Abstract
Individual role specialization during group hunting is extremely rare in mammals. Observations on two groups of bottlenose dolphins (Tursiops truncatus) in Cedar Key, Florida revealed distinctive behavioural roles during group feeding. In each group, one individual was consistently the ‘driver’, herding the fishes in a circle toward the remaining ‘barrier’ dolphins. Aerial fish-capture rates differed between groups, as well as between the driver and barrier dolphins, in one group but not in the other. These differences between the two groups may reflect differences in group stability or in prey school size.
Keywords: dolphin, feeding, cooperation, division of labour
1. Introduction
Cooperative or group hunting has been reported in several mammals and even in one bird species (e.g. Bednarz 1988; Creel & Creel 1995; Kitchen & Packer 1999). Group hunts that are considered cooperative range from simultaneous chases to hunts that are clearly coordinated (Kitchen & Packer 1999). Bednarz (1988) describes apparent coordinated behaviour among Harris’ hawks (Parabuteo unicinctus) that converged on their rabbit quarry from different directions. Bednarz further describes different roles for individual birds during hunts. During ‘flush and ambush’ hunts, one to two birds penetrated the bush to flush out a hiding rabbit while others surrounded the bush and made the kill once the rabbit emerged. The ‘flush and ambush’ strategy of the hawks involves a division of labour, defined by Anderson & Franks (2001) as occurring when individuals, working as a team to complete a task, perform different subtasks. During group hunts, individual chimpanzees (Pan troglodytes) hunting in the Tai National Forest, Ivory Coast, may engage in particular subtasks such as ‘driving’ or ‘blocking’ their red colobus monkey (Procolobus badius) prey (Boesch & Boesch 1989).
Role specialization is found when individuals specialize in their subtasks during repeated team tasks. Group hunting with a division of labour and role specialization is extremely rare. To our knowledge, the only well documented case in mammals is Stander’s (1992) study of coordinated group hunts in African lionesses (Panthera leo). Females in ‘centre’ roles waited for prey to move towards them while those in ‘wing’ positions initiated an attack on the prey (Stander 1992). Hunting success was higher when lionesses occupied preferred stalking positions.
Cooperative hunting has been described in several cetaceans, including bottlenose dolphins (Tursiops truncatus), killer whales (Orcinus orca) and humpback whales (Megaptera novaeangliae) (reviewed in Connor 2000). Accounts of apparent cooperative behaviour in feeding bottlenose dolphins include fishes being herded into a ball (Caldwell & Caldwell 1972; Leatherwood 1975; Rossbach 1999), fishes driven ahead of dolphins swimming in a crescent formation (Leatherwood 1975; Wursig 1986), against mud banks (Leatherwood 1975) or trapped between dolphins attacking from either side (Wursig 1986). Groups of dolphins may even beach themselves to feed on fishes that they have chased onto mud banks (Hoese 1971; Rigley et al. 1981; Petricig 1995). However, none of the previously described cases of group hunting in cetaceans demonstrates a division of labour with role specialization (reviewed by Connor 2000).
In Cedar Key, Florida, group-hunting dolphins engage in two types of behaviours while herding fishes. One individual in a group of three to six dolphins, the ‘driver’, herds the fishes in circles, as well as towards the tightly grouped ‘barrier’, or ‘non-driving’ dolphins that are less than one body-length apart and often touching. The driver may perform fluke-slaps (when a dolphin lifts its fluke, or tail, out of the water and slaps it against the water surface forcefully) during the drive. Fishes being herded in this fashion leap into the air, where some are captured by driver and barrier dolphins. The driver often surfaces alongside the barrier dolphins as the fishes begin to leap. A fish capture is characterized by a lurch or a lunge by the dolphin at a leaping fish, which is seized in the air, followed by repeated biting motions as the dolphin further manipulates the fish in its mouth before swallowing. Observations of two such feeding groups allow us to test the hypothesis that individual dolphins herding fishes in Cedar Key specialize in the roles of driver and barrier, thus meeting the criteria for a division of labour with role specialization. The alternative hypothesis, that there is no role specialization, predicts that individuals will not occupy the same role, either driver or barrier, during repeated hunts.
2. Material and methods
2.1 Study area
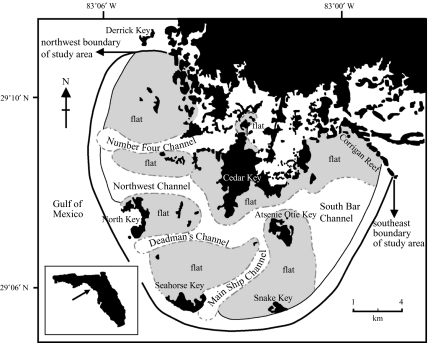
The area of the Cedar Keys (29°05′49″N, 83°03′58″W) comprises five major islands, numerous smaller islands, and wetland areas connected to the mainland off the northwest coast of Florida. One of the two feeding groups (A group) was observed consistently at Seahorse Key over a seagrass bed substrate, and the other (B group) was observed at Corrigan Reef with a muddy/sandy substrate (see figure 1).
Figure 1.
Map of Cedar Key, Florida. Courtesy of Ester Quintana, Laurie Waltz and Joel Bellucci.
2.2 Behavioural observations
Observations were made from a 14-foot Carolina Skiff boat with a 45 horsepower Yamaha outboard motor, from June to August 2001. Daily attempts were made to locate dolphins participating in the fish-herding behaviour.
Individuals were photographed using a Nikkoromat camera fitted with an 80–250 mm zoom lens. Once a dolphin was encountered in the study site, the markings on the dorsal fin were photographed for individual identification (Caldwell 1955) using the methods described by Defran et al. (1990). Individuals were included in the group if they were within 10 m of any other group member (Smolker et al. 1992).
The feeding behaviour was recorded using a Panasonic digital zoom S-VHS camcorder, and the driver’s dorsal fin was photographed during each bout. A blank photograph was taken in between bouts to demarcate sequential feeding bouts. For each bout, the start time, longitude and latitude, water depth and substrate composition were recorded. The fishes identified leaping during feeding bouts were mullet (Mugil cephalus), but we could not be certain that every leaping fish was a mullet. Barros & Odell (1990) suggested that observations of mullet leaping when pursued by bottlenose dolphins have led to an overestimate of the importance of mullet as a dolphin prey item.
A feeding bout began when the driving dolphin began swimming rapidly in tight circles—either with or without fluke slaps—and was considered to be finished when the participating dolphins put their heads back under water and rolled upright.
Non-driving dolphins are defined as all group members that did not drive. This includes the barrier dolphins that were tightly bunched and raised their heads out of the water attempting to catch leaping fishes, as well as any other dolphins in the group that did not drive or form the barrier. Only aerial fish capture was recorded and only dolphins that had their heads up (i.e. drivers and barrier dolphins) were used to calculate capture success. Fish-capture success was determined by counting the number of fishes that each individual caught in air, indicated by either observing the fish in the dolphin’s mouth or observing the dolphin’s lunge followed by repeated biting motions. A lunge that was not followed by biting motions was not counted, because dolphins sometimes missed fishes that they lunged at. Recording the capture rates of individual barrier dolphins was not possible since the dolphins frequently changed positions and their dorsal fins were often submerged. Therefore, for each bout, an average number of fishes captured by the nondrivers was calculated from the number of nondrivers and the total number of fishes that they captured.
Fish-capture success might relate to the number of leaping fishes; therefore, the number of fishes leaping per feeding bout was counted from the videotape. Some leaps occurred after a leaping fish fell back into the water and thus could have been a fish leaping for a second time. These cases were not included in the total of fishes leaping per bout.
Fish-capture success was calculated for bouts for which we could determine: (i) the identity of the driver; (ii) the number of fishes captured by the driver; and (iii) the number of fishes captured by nondrivers. These criteria were met in 58 out of 126 group-feeding bouts, including 28 out of 60 bouts for the A group and 30 out of 66 bouts for the B group.
2.3 Statistical analysis
Nonparametric statistical analyses were used to evaluate hypotheses about group behaviour. Differences in means between groups involving paired observations were made using the sign test (table 2; Sokal & Rohlf 1995). Statistical analysis of unpaired observations within and between A and B groups employed the Mann–Whitney U-test (Sokal & Rohlf 1995). Variables were assessed for an independence of errors (observations) using the Wald–Wolfowitz runs test (StatisiXL, v. 1.4, 2004), and where these tests were significant they are reported along with the U-test.
Table 2.
Sign test results of fish capture success compared within groups.
| npositive | nties | nnegative | p-value | |
| all drivers versus all nondrivers | 24 | 17 | 17 | 0.211 |
| A-group driver versus A-group nondrivers | 17 | 5 | 6 | 0.011 |
| B-group driver versus B-group nondrivers | 8 | 12 | 10 | 0.815 |
3. Results
The herding behaviour was seen 155 times, and the driver was identified in 145 bouts (93.5% of the time). Nineteen of the bouts with an identified driver involved a single dolphin driving without other dolphins present. These bouts were not included in analyses comparing drivers and nondrivers and are considered separately. Average bout duration was 19.9s (range of 11.0–28.0s). The interval between successive bouts on the same day ranged from 30 to 2441s (mean 281s).
The A group accounted for 60 and the B group for 66 of the remaining 126 bouts. In all 60 A-group bouts the same dolphin (TLFN) was the driver, and in all 66 B-group bouts the same dolphin (PNT) was the driver. This finding is significantly different from a distribution derived from a hypothesis that the driving individual is randomly selected for each bout.
The A group drove only at Seahorse Key, and the B group drove only around the Corrigan Reef area. TLFN drove using a series of driving fluke slaps at the beginning of each herding bout, but PNT did not. Members of the B group were observed to hit fishes into the air with their flukes (‘fish kick’; see Wells et al. 1987) while feeding during the bouts, after the driver had ceased herding (n=14 bouts out of 30 recorded bouts, or 47% of bouts). ‘Fish kicking’ was not observed in the A group.
The A group had a stable membership in all 60 bouts, consisting of only three dolphins: TLFN, SFSK and VFSK. The B group varied in size from two to six dolphins, including the driver, PNT.
In all 14 bouts observed with a single dolphin driving, the individual was identified as CECR. CECR drove in the same area as the B group and was observed on several occasions to be one of the non-driving members of the B group. CECR did not drive fishes while others were present.
Significantly more fishes leaped on average during the A-group bouts than the B-group bouts (table 1; meanA-group=16.4±16.0, meanB-group=8.7±9.3, U=191, nA-group=28, nB-group=22, p=0.022), between A group and the single animal CECR (table 1: meanA-group=16.4±16.0, meanCECR=0.3±0.7, U=390, nA-group=28; nCECR=14, p<0.001) and between B group and CECR (table 1: meanB group=8.7 ±9.3, meanCECR=0.3±0.7, U=269, nB-group=22; nCECR=14, p<0.001).
Table 1.
Mann–Whitney U-test results of fishes leaping per bout compared between groups.
| groups compared | mean (±s.d.) | n1 | n2 | U | p-value |
| A group | 16.4±16.0 | 0.022 | |||
| B group | 8.7±9.3 | 28 | 22 | 191 | |
| A group | 16.4±16.0 | <0.001 | |||
| CECR | 0.3±0.7 | 28 | 14 | 390 | |
| B group | 8.7±9.3 | <0.001 | |||
| CECR | 0.3±0.7 | 22 | 14 | 269 |
When the data from both groups were pooled, average driver fish-capture success did not differ significantly for nondriver fish-capture success (table 2; sign test [all drivers–all nondrivers]: npositive=24, nties=17, nnegative=17, p=0.21). The A-group driver captured, on average, significantly more fishes than the A-group nondrivers (table 2; sign test [A-group drivers–A-group nondrivers]: npositive=17, nties=5, nnegative=6, p=0.011). There was no significant difference between the average capture successes of the B-group driver compared to the B-group nondrivers (table 2; sign test [B-group drivers−B-group nondrivers]: npositive=8, nties=12, nnegative=10, p=0.815).
The A-group driver captured, on average, significantly more fishes than did the B-group driver (table 3; meanA-group driver: 1.14±0.97, meanB-group driver: 0.37±0.61, U=214; nA-group driver=28, nB-group driver=30, p=0.001), and the A-group nondrivers captured, on average, significantly more fishes than did the B-group nondrivers (table 3; meanA-group nondrivers=0.69±0.10, meanB-group nondrivers=0.38±0.41, U=291; nA-group nondrivers=28, nB-group nondrivers=30, p=0.04).
Table 3.
Mann–Whitney U-test results of fish capture success compared between groups.
| groups compared | mean (± s.d.) | n1 | n2 | U | p-value |
| A-group driver | 1.14±0.97 | 0.001 | |||
| B-group driver | 0.37±0.61 | 28 | 30 | 214 | |
| A-group nondrivers | 0.69±0.10 | 0.040 | |||
| B-group nondriversa | 0.38±0.41 | 28 | 30 | 291 | |
| A-group driver | 1.14±0.97 | < 0.005 | |||
| CECR | 0.29±0.19 | 28 | 14 | 304 | |
| B-group driver | 0.37±0.61 | > 0.200 | |||
| CECR | 0.29±0.19 | 30 | 14 | 236 |
a Observations in this variable were not independent (p-value≈0.05).
During 14 bouts, CECR was observed to capture four fishes, at a mean rate of 0.29±0.19 fishes captured per bout. The A-group driver caught significantly more fishes, on average, than CECR (table 3: meanA-group driver 1.14±0.97, meanCECR=0.29±0.19, U=304; nA-group driver=28; nCECR=14, p<0.005), but the B-group driver did not (table 3: meanB-group driver=0.37±0.61, meanCECR=0.29±0.19 U=236; nB-group driver=30; nCECR=14, p>0.2).
To test for independence of observations within the datasets, a Wald–Wolfowitz runs test was used (StatistiXL, v. 1.4, 2004). The null hypothesis of independence was rejected in only one case—that of the B-group fish capture success of nondrivers (table 3). In this case the p-value of independence was ca. 0.05. It should be noted that there were long periods when no fishes were caught within the datasets of group B, and the analysis is very sensitive to this timeframe. If there had been just one capture that had interrupted the lack of fish captures, the assumption of independence would have been met.
4. Discussion
4.1 Driver identity and role specialization
The identity of the driver in both groups did not change. TLFN was the sole driver in A group, and PNT was the sole driver in B group. Because drivers and nondrivers benefit, this fish-herding behaviour should be considered a by-product mutualism (sensu Connor 1995) with a division of labour and role specialization. Cooperative feeding and foraging within groups of marine mammal species has been noted before (reviewed by Connor 2000), but, to our knowledge, the consistent role-playing in cooperative herding that was seen in Cedar Key has not.
We emphasize that a dolphin’s choice of roles in a bout (driver versus barrier) is not at all constrained by the role that they played in the previous bout. After a bout, all the dolphins are typically within a few metres of each other. The average time between bouts (four to five minutes) is vastly more than would be required for such closely grouped dolphins to switch roles if there were no preferences.
Anderson & Franks (2001) define a ‘team’ as cooperative behaviour with a division of labour. Individuals in a team perform different subtasks that must be performed at the same time for successful completion (Anderson & Franks 2001). In this case, there would be two subtasks: driving and barrier formation. Driving alone, the dolphin CECR had some success in catching fishes. Although significantly fewer fishes jumped during bouts of driving by CECR (table 1), perhaps indicating the importance of barrier dolphins in trapping fishes, CECR was as successful as the driver in B group at catching fishes (table 3). Thus, the nondriver subtask is not essential for feeding in this manner, but further observation is required to quantify the relative benefits to drivers of working with nondrivers. Stander (1992) also observed solitary hunts by lionesses but their success rate was lower than that for individuals participating in group hunts.
There are numerous reports of bottlenose dolphins using barriers to trap fishes. The barriers may be the sea surface, the shore or other dolphins that form a circle around the fishes or attack from either side (reviewed in Connor 2000). The behaviour described here is unique because of the division of labour and role specialization that accompany the barrier feeding. It is worth noting, however, that there are other cases where group-hunting dolphins participate in a team task with role specialization, but they belong in the category of interspecific mutualism where role specialization is common. In Mauritania and Brazil, dolphins foraging in shallow water drive fishes into barriers provided by fishermen with nets, to the mutual benefit of both parties (Busnel 1973; Pryor et al. 1990; Connor 1995).
The alternative ‘noncooperative’ explanation for the behaviour described here is that the ‘barrier’ dolphins are ‘scroungers’ in a producer–scrounger system (Barnard & Sibly 1981; Hamilton & Dill 2002). This is unlikely for several reasons. First, the barrier dolphins perform an important role in the behaviour: they serve as the barrier against which fishes are trapped just as the shore or sea surface does in other cases. Second, after the barrier dolphins stop, the driver continues to move toward them, whether the barrier dolphins are oriented toward the shore, as is usually the case, or parallel to the shore. The driver should not continue to move toward the barrier dolphins if the latter are simply ‘scrounging’. Third, the groups moved slowly in a cohesive manner along the channels during the search for fish schools, and no obvious avoidance behaviours were observed on the part of the driver (although avoidance isn’t expected in all cases; Hamilton & Dill 2002). Fourth, members of the A group were observed together repeatedly during research conducted 4 to 5 years before this study (E. Quintana-Rizzo, personal communication.).
4.2 Costs and benefits
The data are mixed as to whether the driver obtains a larger benefit; in the A group it did, but in the B group it did not. This difference between the A and B groups may relate to such factors as group stability or habitat.
The stable A group had a higher fish-capture success than the unstable B group (table 3). The A group consistently comprised three individuals, while the size of the B group varied from two to six individuals. It is possible that nondrivers only joined B group when the expected payoff was high.
Significantly more fishes leapt in A-group than in B-group feeding bouts, and in both groups compared to the solo efforts of CECR (see table 1). The difference between the A and B groups may be a function of the local habitat, because the area where the A group fed, Seahorse Key, has a seagrass bed substrate, but the area where the B group fed, Corrigan Reef, has a muddy/sandy substrate. Fish schools may be larger over seagrass beds in which fishes may hide or forage (Sogard et al. 1989). Habitat may also explain the differences in driving technique. The A-group driver, TLFN, began each driving bout with two to three driving fluke slaps. whereas the B-group driver, PNT, did not use driving tail slaps. The driving fluke slaps appeared somewhat similar to ‘kerplunks’ described by Connor et al. (2000) in Shark Bay, Western Australia. ‘Kerplunking’ is thought to startle fishes out of their hiding places in seagrass beds (Connor et al. 2000). The fluke-slaps during driving in Cedar Key may have a similar function.
It seems likely that the driver incurs a higher energetic cost than do the nondrivers. The driver performs the majority of the herding behaviour, while the nondrivers wait for the driver to herd the fishes in a circle towards them and, in the process, may help to provoke the fish to leap out of the water.
It remains unclear why a division of labour with role specialization is so rare in species that hunt cooperatively. It may be simply that it rarely pays to specialize; practice may not improve performance sufficiently to warrant role specialization. We suggest, however, that this may be the case less for the marine than the terrestrial habitat. It has become increasingly clear that individual foraging specializations (differences in the types of food or methods used to procure food among two or more individuals of the same age, sex and reproductive state that have overlapping home ranges) are more common among marine than terrestrial mammals (Connor 2001). Connor (2001) outlined a series of hypotheses to explain this difference; the terrestrial and marine habitats may differ in prey diversity, biomass, seasonality, predator mobility or rewards in foraging efficiency acquired through practice. To the extent that practice is an important element of the terrestrial versus marine differences in individual foraging specializations, we predict that a division of labour with role specialization will also turn out to be more common in group-hunting marine mammals.
Acknowledgments
The authors thank Dan Odell, for showing R.C.C. the video footage taken by Frank Cox of the driving behaviour; the Cox family, especially Michael Cox (for lending a boat) and Harvey Cox (technical assistance in the field); Katie Davis (research assistant); Anita Kim, Johanna Blasi, and Jeffrey Holcomb (editorial feedback); and Kelvin Dalton (mentor). This project was funded through private donations from Helen Brosseau as well as Richard and Everette Gazda. We also thank the anonymous reviewers for their valuable feedback that increased the strength of this paper.
References
- Anderson C., Franks N.R. Teams in animal societies. Behav. Ecol. 2001;12:534–540. [Google Scholar]
- Barnard C.J., Sibly R.M. Producers and scroungers: a general model and its application to captive flocks of house sparrows. Anim. Behav. 1981;29:543–550. [Google Scholar]
- Barros N.B., Odell D.K. Food habits of bottlenose dolphins in the southeastern United States. In: Leatherwood S., Reeves R.R., editors. The bottlenose dolphin. Academic; San Diego, CA: 1990. pp. 309–328. [Google Scholar]
- Bednarz J.C. Cooperative hunting in Harris’ hawks (Parabuteo unicinctus) Science. 1988;239:1525–1527. doi: 10.1126/science.239.4847.1525. [DOI] [PubMed] [Google Scholar]
- Boesch C., Boesch H. Hunting behavior of wild chimpanzees in the Tao National Park. Am. J. Phys. Anthropol. 1989;78:547–573. doi: 10.1002/ajpa.1330780410. [DOI] [PubMed] [Google Scholar]
- Busnel R.G. Symbiotic relationship between man and dolphins. N. Y. Acad. Sci. Trans. 1973;35:112–131. [Google Scholar]
- Caldwell D.K. Evidence of home range of an Atlantic bottlenose dolphin. J. Mamm. 1955;36:304–305. [Google Scholar]
- Caldwell D.K., Caldwell M.C. The world of the bottlenose dolphin. Lippincott; Philadelphia, PA: 1972. [Google Scholar]
- Connor R.C. The benefits of mutualism: a conceptual framework. Biol. Rev. 1995;70:427–457. [Google Scholar]
- Connor R.C. Group living in whales and dolphins. In: Mann J., Connor R.C., Tyack P.L., Whitehead H., editors. Cetacean societies: field studies of dolphins and whales. The University of Chicago Press; Chicago, IL: 2000. [Google Scholar]
- Connor R.C. Individual foraging specializations in marine mammals: culture and ecology. Behav. Brain Sci. 2001;24:329–330. [Google Scholar]
- Connor R.C., Heithaus M.R., Berggren P., Miksis J.L. ‘Kerplunking’: surface fluke-splashes during shallow-water bottom foraging by bottlenose dolphins. Mar. Mamm. Sci. 2000;16:646–653. [Google Scholar]
- Creel S., Creel N.M. Communal hunting and pack size in African wild dogs. Lycaon pictus. Anim. Behav. 1995;50:1325–1339. [Google Scholar]
- Defran R.H., Shultz G.M., Weller D.W. A technique for the photographic identification and cataloging of dorsal fins of the bottlenose dolphin (Tursiops truncatus) In: Hammond P.S., Mizroch S.A., Donovan G.P., editors. Individual recognition of cetaceans: use of photo-identification and other techniques to estimate population parameters. International Whaling Commission; Cambridge, UK: 1990. pp. 53–55. Reports of the International Whaling Commission, special issue 12. [Google Scholar]
- Hamilton I.M., Dill L.M. Three-player social parasitism games: implications for resource defense and group formation. Am. Nat. 2002;159:670–686. doi: 10.1086/339994. [DOI] [PubMed] [Google Scholar]
- Hoese H.D. Dolphin feeding out of water in a salt marsh. J. Mamm. 1971;52:222–223. [PubMed] [Google Scholar]
- Kitchen D.M., Packer C. Complexity in vertebrate societies. In: Keller L., editor. Levels of selection in evolution. Princeton University Press; 1999. pp. 176–196. [Google Scholar]
- Leatherwood S. Some observations of feeding behavior of bottle-nosed dolphins (Tursiops truncatus) in the northern Gulf of Mexico and (Tursiops cf. T. gilli) off southern California, Baja California and Nayarit, Mexico. Mar. Fish. Rev. 1975;37:10–16. [Google Scholar]
- Petricig R.O. Ph D dissertation. University of Rhode Island; USA: 1995. Bottlenose dolphin (Tursiops truncatus) in Bull Creek, South Carolina. [Google Scholar]
- Pryor K., Lindbergh J., Lindbergh S., Milano R. A dolphin-human fishing cooperative in Brazil. Mar. Mamm. Sci. 1990;6:77–82. [Google Scholar]
- Rigley L., Vandyke V.G., Cram P., Rigley I. Shallow water behavior of the Atlantic bottlenose dolphin (Tursiops truncatus) Proc. Pennsylvania Acad. Sci. 1981;55:157–159. [Google Scholar]
- Rossbach K.A. Cooperative feeding among bottlenose dolphins (Tursiops truncatus) near Grand Bahama Island, Bahamas. Aquat. Mammals. 1999;25:163–167. [Google Scholar]
- Smolker R.A., Richards A.F., Connor R.C., Pepper J.W. Sex differences in patterns of association among Indian Ocean bottlenose dolphins. Behaviour. 1992;123:38–69. [Google Scholar]
- Sogard S.M., Powell G.V.N., Holmquist J.G. Utilization by fishes of shallow, seagrass-covered banks in Florida Bay: 1. Species composition and spatial heterogen eity. Environ. Biol. Fish. 1989;24:53–65. [Google Scholar]
- Sokal R.R., Rohlf F.J. Biometry: the principles and practice of statistics in biological research. 3rd edn. W. H. Freeman & Co; New York: 1995. [Google Scholar]
- Stander P.E. Cooperative hunting in lions: the role of the individual. Behav. Ecol. Sociobiol. 1992;29:445–454. [Google Scholar]
- Wells R.S., Scott M.D., Irvine A.B. The social structure of free-ranging bottlenose dolphins. In: Genoways H.H., editor. Current mammalogy. vol. 1. Plenum; New York: 1987. pp. 247–305. [Google Scholar]
- Wursig B. Delphinid foraging strategies. In: Schusterman R.J., Thomas J.A., Wood F.G., editors. Dolphin cognition and behavior: a comparative approach. Lawrence Erlbaum Associates; Hillsdale, NJ: 1986. pp. 347–359. [Google Scholar]