Abstract
Mating success is often determined by multiple traits, but why this occurs is largely unknown. Much attention has been paid to female preferences for multiple traits, but surprisingly few researchers have addressed the possibility that multiple traits are important because they serve different functions in female choice and male–male competition. Differential trait function could result from a conflict of interest between the sexes or from constraints forcing the sexes to pay attention to different traits. I show that traits determined at distinct life-history stages differ in their importance in female choice and male–male competition in a water boatman Sigara falleni. Juvenile conditions determined body and foreleg pala size and were the main determinants of mating success under female choice, whereas adult conditions determined body mass and influenced mating success when male competition was included. This differential use of condition-dependent traits under the two selection regimes appeared to arise partly from a conflict between the sexes, since the two selection forces (female choice and male competition) conflict for selection on pala size, and partly from constraints, as females appeared unable to assess adult condition.
Keywords: condition dependence, Corixidae, mate choice, multiple cues, sexual selection, Sigara falleni
1. Introduction
An increasing number of studies find that mating success is determined by multiple traits, such as vigorous courtship behaviours combined with bright colours, or elaborate songs that are evaluated together with conspicuous ornaments (Andersson 1994; Candolin 2003). Why this occurs is disputed and many different hypotheses have been proposed. Multiple traits may be important if (i) mate choice is based on multiple traits, (ii) if the success in the competition for the access to mates is determined by multiple traits, or (iii) if mate choice and success in the competition for mates are based on different traits.
Of these three possibilities, the first has received the most theoretical and empirical interest (Candolin 2003). Mate choice is frequently based on multiple cues, which may be adaptive and reduce mate-choice errors or costs (Møller & Pomiankowski 1993; Johnstone 1996), may have no effect on fitness if the preferences are remnants from past selection (Møller & Pomiankowski 1993; Ryan & Rand 1993; Holland & Rice 1998), or may be maladaptive if the signaller manipulates the mating resistance of the receiver (the choosing sex) by taking advantage of pre-existing sensory biases (Holland & Rice 1998).
By contrast, the second and the third possibilities have received almost no attention (but see Howard et al. 1997; Kraak et al. 1999; Andersson et al. 2002). Of particular importance is the third possibility, that different traits are used in female mate choice and in male–male competition. These two selection pressures can work in concert or be in conflict, which could influence the maintenance of genetic variation of traits (Qvarnström & Forsgren 1998; Moore & Moore 1999; Andersson et al. 2002; Lopez et al. 2002). For example, traits that are favoured in male competition may correlate negatively with male parental effort and be counteracted by female choice for direct benefits (Smith 1995; Forsgren 1997; Qvarnström 1997; Magrath & Komdeur 2003). This could relax the strength of directional selection, enforcing stabilizing selection and counteracting the loss of genetic variation (Kirkpatrick & Ryan 1991; Roff 1997).
Traits used as cues in mate choice are generally assumed to be condition dependent (Andersson 1986; Price et al. 1993; Johnstone 1995; Rowe & Houle 1996; Griffith et al. 1999; David et al. 2000; Kotiaho et al. 2001). This can ensure that the traits reflect mate quality by preventing low-condition individuals from developing traits as large or pronounced as males in good condition, presuming that condition correlates with quality. However, different traits may develop at different times of the lifespan and thus reflect condition at different life stages (Møller et al. 1998; Birkhead et al. 1998; Hill et al. 1999; Scheuber et al. 2003). The use of multiple cues could then give a more complete picture of overall male quality than a single cue reflecting condition at only one life stage.
Using the water boatman Sigara falleni (Corixidae) as a model, I investigated (i) how conditions at distinct life stages affect different male traits, and (ii) the subsequent importance of the traits in female mate choice and male competition, the two major forces of sexual selection. Sigara falleni is a semi-aquatic insect that inhabits rivers and lakes of Europe (Jansson 1996). The insects aggregate in patches along the shores of rives and lakes in early spring. The mating attempts of males take place under competition as males try to disrupt each other’s mounting attempts. Females resist most copulation attempts, and both female choice (or resistance) and male competition determine mating success. In contrast to other species of water boatmen, S. falleni do not stridulate. Instead, males have enlarged foreleg palae, i.e. clawlike tarsal segments (Jansson 1996). Males court females by shaking their bodies and enlarged foreleg palae in front of the females. Thus, the sexually dimorphic palae could be sexually selected and used both as visual signals to the female and as structures for clasping the female during mating. An earlier study showed that female choice and male competition exert opposing selection on male foreleg pala size, with female choice favouring large palae, whereas male competition favours smaller palae (Candolin 2004).
2. Material and methods
Water boatmen were collected with dip nets from the shores of Wohlensee, near Bern, Switzerland, before the breeding season in January 2002. The insects were brought to the laboratory, and species and sex identified. Pairs of S. falleni, one male and one female, were placed in plastic jars with 0.5 l of water. They were kept at 22°C under a 16L:8D cycle to stimulate reproduction. A piece of plastic netting served as oviposition substrate. The insects were fed daily with frozen chironomid larvae.
Females started ovipositing within two weeks and usually laid between two and five eggs per day. Every third day, the plastic nettings with newly laid eggs were transferred to separate jars. When the nymphs started to hatch, chironomid larvae and a mixture of phytoplankton were added as food. Nymphs that reached the second instar were transferred to new jars and subjected to two juvenile food treatments and two adult food treatments, according to a full factorial design (see § 2a). Full-sib offspring from 28 pairs, who had produced at least 50 hatched larvae, were used in the experiments.
2.1 Juvenile food treatment
The offspring of a wild-caught female were divided among four 0.5 l jars and submitted to two food rations. Two jars were maintained under high food ration, with frozen chironomid larvae added each day, one chironomid per corixid nymph, and two jars under low food ration with the same amount of chironomids added every second day. When the nymphs reached the fourth instar, the number of chironomids added was increased from one to two chironomids per nymph. The density of nymphs was kept approximately equal among jars by removing nymphs from jars with a higher survival rate. Emerging adults were immediately sexed and males were transferred to the adult food manipulation treatments.
2.2 Adult food treatment
Full-sib males from the two juvenile jars with the same food ration were randomly assigned to two adult food rations: high ration with chironomid larvae added daily (two chironomids per adult), and low ration with the same food added every second day. Two 1 l jars were used for each food treatment (four jars in total). Each jar contained males from both juvenile jars, to eliminate possible effects of jar on the results. The males were maintained on the adult food rations for two weeks before being used in the female preference and male competition experiments.
2.3 Female mate choice
Unmated S. falleni females were caught from the same area as described above, before the breeding season in February and March 2002. They were maintained in the laboratory at 22°C under a 16L:8D cycle to stimulate sexual maturation. They were fed daily on frozen chironomids.
A randomly chosen male whose condition had been manipulated through the juvenile–adult food treatments was placed in a 0.2 l jar. After 30 min of acclimation, an unmated female was added and the insects were observed once every 10 min until mating occurred or 3 h had passed without copulation. Copulation generally lasts 20–40 min (personal observation). Twenty unrelated males were tested for each food treatment combination. New females were used each time. Copulation does not succeed without the agreement of the female and the results give the willingness of the female to mate with a male.
2.4 Inclusion of male–male competition
Two males from the same family that either (i) differed in juvenile feeding history and not in adult feeding history (which could be low or high food ration), or (ii) differed in adult feeding history and not in juvenile feeding history (which could be low or high food ration), were placed in a 0.2 l jar. The paired males had not been raised in the same jar and had therefore no prior experience of each other. The males were marked with different colours of acryl paint on the pronotum to facilitate individual recognition. After 30 min of acclimation, an unmated female was added to the jar and the insects were observed once every 10 min until mating occurred or 3 h had passed without copulation. One male from a pair was randomly selected as the focal male for the analyses on dependence of mating success on food rations and male traits. New males and females were used each time. Twenty pairs of each combination were tested.
2.5 Body measurements
After the trials, the insects were dried on blotting paper for 1 min and weighed to the nearest 0.001 g. Body size was measured by photographing the males with a video camera mounted on a microscope and connected to a computer. Male body length and the areas of the two foreleg palae were measured with ImageJ v.1.28 u (http://rsb.info.nih.gov/ij/). Body length was measured from the front of the pronotum to the end of the left forewing corium. Each character was measured twice and the average value was used in the analyses. For foreleg palae, the average size of the two palae was calculated.
2.6 Analyses
The effects of food treatments on the measured male traits were analysed with mixed model analyses of variance, with food treatments as fixed factors and family as a random factor. Non-significant interactions terms (p > 0.1) were excluded from the models. To analyse for effects of food treatments on mating success, logistic regression models were fitted with mating success as a binary response variable and family and larvae and adult food treatments as categorical covariates. Interaction terms that did not significantly improve the fit of the models were deleted.
The dependence of mating success on male traits (body length, body weight and pala size) were analysed with logistic regression. Correlation between the traits, collinearity, which can decrease the reliability of individual regression coefficients, did not pose a problem as the tolerance of all variables were greater than 0.11 (VIF<9) (Kleinbaum et al. 1988).
Differences between paired males in body traits were calculated as: (a − b)/(a + b), where a is the trait value of the male on high ration, and b is the trait value of the male on low ration. Values given are means ± s.e.m.
3. Results
3.1 Effects of food treatments on male body traits
Juvenile food rations determined body size and pala size: males on a high ration grew larger and heavier, and developed larger foreleg palae than males on a low ration (high ration: 0.01142 ± 0.0001 g (body weight), 6.55 ± 0.02 mm (body length), 0.422 ± 0.003 mm2 (pala area); low ration: 0.0105 ± 0.0001 g, 6.29 ± 0.02 mm, 0.392 ± 0.002 mm2; table 1). The significant effects remained after a sequential Bonferroni correction was applied to maintain a table-wide α = 0.05. Juvenile food rations had no significant effect on pala size in relation to body size, since pala size was mainly determined by body length (table 1).
Table 1.
Effects of food rations and family on male traits.
(n = 240. Mixed-model ANOVA was used for analyses. Non-significant interaction terms were excluded from the models.)
| body mass |
body length |
pala area |
relative pala areaa |
|||||
| F | p | F | p | F | p | F | p | |
| juvenile food | 78.05 | <0.0001 | 79.98 | <0.0001 | 68.39 | <0.0001 | 0.53 | 0.466 |
| adult food | 4.08 | 0.045 | 0.911 | 0.341 | 2.18 | 0.141 | 1.78 | 0.184 |
| family | 1.86 | 0.018 | 1.328 | 0.167 | 1.32 | 0.174 | 1.98 | 0.011 |
| body length | 738.04 | <0.001 | ||||||
| juvenile×adult | 5.33 | 0.022 | ||||||
a Body length was included as a covariate in the model to scale pala size to body size.
Adult food rations determined body mass, with males on a high ration becoming heavier than males on a low ration (0.0111 ± 0.0001 g and 0.0109 ± 0.0001 g, respectively). Adult rations had no effect on body length or pala size, since these traits do not change after the last moult. Juvenile and adult food rations interacted in determining body mass, with the effect of an adult food ration depending on larvae ration (low juvenile, low adult: 0.0105 ± 0.0001 g, low juvenile, high adult: 0.0105 ± 0.0001 g, high juvenile, low adult: 0.0112 ± 0.0001 g, high juvenile, high adult: 0.0116 ± 0.0001 g).
Body mass and relative pala size differed among families, and the significant effects remain under sequential Bonferroni correction.
3.2 Female mate choice
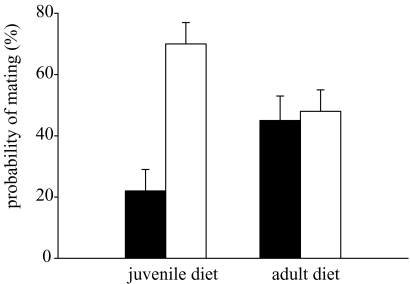
Juvenile food ration, but not adult food ration, determined the probability of mating in the single male trials; males raised on a high juvenile ration had a higher mating probability than males raised on a low juvenile ration (table 2; figure 1). No significant effect of family was detected (table 2).
Table 2.
The effects of food rations and family on the probability of mating of single males.
(n = 80. Logistic regression was used for analyses. Non-significant interaction terms were deleted from the models.)
| probability of mating |
||
| Wald | p | |
| juvenile food | 16.58 | <0.001 |
| adult food | 0.06 | 0.800 |
| family | 1.01 | 0.316 |
Figure 1.
The effects of juvenile and adult food rations on the probability of mating (mean±s.e.m.) of single males. Low food, filled bars; high food, open bars.
The probability of mating increased with increasing pala size (table 3). Thus, the effect of juvenile conditions on mating could be mediated by effects on pala size (table 1). A non-significant marginal effect of family on mating probability was detected.
Table 3.
The dependence of mating success on male traits for single males.
(n = 80. Backward stepwise logistic regression was used for analyses.)
| probability of mating |
||
| Wald | p | |
| body length | 1.11 | 0.291 |
| body mass | 0.18 | 0.893 |
| pala size | 14.93 | <0.001a |
| family | 2.75 | 0.097a |
| χ2 = 67.17, d.f. = 2, p < 0.001 | ||
a Terms that were maintained in the final model.
3.3 Inclusion of male–male competition
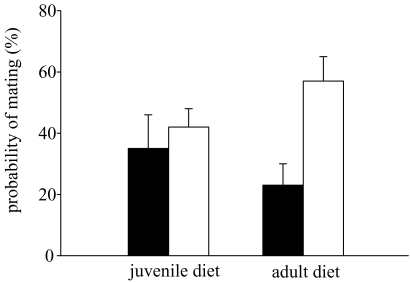
Mating success depended on adult food conditions but not on juvenile conditions under male competition (table 4; figure 2). A male on a high adult food ration had a higher probability of mating than a male on a low ration. These results differ from those gained in the absence of competition (interaction between food rations and presence or absence of male competition: juvenile conditions: Wald=5.26, p = 0.022, adult conditions: Wald=2.20, p = 0.046).
Table 4.
The effects of food rations and family on the probability of mating of randomly selected competing males.
(n = 80. Logistic regression was used for analyses. Non-significant interaction terms were deleted from the models.)
| probability of mating | ||
| Wald | p | |
| juvenile food | 0.32 | 0.572 |
| adult food | 9.58 | 0.002 |
| family | 0.16 | 0.684 |
Figure 2.
The effects of juvenile and adult food rations on the probability of mating (mean±s.e.m.) of competing males. Low food, filled bars; high food, open bars.
Mating success of competing males was related to their absolute and relative body mass (table 5). Thus, the effect of adult food conditions on mating success of competing males could be mediated by effects on body mass.
Table 5.
The dependence of mating success on absolute male traits values, and difference between males in trait values, for a randomly selected competing male, n = 80.
(Backward stepwise logistic regression was used for analyses.)
| absolute male traits |
relative male traits |
|||
| Wald | p | Wald | p | |
| body length | 0.01 | 0.955 | 0.05 | 0.820 |
| body weight | 16.34a | <0.001a | 8.91a | 0.003a |
| pala size | 0.02 | 0.884 | 0.16 | 0.693 |
| family | 0.373a | 0.542a | 0.55a | 0.458a |
| χ2 = 34.90, d.f. = 2, p <0.001 | χ2 = 11.39, d.f. = 2, p = 0.003 | |||
a Terms that were maintained in the final model.
4. Discussion
Food conditions during distinct life-history stages had differential effects on male traits and mating success under female choice and male competition. Juvenile conditions determined body morphology, such as pala size, and were the main determinants of mating success under female choice. Adult conditions, by contrast, determined body mass and influenced mating success when male competition was included. Surprisingly, juvenile conditions had no significant effect on mating success under male competition, which suggests that present condition, and perhaps energy levels, are more important in determining mating success under competition. However, it is not known whether the investigated male traits, pala and body size, or other correlated traits were the ones that influenced mating success. Nevertheless, the results demonstrate that mating success is affected by different condition-dependent traits under the two selection regimes, female choice and male competition.
Studies on a wide variety of animals have found mating success to be determined by multiple traits (Candolin 2003). Why this occurs is largely unknown and many hypotheses have been proposed. This study supports the hypothesis that multiple traits determine mating success because female choice and male competitive ability are based on different traits. This could arise owing to constraints that force the sexes to pay attention to different traits, or to a conflict of interest between the sexes, if the target of female choice does not coincide with male competitive ability.
Constraints in the ability of females to judge adult male condition could be one reason for the differential use of the traits in S. falleni, since adult conditions had no significant effect on mating success when only female choice was operating. This could arise from an inability to assess adult male condition, or from excessive costs of mate assessment. Interestingly, the importance of adult condition increased when male competition was included, resulting in the ‘choice’ of the male in the best overall condition. Condition is generally assumed to reflect mate quality by reflecting direct or indirect genetic benefits of mate choice (Andersson 1986; Price et al. 1993; Johnstone 1995; Rowe & Houle 1996; Griffith et al. 1999; David et al. 2000; Kotiaho et al. 2001). Thus, females could benefit from mating with males in good condition and consequently from the inclusion of male competition. This could be of particular importance in environments where conditions fluctuate and male condition and juvenile viability do not necessarily reflect viability as adult. The inclusion of several condition-dependent traits in the choice process, through the inclusion of male competition, could then facilitate female choice for the male in the best overall condition and reduce mate-choice errors or costs.
The other possibility, that several traits determined mating success because of a conflict of interest between the sexes, is also plausible. An earlier study found the two selection forces, male competition and female choice, to conflict in selection on male foreleg pala size, although not on body size (Candolin 2004). Female choice favoured larger palae, whereas male competition favoured smaller palae, probably owing to costs to males of large palae. Whether the two forces, female choice and male competition, act in the same or opposite direction is currently debated, with differing results for different species (Berglund et al. 1996; Wiley & Poston 1996; Qvarnström & Forsgren 1998; Candolin 1999; Moore & Moore 1999; Wollerman 1999; Andersson et al. 2002; Lopez et al. 2002). In the present species, enforcing or counteracting female choice and male competition depend on whether individuals vary in their ability to cope with both juvenile and adult conditions. A conflict situation arises if phenotypes well adapted to juvenile conditions do less well as adults, owing to a gene–environment interaction, and if juvenile conditions correlate better than adult conditions with fitness benefits to females. Male competition and a corresponding increase in the importance of adult conditions could then hamper adaptive female choice. By contrast, if juvenile and adult conditions are generally correlated, then a conflict situation does not arise and male competition could facilitate mate choice. In a related study on water boatmen caught from the field, where condition was not manipulated, both female choice and male competition favoured larger males, suggesting that condition at the two stages generally does not conflict (Candolin 2004). To what degree sexual conflict occurs and its effects on male and female fitness is disputed (Chapman et al. 2003; Cordero & Eberhard 2003; Cordoba-Aguilar & Contreras-Garduno 2003; Kokko et al. 2003). To determine whether male competition ultimately facilitates or hampers female choice in water boatmen, the target of female choice and the value of juvenile and adult conditions as indicators of mate quality need to be determined.
The results of this study differ from those of other studies where females have been found to be able to use one cue as a signal of a male’s lifetime fitness (Sullivan 1990; Emlen 1994; Møller et al. 1998; David et al. 2000). For example, in lekking black grouse Tetrao tetrix females can use territory position as a signal of a male’s lifetime performance (Kokko et al. 1999). The present study on water boatmen suggests that the pattern can be more complicated than generally assumed. Multiple traits could give a more complete picture of mate quality and become operative under different selection regimes.
In conclusion, traits determined at distinct life-history stages differed in their importance in female choice and male–male competition. This implies that the two selection forces (female choice and male competition) could counteract each other if males vary in their ability to cope with conditions at different life stages. Whether the inclusion of male competition in the mating process then facilitates or hampers choice depends on the target of female choice and to what extent condition at different life stages reflects male quality.
Acknowledgments
The author thanks Martin Brinkhof and Hannes Scheuber for discussions, and Hanna Kokko and two anonymous reviewers for helpful comments on the paper. This work was supported by the Academy of Finland.
References
- Andersson M. Evolution of condition-dependent sex ornaments and mating preferences: sexual selection based on viability differences. Evolution. 1986;40:804–816. doi: 10.1111/j.1558-5646.1986.tb00540.x. [DOI] [PubMed] [Google Scholar]
- Andersson M. Sexual selection. Princeton University Press; Princeton, NJ: 1994. [Google Scholar]
- Andersson S., Pryke S.R., Ornborg J., Lawes M.J., Andersson M. Multiple receivers, multiple ornaments, and a trade-off between agonistic and epigamic signaling in a widowbird. Am. Nat. 2002;160:683–691. doi: 10.1086/342817. [DOI] [PubMed] [Google Scholar]
- Berglund A., Bisazza A., Pilastro A. Armaments and ornaments: an evolutionary explanation of traits of dual utility. Biol. J. Linn. Soc. 1996;58:385–399. [Google Scholar]
- Birkhead T.R., Fletcher F., Pellatt E.J. Sexual selection in the zebra finch Taeniopygia guttata: condition, sex traits and immune capacity. Behav. Ecol. Sociobiol. 1998;44:179–191. [Google Scholar]
- Candolin U. Male–male competition facilitates female choice in sticklebacks. Proc. R. Soc. Lond. B. 1999;266:785–789. doi:10.1098/rspb.1999.0706 [Google Scholar]
- Candolin U. The use of multiple cues in mate choice. Biol. Rev. 2003;78:575–595. doi: 10.1017/s1464793103006158. [DOI] [PubMed] [Google Scholar]
- Candolin U. Opposing selection on a sexually dimorphic trait through female choice and male competition in a water boatman. Evolution. 2004;58:1861–1864. doi: 10.1111/j.0014-3820.2004.tb00470.x. [DOI] [PubMed] [Google Scholar]
- Chapman T., Arnqvist G., Bangham J., Rowe L. Sexual conflict. Trends Ecol. Evol. 2003;18:41–47. [Google Scholar]
- Cordero C., Eberhard W.G. Female choice of sexually antagonistic male adaptations: a critical review of some current research. J. Evol. Biol. 2003;16:1–6. doi: 10.1046/j.1420-9101.2003.00506.x. [DOI] [PubMed] [Google Scholar]
- Cordoba-Aguilar A., Contreras-Garduno J. Sexual conflict. Trends Ecol. Evol. 2003;18:439–440. [Google Scholar]
- David P., Bjorksten T., Fowler K., Pomiankowski A. Condition-dependent signalling of genetic variation in stalk-eyed flies. Nature. 2000;406:186–188. doi: 10.1038/35018079. [DOI] [PubMed] [Google Scholar]
- Emlen D.J. Environmental control of horn length dimorphism in the beetle Ontophagus acuminatus (Coleoptera, Scarabaeidae) Proc. R. Soc. Lond. B. 1994;256:131–136. [Google Scholar]
- Forsgren E. Female sand gobies prefer good fathers over dominant males. Proc. R. Soc. Lond. B. 1997;264:1283–1286. doi:10.1098/rspb.1997.0177 [Google Scholar]
- Griffith S.C., Owens I.F., Burke T. Environmental determination of a sexually selected trait. Nature. 1999;400:358–360. [Google Scholar]
- Hill J.-A., Enstrom D.A., Ketterson E.D., Van Nolan V., Jr, Ziegenfus C. Mate choice based on static versus dynamic secondary sexual traits in the dark-eyed junco. Behav. Ecol. 1999;10:91–96. [Google Scholar]
- Holland B., Rice W.R. Perspective: chase-away sexual selection: antagonistic seduction versus resistance. Evolution. 1998;52:1–7. doi: 10.1111/j.1558-5646.1998.tb05132.x. [DOI] [PubMed] [Google Scholar]
- Howard R.D., Moorman R.S., Whiteman H.H. Differential effects of mate competition and mate choice on eastern tiger salamanders. Anim. Behav. 1997;53:1345–1356. doi: 10.1006/anbe.1996.0359. [DOI] [PubMed] [Google Scholar]
- Jansson A. Heteroptera Nepomorpha, aquatic bugs. In: Nilsson A., editor. The aquatic insects of north Europe. Apollo Books Aps; Stenstrup: 1996. pp. 91–104. [Google Scholar]
- Johnstone R.A. Sexual selection, honest advertisement and the handicap principle: reviewing the evidence. Biol. Rev. 1995;70:1–65. doi: 10.1111/j.1469-185x.1995.tb01439.x. [DOI] [PubMed] [Google Scholar]
- Johnstone R.A. Multiple displays in animal communication: ‘backup signals’ and ‘multiple messages’. Phil. Trans. R. Soc. Lond. B. 1996;351:329–338. [Google Scholar]
- Kirkpatrick M., Ryan M.J. The evolution of mating preferences and the paradox of the lek. Nature. 1991;350:33–38. [Google Scholar]
- Kleinbaum D.G., Kupper L.L., Muller K.E. Applied regression analysis and other multivariable methods. 2nd edn. PWS-Kent; Boston, MA: 1988. [Google Scholar]
- Kokko H., Rintamäki P.T., Alatalo R.V., Höglund J., Karvonen E., Lundberg A. Female choice selects for lifetime lekking performance in black grouse males. Proc. R. Soc. Lond. B. 1999;266:2109–2115. doi:10.1098/rspb.1999.0895 [Google Scholar]
- Kokko H., Brooks R., Jennions M.D., Morley J. The evolution of mate choice and mating biases. Proc. R. Soc. Lond. B. 2003;270:653–664. doi: 10.1098/rspb.2002.2235. doi:10.1098/rspb.2002.2235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotiaho J.S., Simmons L.W., Tomkins J.L. Towards a resolution of the lek paradox. Nature. 2001;410:684–686. doi: 10.1038/35070557. [DOI] [PubMed] [Google Scholar]
- Kraak S.B.M., Bakker T.C.M., Mundwiler B. Sexual selection in sticklebacks in the field: correlates of reproductive, mating, and paternal success. Behav. Ecol. 1999;10:696–706. [Google Scholar]
- Lopez P., Munoz A., Martin J. Symmetry, male dominance and female mate preferences in the Iberian rock lizard, Lacerta monticola. Behav. Ecol. Sociobiol. 2002;52:342–347. [Google Scholar]
- Magrath M.J.L., Komdeur J. Is male care compromised by additional mating opportunity? Trends Ecol. Evol. 2003;18:424–430. [Google Scholar]
- Møller A.P., Pomiankowski A. Why have birds got multiple sexual ornaments? Behav. Ecol. Sociobiol. 1993;32:167–176. [Google Scholar]
- Møller A.P., Saino N., Taramino G., Galeotti P., Ferrario S. Paternity and multiple signaling: effects of a secondary sexual character and song on paternity in the barn swallow. Am. Nat. 1998;151:236–242. doi: 10.1086/286114. [DOI] [PubMed] [Google Scholar]
- Moore A.J., Moore P.J. Balancing sexual selection through opposing mate choice and male competition. Proc. R. Soc. Lond. B. 1999;266:711–716. doi:10.1098/rspb.1999.0694 [Google Scholar]
- Price T., Schluter D., Heckman N.E. Sexual selection when the female directly benefits. Biol. J. Linn. Soc. 1993;48:187–211. [Google Scholar]
- Qvarnström A. Exeprimentally increased badge size increases male competition and reduces male parental care in the collared flycatcher. Proc. R. Soc. Lond. B. 1997;264:1225–1231. doi:10.1098/rspb.1997.0169 [Google Scholar]
- Qvarnström A., Forsgren E. Should females prefer dominant males? Trends Ecol. Evol. 1998;13:498–501. doi: 10.1016/s0169-5347(98)01513-4. [DOI] [PubMed] [Google Scholar]
- Roff D. Evolutionary quantitative genetics. Chapman & Hall; New York: 1997. [Google Scholar]
- Rowe L., Houle D. The lek paradox and the capture of genetic variance by condition dependent traits. Proc. R. Soc. Lond. B. 1996;263:1415–1421. [Google Scholar]
- Ryan M.J., Rand A.S. Sexual selection and signal evolution: the ghost of biases past. Phil. Trans. R. Soc. Lond. B. 1993;340:187–195. [Google Scholar]
- Scheuber H., Jacot A., Brinkhof M.W.G. The effect of past condition on a multicomponent sexual signal. Proc. R. Soc. Lond. B. 2003;270:1779–1784. doi: 10.1098/rspb.2003.2449. doi:10.1098/rspb.2003.2449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H.G. Experimental demonstration of a trade-off between mate attraction and paternal care. Proc. R. Soc. Lond. B. 1995;260:45–51. [Google Scholar]
- Sullivan M.S. Assessing female choice for mates when the males characters vary during the sampling period. Anim. Behav. 1990;40:780–782. [Google Scholar]
- Wiley R.H., Poston J. Indirect mate choice, competition for mates, and coevolution of the sexes. Evolution. 1996;50:1371–1381. doi: 10.1111/j.1558-5646.1996.tb03911.x. [DOI] [PubMed] [Google Scholar]
- Wollerman L. Acoustic interference limits call detection in a neotropical frog Hyla ebraccata. Anim. Behav. 1999;57:529–536. doi: 10.1006/anbe.1998.1013. [DOI] [PubMed] [Google Scholar]