Abstract
The evolution of group-beneficial traits potentially allows the survival of ‘cheaters’ that would otherwise be unfit. Here we describe experimental work on group-beneficial traits and the consequences of frequency-dependent selection in the context of bacterial antibiotic resistance. We constructed a ‘self-limited antibiotic resistant’ (SLAR) strain of Escherichia coli in which a TEM-1 β-lactamase was anchored to the inner membrane. In pairwise competition experiments between the SLAR strain and ampicillin-sensitive strains, only the SLAR strain survived in the presence of ampicillin. We also constructed a ‘shared antibiotic resistant’ (SAR) strain in which TEM-1 β-lactamase protected both the SAR strain and nearby sensitive cells, thus acting as a model for a genetically defined group-beneficial trait. In pairwise competition experiments of the SAR strain against two different sensitive strains of E. coli, we found that the sensitive strains maintained themselves at frequencies of 5–12% in the presence of ampicillin. When the relative cost of the SAR strain was lowered, its equilibrial frequency rose. Sensitive strains also arose from pure cultures of the SAR strain. In these cases, too, the sensitive ‘cheaters’ were maintained in ampicillin at frequencies comparable to those observed in the previous competitions. These results suggest that traits which benefit other group members can permit survival of genotypes that otherwise would be eliminated by natural selection, and allow the maintenance of greater genetic variation upon which evolution can operate.
Keywords: frequency dependence, group-beneficial traits, antibiotic resistance
1. Introduction
The evolution of group-beneficial traits has been one of the more contentious topics in evolutionary biology (Williams 1966; Wilson 1975; Dawkins 1976; Wilson 1980; Sober & Wilson 1998). Experimental evidence for the presence or absence of genes coding for group-beneficial traits has been difficult to obtain, in part because investigators often focus on behaviours—such as alarm calling in mammals—whose genetic basis is unknown. Questions surrounding group-beneficial traits have long plagued behavioural studies in mammalian and other vertebrate systems (Levin 1988; Rainey & Travisano 1998; Sober & Wilson 1998; Bohannan & Lenski 2000; Massey & Peacock 2002; Travisano 2001; Velicer & Yu 2003; West & Buckling 2003; Greig & Travisano 2004). Bacterial models afford the ability to work with large population sizes and rapid generation time so as to provide a system with relative ease of manipulation (Eberhard 1990; Bohannan & Lenski 2000). Karthikeyan et al. (1998) for example, were able to follow the protection afforded by strains of Pseudomonas fluorescens that degrade benzoate in the medium, finding that protection to benzoate-sensitive organisms in a biofilm occurred as a function of distance from the benzoate degraders, and thus demonstrating that benzoate degraders are capable of producing a protective microenvironment for other members of the biofilm community.
In this paper, we present data that use bacterial populations and β-lactamase-mediated resistance to ampicillin to address long-standing questions about frequency-dependent selection and group-beneficial traits. In particular, we wished to examine whether traits that benefit other group members could permit survival of genotypes that otherwise would be eliminated by natural selection, and thereby allow the maintenance of greater genetic variation upon which evolution can operate (Fiegna & Velicer 2003; Velicer & Yu 2003; Greig & Travisano 2004). The maintenance of genetic diversity in microbial communities may be important in the face of changing environmental conditions, such as new antibiotic treatments, and as such, our experimental work may have implications for medical science, as well as evolutionary biology.
For our experiments on antibiotic resistance and the evolution of group-beneficial traits via frequency-dependent selection, we began by creating two strains of otherwise isogenic Escherichia coli, a Gram-negative bacterium. The first strain, which we label ‘self-limited antibiotic resistant’ (SLAR), was one in which only cells containing the plasmid-borne blaTEM–1 gene were resistant to ampicillin. For the second strain, which we label ‘shared antibiotic resistant’ (SAR), cells containing the blaTEM–1 gene, as well as cells in their vicinity, were protected against ampicillin. The blaTEM–1 gene present on transposon Tn3 encodes the class A TEM-1 β-lactamase. TEM-1 is the most common plasmid-mediated β-lactamase among Gram-negative bacteria, and is often found encoded on commercially available plasmid vectors.
Like most class A enzymes, TEM-1 is able to hydrolyse both penicillins and cephalosporins by cleaving the amide bond in the β-lactam ring (Petrosino et al. 1998). It has a high affinity for ampicillin, and the blaTEM–1 gene confers high-level bacterial resistance to this drug. In Gram-negative bacteria, TEM-1 β-lactamase can be completely cell-associated such that only cells producing this enzyme are protected from ampicillin. By contrast, for Gram-positive bacteria, β-lactamases are often transported out of the cell into the medium, so that the drug concentration in the medium actually declines for all cells in the vicinity of the β-lactamase-producing bacteria (Petrosino et al. 1998; Nordmann 2003).
In addition to our SLAR and SAR strains of E. coli, we also constructed an ampicillin-sensitive strain—ampsens1—that was isogenic with the SLAR and SAR strains, with one exception: a kanamycin resistance gene the same size as the blaTEM-1 gene was inserted into the plasmid and the blaTEM-1 gene was deleted. Thus, the ampsens1 strain contained a plasmid that was approximately the same size as that found in the SAR and SLAR strains, but the ampsens1 strain was sensitive to ampicillin. The construction of ampsens1 allowed us to manipulate the relative cost–benefit ratio of genotypes in our experimental populations, and examine the consequent change in genotype frequencies (Lenski & Hattingh 1986; Levin 1988; Smith 2001; Dugatkin et al. 2003).
2. Materials and methods
2.1 Bacterial strains, plasmids, and antibiotic resistance genes
Two otherwise isogenic strains of E. coli—REL607 (also known as JB12; Ara+) and REL606 (also known as JB11; Ara−) (Lenski et al. 1994a)—were used in our experiments. The only difference between these strains is a mutation in the ara operon that prevents use of arabinose. This mutation has been shown to have no observable effects on fitness, but provides a quick and easy way to distinguish the Ara− from the Ara+ cells, as the former are light red and the latter are white on arabinose–tetrazolium agar plates (Lenski et al. 1994a). All plasmid constructs were transformed into both strains, so that in competitions all pairwise combinations could be tested. We were thus able to demonstrate with each set of experiments that the strain background (i.e., Ara+ or Ara−) did not play a role in the outcome of the competition. Also, no Ara+ revertants were obtained from Ara− cells. These host strains also were used in competitions as ‘ampsens2’ strains. They were otherwise isogenic with plasmid-bearing competitors, but lacked both antibiotic resistance and the plasmids normally carrying the genes for such resistance. Similar results were obtained with a second set of strains—strains REL373 (Lac−) and REL383 (Lac+) (Lenski et al. 1994b)—but here we only report those with the REL607 and REL606 backgrounds.
All plasmids were derived from the small, non-conjugative pCR2.1 TOPO (Invitrogen, Carlsbad, CA); in each case the resident ampicillin-resistance gene of the original vector has been inactivated by a deletion. Controlled expression of the blaTEM−1 gene variants is provided by the lac promoter or operator located upstream of the cloning site. The pSLAR1 plasmid contains the blaTEM−1 gene with mutations that interfere with signal peptide cleavage (mutant 7–4) (Palzkill et al. 1994). These mutations prevent the β-lactamase from being fully transported into the periplasm. The protein is essentially ‘locked’ into the cytoplasmic membrane facing into the periplasm. It is active there, and only ca. 2% of the protein is processed and released freely into the periplasmic space (Palzkill et al. 1994). Thus, this plasmid provides resistance only to those cells that possess it, hence it is a ‘SLAR’ strain in terms of β-lactamase retention. The second plasmid used in our studies is pSAR1, which is identical to pSLAR1, except that the blaTEM−1 gene has been fused at its 5′ end to a DNA fragment encoding the ompA and lpp signals (Ghrayeb et al. 1984; Lunn et al. 1986). This allows the enzyme to be transported to the outer membrane, where it is tethered to the cell, extending into the growth medium, where it destroys ampicillin in the surrounding medium. Thus, other cells nearby benefit. Hence, strains bearing this plasmid may be considered ‘SAR’. The third plasmid, pAmpsens1, is isogenic with the first two, except a kanamycin resistance gene, aphA-2, roughly the same size as the blaTEM–1 gene, has been inserted in place of this latter gene. This plasmid is essentially the same size overall as plasmids pSLAR1 and pSAR1, but provides no ampicillin resistance. In all cases, the cloned gene is under control of the lac promoter or operator and is inducible with isopropyl b-d-thiogalactopyranoside (IPTG).
2.2 Growth conditions
We have chosen to use liquid culture media for these studies because this will allow an assessment of the contributions of free-floating, well-mixed individual cells to group survival and protection from antibiotic. This design helps avoid aggregate effects to which biofilm experiments might be prone. This system is neither strictly batch culture nor chemostat. Rather, a special experimental flask system was built for our competition experiments. Two 250 ml flasks were connected, but separated by a membrane. One flask housed freshly supplied nutrients (which were replenished every 24 h), while the other flask housed the bacteria in competition experiments. The dialysis membrane between the growth and exchange flasks had a size exclusion limit of 6000 Da. In this way β-lactamase could accumulate in the growth flask with each generation, so that any benefit provided to the group had a chance to accumulate. These flasks provided for the exchange (across the dialysis membranes) of fresh broth, antibiotic and IPTG, which was used to induce expression of the blaTEM–1 gene via the lac promoter.
Cells were grown in Davis minimal media supplemented with 25μg ml−1 glucose for at least 100 generations and kept in a steady state of growth with constant shaking and by constant exchange with fresh medium. The optical density at 600 nm was never allowed to exceed 0.6. Cultures were started with an inoculum of at least 104 cells and IPTG at 40μg ml−1. A concentration of 100μg ml−1 ampicillin was used. This concentration of antibiotic was maintained throughout the experiment with ampicillin freshly supplied to the exchange flask, as described above. At appropriate time points (approximately every 24 h), aliquots from cultures with and without antibiotic were plated onto drug-free arabinose tetrazolium plates. The numbers of light red versus white colonies were counted in each case.
3. Results and discussion
Experiments in which each strain was grown in isolation found that all strains grew in the absence of antibiotic, but only the SLAR and SAR lines grew when ampicillin (100μg ml−1) was present. Cells in the ampsens1 strain were killed rapidly in these concentrations of ampicillin, so that no survivors were detected after 1 day into the experiment when this strain alone was inoculated.
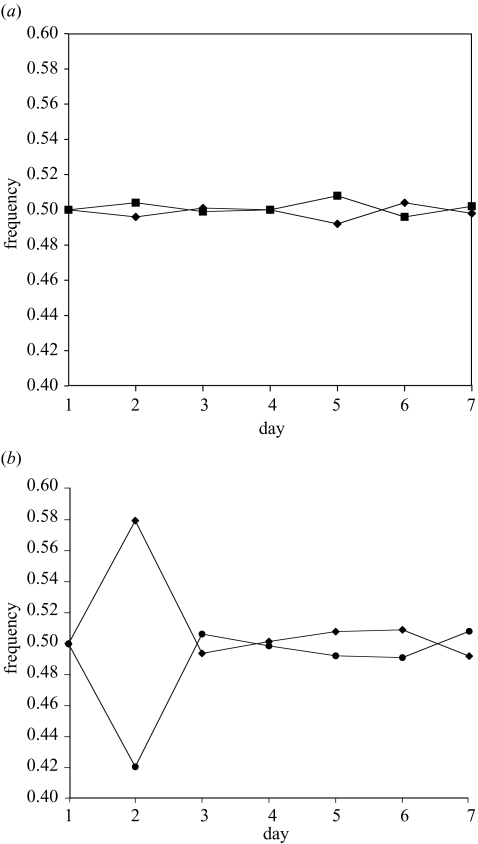
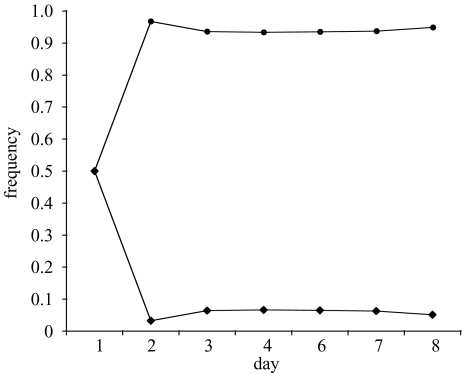
Pairwise competition trials, in which each strain was initiated at a frequency of 0.50, were run for 6–7 days, and strain frequencies were censused daily. We predicted that in the absence of ampicillin, the ampsens1 strain should stabilize at a frequency of ca. 0.5 in competition experiments with either the SAR or SLAR strains. Our results support this prediction (figure 1a,b). In the presence of ampicillin, the ampsens1 strain dropped to a frequency of zero in all competition experiments in which it was pitted against the SLAR strain. However, the ampsens1 strain—even though sensitive to ampicillin—was able to maintain itself at a low frequency in competition experiments with the SAR strain in the presence of ampicillin (figure 2; frequency of ampsens1 when in competition with SAR was greater than the frequency of ampsens1 when in competition with the SLAR strain; repeated-measures ANOVA: F185=143.58 p<0.0001; no effect of day, F585=1.54 p<0.15).
Figure 1.
The frequency of the ampsens1 strain (diamonds) in competition with: (a) the SLAR strain (squares, n=4 replicates), and (b) the SAR strain (circles, n=4 replicates). No ampicillin was present in these competition experiments. On day 1, strains were initialized at a frequency of 0.5.
Figure 2.
The frequency of the ampsens1 (diamonds) and SAR strains (circles) in competition experiments carried out in the presence of ampicillin (n=8 replicates). On day 1, strains were initialized at a frequency of 0.5.
Evolutionary theory predicts that as the relative cost of the SAR strain of E. coli increases, its equilibrium frequency should decrease (Lenski & Hattingh 1986; Levin 1988; Dugatkin et al. 2003). To test this prediction, we created a fourth strain, labelled ampsens2, that was isogenic with the other three strains except that the entire plasmid on which the blaTEM−1 (or kanamycin resistance) gene resided was eliminated. We undertook pairwise competition experiments with the ampsens2 strain against the SAR and SLAR strains, in both the presence and absence of ampicillin. When no ampicillin was present, the frequency of the SAR strain was significantly lower in competition experiments against the ampsens2 strain than in competition experiments against the ampsens1 strain (repeated-measures ANOVA: F161=29.07 p<0.0001). Furthermore, in the absence of ampicillin, the SLAR strain settled at a lower frequency in competition experiments with the ampsens2 strain than in competitions against the ampsens1 strain (repeated-measures ANOVA: F142=14.11 p<0.0005).
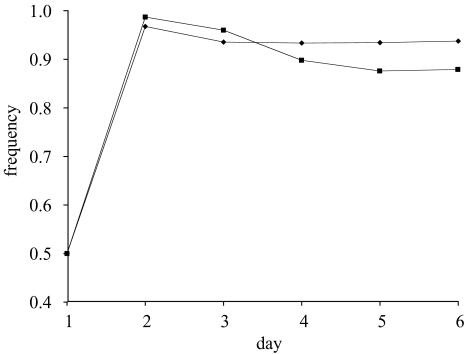
Because it allows us to manipulate the cost–benefit ratio in a controlled manner, the most critical comparison with respect to the evolution of group-beneficial traits is that of the SAR strain when competed against either the ampsens1 strain or the ampsens2 strain in the presence of ampicillin. Although there were no statistically significant differences between treatments on days 2–4, in the presence of ampicillin the frequency of the SAR strain was significantly lower in competition experiments with ampsens2 strains as compared with competition experiments with the ampsens1 strains on days 5 and 6 (figure 3: day 5, least-squares contrast: F140=6.63 p<0.05; day 6, least-squares contrast: F140=6.65 p<0.05).
Figure 3.
The frequency of the SAR strain in competition experiments against the ampsens1 strain (diamonds, n=8 replicates) and against the ampsens2 strain (squares, n=2 replicates). These competition experiments were carried out in the presence of ampicillin. On day 1, strains were initialized at a frequency of 0.5. Differences were found to be significant on days 4 and 5 only.
We conducted another experiment to address whether sensitive ‘cheater’ strains (Travisano & Velicer 2004) could arise from pure cultures of resistant individuals (Rosenweig et al. 1994). In these experiments, we examined the frequency with which the SAR strain yields mutants that fail to produce shared resistance and, thus, are ‘cheaters’, which survive antibiotic treatment with the help of the wild-type SAR cells in their midst. In two of four replicate growth experiments, cheaters arose when the SAR strain was grown in the presence of ampicillin over a period of 27 days (figure 4). In these two replicates cheaters rose to a frequency of ca. 10%, and all of the cheaters we examined lost their plasmids. These preliminary results lend additional insights into the dynamics of the interactions between SAR bacteria and their normally antibiotic-sensitive cohorts. It appears that the selective conditions necessary for the evolutionary emergence of cheaters exist, and that such cheaters can arise in a less ‘contrived’ setting more akin to what would go on in nature.
Figure 4.
The frequency of SAR cells lacking a pSAR1 plasmid. All four replicates were initiated with a population of cells, all of which contained a pSAR1plasmid. Diamonds, replicate 1; triangles, replicate 2. Replicates 3 and 4 never contained any cells lacking the pSAR1 plasmid.
3.1 Implications of group-beneficial traits
Recent work suggests that the evolution of group-beneficial traits may help explain the evolution of multicellularity. For example, Rainey & Rainey (2003) found that defecting (cheating) genotypes evolved in populations founded by the cooperating type and were fitter in the presence of this type than in its absence. Moreover, Velicer & Yu (2003) demonstrated that micro-organisms can align their evolutionary interests by evolving primitive forms of cooperation. Our findings indicate that traits which potentially benefit the whole population can and do evolve in other contexts, and that an understanding of such evolution may have implications for medically relevant issues such as antibiotic resistance.
Massey & Peacock (2002) reported that, in populations of Staphylococcus aureus, mutants arose which, although slower growing than their wild-type counterparts, nevertheless were better suited to survive in the presence of aminoglycoside antibiotics. Further, the mutants changed their environment so as to benefit wild-type individuals normally sensitive to these drugs. In our study, however, it is the wild-type individuals that are already resistant to the antibiotics, and the emphasis is on cheaters that arise via mutation, as well as the ability to experimentally manipulate the costs and benefits of different strains and examine genotype frequencies in accordance with theory.
Velicer and colleagues (Velicer et al. 1998, 2000, 2002; Velicer & Stredwick 2002; Fiegna & Velicer 2003; Velicer & Yu 2003) and Strassmann and colleagues (Strassmann et al. 2000; Foster et al. 2002; Fortunato et al. 2003a,b; Queller et al. 2003) have shown that cheaters may be common in the bacterium Myxococcus xanthus and in the social amoeba, Dictyostelium discoideum, respectively. In these cases, when the organisms are starved they amass into a non-migrating fruiting body or a slug, respectively, and cheater clones make up a small fraction of stalk-like reproductive structures. Cheaters—individuals who receive, but do not produce, shared benefits—could emerge in our antibiotic system in two different ways. First, SAR individuals may lose the plasmid carrying the gene for ampicillin resistance. We attempted to mimic this with our ampsens2 strain. Second, the plasmid bearing the gene for ampicillin resistance may remain, but a mutation may cause the gene to no longer detoxify ampicillin. Our ampsens1 strain was constructed to mimic this situation. In both cases, we found that even with ampicillin present, sensitive cells remained in our populations, albeit at low frequencies (ca. 5–12%, depending upon treatment).
Our results also suggest that group-beneficial traits can act to preserve, or even increase, microbial genetic diversity, by allowing the continued existence of genotypes (such as ampsens1 and ampsens2) that would otherwise disappear under certain selective regimes (e.g. in the presence of antibiotics). Moreover, when SAR strains were grown in pure culture, in two of four replicates, mutants arose de novo that were able to exploit the ‘altruism’ of the SAR strains. No such mutants arose from the SLAR strains, indicating that the group-beneficial trait was necessary for such cheaters to evolve. Together, these results are consistent with those recently reported by Rainey & Rainey (2003), who found that a range of niche specialist genotypes are maintained by negative frequency-dependent selection, as well as Greig & Travisano’s (2004) work on the maintenance of yeast cells that digest sucrose outside the cell, and the cheaters that parasitize such behaviour (also see West & Buckling (2003) for more on group-beneficial traits and sideophores).
The maintenance of genetic wdiversity in microbial communities may be important as a genetic reserve in the face of changing environmental conditions, such as new antibiotic treatments or regimes (Bohannan & Lenski 2000; Travisano 2001). Moreover, the frequency-dependent selection that we observed establishes a basis for the maintenance of high genetic diversity, even in the presence of very strong selective pressures that otherwise would lead to a significant reduction of genotypes. By permitting the survival of genotypes that otherwise would be ‘unfit’ and become extinct as a result of natural selection, group-beneficial traits leave the door open, via increased genetic variation, for new ‘fit’ recombinants to potentially evolve in the future, including bacterial strains with multiple antibiotic resistances.
Acknowledgments
We thank Dana Dugatkin for proofreading this paper and acknowledge funding through a grant from the Arts and Sciences Research Program at the University of Louisville. We also thank Brian Smith for his work on pilot experiments that led to the work published here.
References
- Bohannan B.J.M., Lenski R.E. Linking genetic change to community evolution: insights from studies of bacteria and bacteriophage. Ecol. Lett. 2000;3:362–377. [Google Scholar]
- Dawkins R. The selfish gene. Oxford University Press; 1976. [Google Scholar]
- Dugatkin L.A., Perlin M., Atlas R. The evolution of group-beneficial traits in the absence of between-group selection: a model. J. Theor. Biol. 2003;220:67–74. doi: 10.1006/jtbi.2003.3149. [DOI] [PubMed] [Google Scholar]
- Eberhard W.G. Evolution in bacterial plasmids and levels of selection. Q. Rev. Biol. 1990;65:3–22. doi: 10.1086/416582. [DOI] [PubMed] [Google Scholar]
- Fiegna F., Velicer G.J. Competitive fates of bacterial social parasites: persistence and self-induced extinction of Myxococcus xanthus cheaters. Proc. R. Soc. Lond. B. 2003;270:1527–1534. doi: 10.1098/rspb.2003.2387. doi:10.1098/rspb.2003.2387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortunato A., Queller D.C., Strassmann J.E. A linear dominance hierarchy among clones in chimeras of the social amoeba Dictyostelium discoideum. J. Evol. Biol. 2003a;16:438–445. doi: 10.1046/j.1420-9101.2003.00545.x. [DOI] [PubMed] [Google Scholar]
- Fortunato A., Strassmann J.E., Santorelli L., Queller D.C. Co-occurrence in nature of different clones of the social amoeba, Dictyostelium discoideum. Mol. Ecol. 2003b;12:1031–1038. doi: 10.1046/j.1365-294x.2003.01792.x. [DOI] [PubMed] [Google Scholar]
- Foster K.R., Fortunato A., Strassmann J.E., Queller D.C. The costs and benefits of being a chimera. Proc. R. Soc. Lond. B. 2002;269:2357–2362. doi: 10.1098/rspb.2002.2163. doi:10.1098/rspb.2002.2163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghrayeb J., Kimura H., Takahara M., Hsiung H., Masui Y., Inouye M. Secretion cloning vectors in Escherichia coli. EMBO J. 1984;3:2437–2442. doi: 10.1002/j.1460-2075.1984.tb02151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greig D., Travisano M. The Prisoner’s Dilemma and polymorphism in yeast SUC genes. Proc. R. Soc. Lond. B. 2004;271:S25–S26. doi: 10.1098/rsbl.2003.0083. doi:10.1098/rsbl.2003.0083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karthikeyan, S., Wolfaardt, G., Krober, D. & Caldwell, G. 1998 Use of gfp as a conservative fluorescent marker to examine the protective interactions between benzoate resistant and sensitive strains of Pseudomonas spp. in static and flowing environments. Paper presented at the 98th General Meeting of the American Society for Metals, Atlanta, GA 11–21 May 1998.
- Lenski R.E., Hattingh S.E. Coexistence of 2 competitors on one resource and one inhibitor: a chemostat model based on bacteria and antibiotics. J. Theor. Biol. 1986;122:83–93. doi: 10.1016/s0022-5193(86)80226-0. [DOI] [PubMed] [Google Scholar]
- Lenski R.E., Simpson S.C., Nguyen T.T. Genetic analysis of a plasmid-encoded, host genotype-specific enhancement of bacterial fitness. J. Bacteriol. 1994a;176:3140–3147. doi: 10.1128/jb.176.11.3140-3147.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenski R.E., Souza V., Duong L.P., Phan Q.G., Nguyen T.N., Bertrand K.P. Epistatic effects of promoter and repressor functions of the Tn10 tetracycline-resistance operon of the fitness of Escherichia coli. Mol. Ecol. 1994b;3:127–135. doi: 10.1111/j.1365-294x.1994.tb00113.x. [DOI] [PubMed] [Google Scholar]
- Levin B.R. Frequency-dependent selection in bacterial populations. Phil. Trans. R. Soc. Lond. B. 1988;319:459–472. doi: 10.1098/rstb.1988.0059. [DOI] [PubMed] [Google Scholar]
- Lunn C.A., Takahara M., Inouye M. Use of secretion cloning vectors for guiding the localization of proteins in Escherichia coli. Meth. Enzymol. 1986;125:138–152. doi: 10.1016/s0076-6879(86)25013-2. [DOI] [PubMed] [Google Scholar]
- Massey R.C., Peacock S.J. Antibiotic-resistant sub-populations of the pathogenic bacterium Staphylococcus aureus confer population-wide resistance. Curr. Biol. 2002;20:R686–R687. doi: 10.1016/s0960-9822(02)01205-8. [DOI] [PubMed] [Google Scholar]
- Nordmann P. Mechanism of resistance to betalactam antibiotics in Pseudomonas aeruginosa. Ann. Fr. Anesth. Reanim. 2003;22:527–530. doi: 10.1016/s0750-7658(03)00170-9. [DOI] [PubMed] [Google Scholar]
- Palzkill T., Le Q., Wong A. Functional signal peptide cleavage sites from a library of random sequences. J. Bacteriol. 1994;176:563–568. doi: 10.1128/jb.176.3.563-568.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrosino J., Cantu C., Palzkill T. β-Lactamases: protein evolution in real time. Trends Microbiol. 1998;68:323–327. doi: 10.1016/s0966-842x(98)01317-1. [DOI] [PubMed] [Google Scholar]
- Queller D.C., Ponte E., Bozzaro S., Strassmann J.E. Single-gene greenbeard effects in the social amoeba Dictyostelium discoideum. Science. 2003;299:105–106. doi: 10.1126/science.1077742. [DOI] [PubMed] [Google Scholar]
- Rainey P.B., Rainey K. Evolution of cooperation and conflict in experimental bacterial populations. Nature. 2003;425:72–74. doi: 10.1038/nature01906. [DOI] [PubMed] [Google Scholar]
- Rainey P.B., Travisano M. Adaptive radiation in a heterogeneous environment. Nature. 1998;394:69–72. doi: 10.1038/27900. [DOI] [PubMed] [Google Scholar]
- Rosenweig R.F., Sharp R.R., Treves D.S., Adams J. Microbial evolution in a simple unstructured environment: genetic differentiation in Escherichia coli. Genetics. 1994;137:903–917. doi: 10.1093/genetics/137.4.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. The social evolution of bacterial pathogenesis. Proc. R. Soc. Lond. B. 2001;268:61–69. doi: 10.1098/rspb.2000.1330. doi:10.1098/rspb.2000.1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sober E., Wilson D.S. Unto others. Harvard University Press; Cambridge, MA: 1998. [Google Scholar]
- Strassmann J.E., Zhu Y., Queller D.C. Altruism and social cheating in the social amoeba Dictyostelium discoideum. Nature. 2000;408:965–967. doi: 10.1038/35050087. [DOI] [PubMed] [Google Scholar]
- Travisano M. Experimental evolution studies yield insights into bacterial diversity. ASM News. 2001;67:403–409. [Google Scholar]
- Travisano M., Velicer G.J. Strategies of microbial cheater control. Trends Microbiol. 2004;12:72–78. doi: 10.1016/j.tim.2003.12.009. [DOI] [PubMed] [Google Scholar]
- Velicer G.J., Stredwick K.L. Experimental social evolution with Myxococcus xanthus. Int. J. Gen. Mol. Microbiol. 2002;81:155–164. doi: 10.1023/a:1020546130033. [DOI] [PubMed] [Google Scholar]
- Velicer G.J., Yu Y.T.N. Evolution of novel cooperative swarming in the bacterium Myxococcus xanthus. Nature. 2003;425:75–78. doi: 10.1038/nature01908. [DOI] [PubMed] [Google Scholar]
- Velicer G.J., Kroos L., Lenski R.E. Loss of social behaviors by Myxococcus xanthus during evolution in an unstructured habitat. Proc. Natl Acad. Sci. USA. 1998;95:12376–12380. doi: 10.1073/pnas.95.21.12376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velicer G.J., Kroos L., Lenski R.E. Developmental cheating in the social bacterium Myxococcus xanthus. Nature. 2000;404:598–601. doi: 10.1038/35007066. [DOI] [PubMed] [Google Scholar]
- Velicer G.J., Lenski R.E., Kroos L. Rescue of social motility lost during evolution of Myxococcus xanthus in an asocial environment. J. Bacteriol. 2002;184:2719–2727. doi: 10.1128/JB.184.10.2719-2727.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West S.A., Buckling A. Cooperation, virulence and siderophore production in bacterial parasites. Proc. R. Soc. Lond. B. 2003;270:37–44. doi: 10.1098/rspb.2002.2209. doi:10.1098/rspb.2002.2209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams G. Adaptation and natural selection. Princeton University Press; 1966. [Google Scholar]
- Wilson D.S. The natural selection of populations and communities. Benjamin Cummings; Menlo Park, CA: 1980. [Google Scholar]
- Wilson E.O. Sociobiology: the new synthesis. Harvard University Press; Cambridge, MA: 1975. [Google Scholar]