longhurst A.R., Pauly D. Ecology of tropical ocean. Academic; San Diego, CA: 1987. [Google Scholar]
- Nelson J.B. The Sulidae, gannets and boobies. Oxford University Press; 1978. [Google Scholar]
- Nevitt G., Veit R.R. Mechanisms of prey-patch detection by foraging seabirds. In: Adams N.J., Slotow R.H., editors. Proc. 22 Int. Ornithol. Congr., Durban. BirdLife South Africa; Johannesburg: 1999. pp. 2072–2082. [Google Scholar]
- Obst B.S., Nagy K.A. Field energy expenditure of southern giant petrels. Condor. 1992;94:801–810. [Google Scholar]
- Pennycuick C.J. The flight of petrels and albatrosses (Procellariiformes) observed in South Georgia and its vicinity. Phil. Trans. R. Soc. Lond. B. 1982;300:75–106. [Google Scholar]
- Pennycuick C.J. Flight of seabirds. In: Croxall J.P., editor. Seabirds, feeding ecology and role in marine ecosystems. Academic; New York: 1987. pp. 43–62. [Google Scholar]
- Pennycuick C.J. Bird flight performance: a practical calculation manual. Oxford Science Publications. Oxford University Press; 1989. [Google Scholar]
- Ropert-Coudert Y., Kato A., Baudat J., Bost C.-A., Le Maho Y., Naito Y. Feeding strategies of free-ranging Adelie penguins, Pygoscelis adeliae, analyzed by multiple data recording. Polar Biol. 2001;24:460–466. [Google Scholar]
- Ropert-Coudert Y., Grémillet D., Ryan P., Kato A., Naito Y., Le Maho Y. Between air and water, the plunge dive of the Cape gannet Morus capensis. Ibis. 2004a;146:281–290. [Google Scholar]
- Ropert-Coudert Y., Grémillet D., Kato A., Ryan P., Naito Y., Le Maho Y. A fine-scale time budget of Cape gannets provides insights into the foraging strategies of coastal seabirds. Anim. Behav. 2004b;65:985–992. [Google Scholar]
- Schreiber E.A., Schreiber R.W., Schenk G.A. Red-footed booby (Sula sula) In: Poole A., Gill F., editors. The birds of North America. no. 241. The Academy of Natural Sciences; Philadelphia, PA: The American Ornithologist’ Union; Washington, DC: 1996. p. 23. [Google Scholar]
- Schreiber E.A., Feare C.J., Harrington B.A., Murray B.G., Jr, Robertson W.B., Jr, Robertson M.J., Woolfenden G.E. Sooty tern (Sterna fuscata) In: Poole A., Gill F., editors. The birds of North America. no. 665. The Academy of Natural Sciences; Philadelphia, PA: The American Ornithologist’ Union; Washington, DC: 2002. p. 32. [Google Scholar]
- Shaffer S., Costa D., Weimerskirch H. Behavioural factors affecting foraging effort of breeding wandering albatrosses. J. Anim. Ecol. 2001;70:864–874. [Google Scholar]
- Spear L.B., Ainley D.G. Flight behaviour of seabirds in relation to wind direction and wing morphology. Ibis. 1997;139:221–233. [Google Scholar]
- Steiner I., Bürgi C., Werffeli S., Dell’Omo G., Valenti P., Tröster G., Wolfer D.P., Lipp H.-P. A GPS logger and software for analysis of homing in pigeons and small mammals. Physiol. Behav. 2000;71:589–596. doi: 10.1016/s0031-9384(00)00409-1. [DOI] [PubMed] [Google Scholar]
- Verner J. Flight behaviour of the red-footed booby. Wilson Bull. 1965;77:229–234. [Google Scholar]
- Watanuki Y., Niizuma Y., Gabrielsen G.W., Sato K., Naito Y. Stroke and glide of wing-propelled divers, deep diving seabirds adjust surge frequency to buoyancy change with depth. Proc. R. Soc. B. 2003;270:483–488. doi: 10.1098/rspb.2002.2252. doi:10.1098/rspb.2002.2252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weimerskirch H., Guionnet T. Comparative activity pattern during foraging of four albatross species. Ibis. 2002;144:40–50. [Google Scholar]
- Weimerskirch H., Wilson R.P. When do wandering albatrosses Diomedea exulans forage? Mar. Ecol. Prog. Ser. 1992;86:297–300. [Google Scholar]
- Weimerskirch H., Wilson R., Lys P. Activity pattern of foraging in the wandering albatross, a marine predator with two modes of prey searching. Mar. Ecol. Prog. Ser. 1997;151:245–254. [Google Scholar]
- Weimerskirch H., Guionnet T., Martin J., Shaffer S.A., Costa D.P. Fast and fuel efficient? Optimal use of wind by flying albatrosses. Proc. R. Soc. B. 2000;267:1869–1874. doi: 10.1098/rspb.2000.1223. doi:10.1098/rspb.2000.1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weimerskirch H., Bonadonna F., Bailleul F., Mabille G., Dell’Omo G., Lipp H.P. GPS tracking of foraging albatrosses. Science. 2002;295:1259. doi: 10.1126/science.1068034. [DOI] [PubMed] [Google Scholar]
- Weimerskirch H., Chastel O., Barbraud C., Tostain O. Frigatebirds ride high on thermals. Nature. 2003;421:333–334. doi: 10.1038/421333a. [DOI] [PubMed] [Google Scholar]
- Weimerskirch H., Le Corre M., Jaquemet S., Potier M., Marsac F. Foraging strategy of a top predator in tropical waters, great frigatebirds in the Mozambique Channel. Mar. Ecol. Prog. Ser. 2004;275:297–308. [Google Scholar]
- Weimerskirch, H., Le Corre, M., Jaquemet, S. & Marsac, F. 2005 Foraging strategy of a tropical seabird, the red-footed booby, in a dynamic marine environment. Mar. Ecol. Prog. Ser (In the press.)