Abstract
Allometry for sexual size dimorphism (SSD) is common in animals, but how different evolutionary processes interact to determine allometry remains unclear. Among related species SSD (male:female) typically increases with average body size, resulting in slopes of less than 1 when female size is regressed on male size: an allometric relationship formalized as ‘Rensch's rule’ . Empirical studies show that taxa with male-biased SSD are more likely to satisfy Rensch's rule and that a taxon's mean SSD is negatively correlated with allometric slope, implicating sexual selection on male size as an important mechanism promoting allometry for SSD. I use body length (and life-history) data from 628 (259) populations of seven species of anadromous Pacific salmon and trout (Oncorhynchus spp.) to show that in this genus life-history variation appears to regulate patterns of allometry both within and between species. Although all seven species have intraspecific allometric slopes of less than 1, contrary to expectation slope is unrelated to species' mean SSD, but is instead negatively correlated with two life-history variables: the species' mean marine age and variation in marine age. Second, because differences in marine age among species render SSD and body size uncorrelated, the interspecific slope is isometric. Together, these results provide an example of how evolutionary divergence in life history among related species can affect patterns of allometry for SSD across taxonomic scales.
Keywords: sexual size dimorphism, allometry, Rensch's rule, Pacific salmon and trout, life history
1. Introduction
Sexual size dimorphism (SSD) is common in animals and results from differences in the cumulative effects of natural and sexual selection acting on male and female body size (Price 1984; Slatkin 1984; Shine 1989; Andersson 1994). Comparisons among related species (Abouheif & Fairbairn 1997; Fairbairn 1997) and populations within species (Fairbairn & Preziosi 1994; Kraushaar & Blanckenhorn 2002) reveal that SSD (male:female throughout) typically increases with body size, resulting in slopes of less than 1 when female body size is regressed on male body size, an allometric relationship sufficiently prevalent to be known as ‘Rensch's rule’ (Rensch 1960). Thus, allometry for SSD results from size-dependent changes in the cumulative effects of natural and sexual selection acting on male and female body size.
Although many studies have identified how different selective forces interact to determine SSD (reviewed in Andersson 1994), less progress has been made towards understanding how different selective forces interact to determine allometry for SSD (e.g. Fairbairn & Preziosi 1994; Reeve & Fairbairn 1996; Fairbairn 1997; Butler & Losos 2002; Kraushaar & Blanckenhorn 2002). Allometry consistent with Rensch's rule results from greater evolutionary divergence in male than female body size and a phenotypic correlation for size between the sexes. In a review of allometry for SSD, Fairbairn (1997) found that taxa with male biased SSD are more likely to display allometry consistent with Rensch's rule and that mean SSD is negatively correlated with allometric slope (i.e. positively related to the degree of departure from isometry). Together, these patterns suggest that variation in the strength of sexual selection on male body size plays an important role in promoting allometry. However, allometry also requires a correlated, but less variable, change in the cumulative effects of selection acting on female body size. The most probable explanation invokes variable sexual selection on male body size, less variable (but not necessarily weaker) natural/fecundity selection on female body size, and correlational selection on female body size associated with changes in optimal male body size (e.g. through assortative mating or selection to produce larger offspring) (see Andersson (1994) and Fairbairn (1997) for discussions of alternative hypotheses). Under this scenario, optimal female body size is determined by the balance of natural/fecundity selection and correlational selection. Allometry occurs because natural/fecundity selection on female body size constrains the response to correlational selection, resulting in correlated but reduced changes in female body size as optimal male body size changes in response to variation in the strength of sexual selection. Understanding allometry for SSD then requires identifying how different evolutionary processes regulate the correlated evolution of male and female body size among related species and populations within species.
In this paper I use data on male and female body length and life history from populations of the seven species of anadromous Pacific salmon and trout (Oncorhynchus spp.) to provide an example of how life-history differences among species appear to regulate allometry for SSD within and between species. Life-history variation among species and populations within species combined with a common mating system makes the genus ideal for such an investigation (Groot & Margolis 1991; Shuster & Wade 2003; Fleming & Reynolds 2004). Juveniles of the seven species rear in fresh water from weeks to years, after which they migrate to the marine environment to rear until returning to fresh water as sexually mature adults. The species, and populations within species, differ in the average length of time spent in the marine environment (Groot & Margolis 1991), which determines mean size at maturity, and in marine-age diversity (see §2), which determines the range of sizes at which individuals mature. Both marine age, if males and females grow at different rates, and marine-age diversity, if they mature at different ages, have the potential to affect the degree to which male and female body size can diverge. Because inter- and intraspecific allometry require such divergence within species or populations, both marine age and marine-age diversity have the potential to constrain or promote departures from isometry under a given suite of selection pressures.
During breeding, males engage in intrasexual competition for access to spawning females and density-dependent sexual selection on male body size is well documented in a number of species (e.g. Chebanov 1984, 1986; Fleming & Gross 1994). Female body size is favoured by fecundity selection and selection on egg size, which increases with body size and is related to offspring size (Einum et al. 2004). Female body size can also be favoured by density-dependent selection related to the ability to acquire and defend quality nest sites (Van den Berghe & Gross 1989; Fleming & Reynolds 2004). The seven species differ dramatically in average population size and breeding density (Groot & Margolis 1991). Consistent with density-dependent sexual selection on male size, average SSD varies from strongly male biased in species with high breeding densities to strongly female biased in species with low breeding densities (figure 1a,b). Body size, which depends on marine growth rate and maturation age, is heritable and evolves rapidly and divergently among geographically adjacent populations (Beacham & Murray 1988; Withler & Beacham 1994; Hendry et al. 2000), which are reproductively isolated because of the strong tendency of individuals to return to their natal stream to reproduce (Quinn 1993).
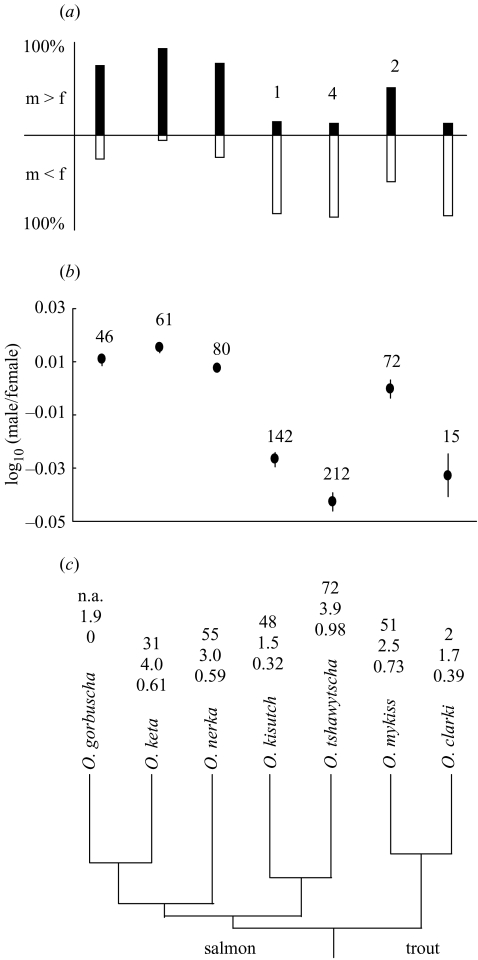
Figure 1.
(a) The proportion of populations of each species in which mean male length was larger or smaller than mean female length (the numbers above bars are the number of populations for which mean male and mean female length were equal). (b) Mean SSD of all sampled populations (± s.e.m.) The numbers above the data points are sample sizes. (c) Phylogenetic relationship of the seven Oncorhynchus species. The branch lengths are proportional to estimated divergence times (McKay et al. 1996). The numbers above species' names are, from top to bottom: the number of populations for which life-history data were available; mean marine age in years; and mean marine-age diversity (see § 2). There are no life-history data from natural populations of O. gorbuscha, which display no variation in marine age at maturation. n.a., not applicable.
2. Material and methods
2.1 Data collection and preliminary analyses
I collected data on mean male and mean female length for 628 wild populations of North American anadromous Pacific salmon (semelparous) and trout (optionally iteroparous) from the published literature, graduate theses, government reports and unpublished government data. Sample sizes varied among species (see figure 1b) because of differences in natural abundance, geographical distribution, commercial importance and availability of data from government agencies. For each population I calculated SSD as log(male length/female length), which is positive for male-biased SSD and negative for female-biased SSD. For each species the proportion of populations with male and female-biased SSD and mean SSD are shown in figure 1a,b, respectively. Life-history data (freshwater age and marine age) from the analysis of scales recovered from breeding adults were available for 259 of the populations (see figure 1c for sample sizes). For each population I calculated two life-history indices which can affect average body size and the degree to which male and female body size are able evolve independently. Mean marine age was calculated as the average time in years individuals spent rearing in the marine environment before returning to fresh water to breed as mature adults. Marine-age diversity was calculated as the Shannon–Wiener index using the proportion of individuals in each marine age class (e.g 1 year, 2 years, etc.); freshwater age was not considered because all species achieve nearly all of their growth in the marine environment. I then calculated the species' means for each of these two life-history indices (figure 1c). These means were significantly correlated (r6=0.74; p<0.05) and the first principal component (from principal components analysis (PCA) on the correlation matrix), loaded equally for the two life-history indices and summarized 87% of the variation in the two variables.
2.2 Allometric slopes and species level analyses
To determine intra- and interspecific allometric slopes I regressed log(female length) on log(male length), using population and species means, respectively. Because male body size (x-axis) is measured with error, ordinary least-squares regression is inappropriate for analysing allometry for SSD (Fairbairn 1997). I used major axis regression (Fairbairn 1997), which calculates the unbiased slope as the first eigenvector axis of the ellipse of points in x–y space (Sokal & Rohlf 1995). Different data sources reported different length measurements (e.g. standard length, fork length, post-orbital-hypural length); unfortunately equations do not exist to transform all populations of all species to a common length measurement. For each species data were combined for major axis slope analysis after meeting two criteria. First, I required that an ANCOVA of log(female length) on log(male length), with measurement type as the class variable, yielded non-significant type and type × male length effects (i.e. equal intercepts and slopes) (p>0.05). Second, I required that the major axis slopes for each measurement type were not significantly different from each other or the major axis slope of the final, combined dataset (overlapping 95% confidence intervals (CIs)). All data met these criteria and were included in the analyses.
I analysed interspecific relationships between intraspecific slope, mean SSD and the three life-history variables (two indices and first principal component score) using parametric correlation and evolutionarily independent contrasts (Felsenstein 1985). Independent contrast analyses were conducted using CAIC (Purvis & Rambaut 1995) with branch lengths (McKay et al. 1996) proportional to those shown in figure 1c. For contrast analyses I report the F-statistic for the null hypothesis that the slope (forced through the origin) of the contrasts is zero.
3. Results and discussion
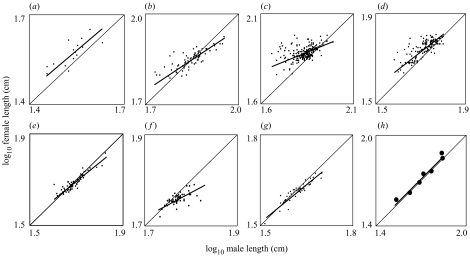
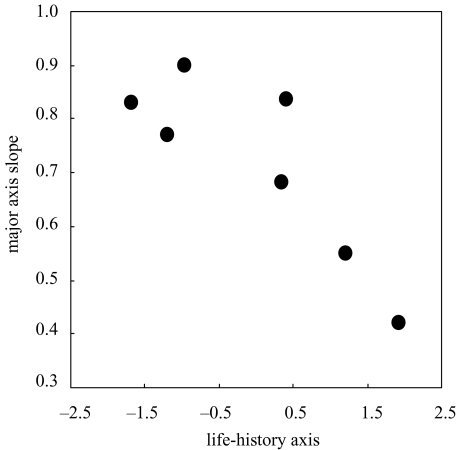
All seven species display allometry consistent with Rensch's rule, and only O. clarki does not have a slope significantly different from isometry (figure 2a–g). For completeness, the variation in the slopes was confirmed using ANCOVA [model: log(female length)=log(male length)+species+log(male length)×species], which revealed significant differences in the least squares slopes (interaction term: F6,620=164, p<0.0001). Contrary to expectation (Abouheif & Fairbairn 1997; Fairbairn 1997), species' mean SSD is unrelated to intraspecific allometric slope (r6=0.18, p>0.5; contrasts: F1,5=0.03, p=0.88). Instead, both mean marine age (r6=−0.82, p<0.02; F1,5=14.6, p=0.01) and mean marine-age diversity (r6=−0.74, p<0.05; F1,5=11.4, p=0.02) are significantly and negatively correlated with intraspecific allometric slope. The single life-history axis (PC1 from the PCA) explains 71% of the variation in intraspecific allometric slope among the seven species (r6=−0.84, p<0.01; F1,5=16.84, p<0.01; figure 3). Thus, for the seven species of Pacific salmon and trout, life history appears more important than mean SSD (i.e. the strength of sexual selection on male size) in determining the intraspecific allometric relationship between female and male body size. For the interspecific relationship between the species' means, differences in mean marine age, which is related to mean length (r6=0.62, p=0.14) but unrelated to mean SSD (r6=0.15, p=0.75), render mean SSD and mean length uncorrelated among the seven species (r6=−0.06, p>0.5; F1,5=0.15, p=0.72). As a result, the interspecific slope is isometric and inconsistent with Rensch's rule (figure 2h; major axis slope of independent contrasts of female and male length=0.89, 95% CI 0.59–1.31, r2=0.94, n=6; data not shown).
Figure 2.
The relationship between mean female length and mean male length among populations of seven species of Oncorhynchus: (a) O. clarki; (b) O. mykiss; (c) O. tshawytscha; (d) O. kisulch; (e) O. nerka; (f) O. keta; and (g) O. gorbuscha, and (h) between the species' means. In all panels the light line is the 1:1 isometric slope and the bold line is the major axis slope of female on male length. (a) Slope=0.89, 95% CI 0.48–1.62, r2=0.60, n=15; (b) slope=0.68, 95% CI 0.56–0.82, r2=0.65, n=72; (c) slope=0.42, 95% CI 0.29–0.55, r2=0.21, n=212; (d) slope=0.77, 95% CI 0.69–0.85, r2=0.61, n=142; (e) slope=0.83, 95% CI 0.74–0.94, r2=0.83, n=80; (f) slope=0.55, 95% CI 0.36–0.77, r2=0.40, n=61; (g) slope=0.83, 95% CI 0.71–0.96, r2=0.85, n=46; and (h) slope=1.02, 95% CI 0.77–1.35, r2=0.94, n=7.
Figure 3.
The negative relationship between the major axis slope of female length on male length (figure 2a–g) and a life-history axis from principal components analysis that summarizes interspecific differences in mean marine age and mean marine-age diversity (see §2).
At the interspecific level, the effect of the relationship between interspecific differences in mean marine age and SSD on slope is fairly clear. Because differences in mean marine age are unrelated to mean SSD, body length and SSD are unrelated, which prevents allometry consistent with Rensch's rule. At the intraspecific level, the correlation between mean marine age and mean marine-age diversity, combined with their similar relationship to intraspecific allometric slope, makes the mechanistic relationship between life history and allometry less obvious. Intraspecific allometry requires that male and female length diverge at the population level; if within populations male and female length cannot diverge, isometry results. Thus, understanding how life-history variation among Oncorhynchus species is related to variation in intraspecific allometry for SSD requires determining how life-history variation among populations within species affects the evolution of male and female size and the magnitude of SSD.
The magnitude of SSD in a population could increase with marine age and/or marine-age diversity. If SSD results primarily from sex-specific growth rates associated with different trade-offs related reproductive roles (Reeve & Fairbairn 1996; Badyaev 2002) or different marine foraging behaviours (Holtby & Healey 1990), an increase in marine age could increase the magnitude of SSD. Alternatively, if the cumulative effects of natural, fecundity, sexual and correlational selection favour different maturation ages or sizes in females and males, higher marine-age diversity could increase the magnitude of SSD. Comparisons among populations within species between mean marine age, marine-age diversity, SSD and the absolute value of SSD (abs(SSD)) (recall SSD is positive in populations with larger males and negative in populations with larger females) reveal life history may affect SSD via both mechanisms. Among populations of O. nerka, abs(SSD) depends more on mean marine age (r54=0.27, p<0.05) than on marine-age diversity (r54=0.2, p=0.13), suggesting differences in the growth rates of males and females may be more important than sex-specific maturation schedules in this species. Alternatively, the second mechanism appears more important in O. tshawytscha. In this species mean marine age is unrelated to abs(SSD) (r71=0.11, p=0.35), but marine-age diversity is positively correlated to abs(SSD) (r71=0.34, p<0.005) and negatively correlated to SSD (r71=−0.44, p<0.0001), indicating that in populations with high marine-age diversity males tend to be smaller than females. Consistent with marine-age diversity allowing male and female length to evolve independently within populations, this species has the highest mean marine-age diversity, the shallowest allometric slope and data from a sufficient number of populations to demonstrate that males typically mature at a younger marine age than females (Roni & Quinn 1995).
I interpret these results as evidence that life-history variation in Pacific salmon and trout affects allometry for SSD in two ways. First, differences in marine age among species render body size and SSD uncorrelated, resulting in interspecific isometry for SSD. Second, life history appears to affect the coevolution of female and male body size at the population level and thus prevent the expected negative relationship between mean SSD (i.e. the strength of sexual selection on male body size) and intraspecific allometric slope. With only seven species, the power to detect interspecific allometry is low. Nevertheless, an exactly isometric interspecific slope (=1.02) combined with the clear biological connection between marine age and body size provide strong evidence that life-history variation unrelated to mean SSD prevents departures from isometry. There are at least two alternative explanations unrelated to marine age and marine-age diversity for the observed variation in intraspecific allometry.
First, density-dependent sexual selection on female body size associated with competition to obtain breeding sites and the ability to bury eggs deeply enough to avoid dig-up by other females (Van den Berghe & Gross 1984, 1989) may be sufficiently strong that in species with high breeding densities, optimal female body size is larger than that favoured by natural/fecundity and correlational selection alone. As a result, in species with high breeding densities and male-biased SSD, those expected to display the most extreme allometry, optimal female and male body size would be more similar, thus preventing intraspecific departures from isometry. Considering the life histories and slopes of the sister species O. gorbuscha and O. keta, which have (often exceptionally) high breeding densities and the highest mean SSD, suggests this mechanism, though perhaps operating, is alone an unlikely explanation for the observed variation in allometric slope. In O. gorbuscha all individuals spend 2 years in the marine environment, there is no marine-age diversity, and this species has the steepest significantly allometric slope. By contrast, O. keta has high marine age and marine-age diversity and has the second shallowest allometric slope. Second, we cannot reject the possibility that the underlying genetic architecture responsible for the phenotypic correlation between female and male size differs among the species. If populations are not at evolutionary equilibrium for size, such differences could theoretically regulate the correlated evolution of male and female size in a manner consistent with the observed relationship between life history and intraspecific allometric slope (Lande 1980).
That life history can constrain the effects of sexual selection in general, and its effect on SSD in particular, has been well-appreciated and empirically demonstrated (Partridge & Endler 1987; Price et al. 1987; Andersson 1994; Watkins 1996). As the most extensive analysis of intraspecific allometry to date, and to my knowledge the first at any taxonomic level using fishes, this study suggests that life history can also constrain the effects of sexual selection on allometry for SSD. Importantly, these results do not invalidate Rensch's rule; all seven intraspecific slopes are allometric in the predicted direction. Rather, Pacific salmon and trout provide a compelling example of how life-history variation within and among related species can regulate allometry for SSD across taxonomic scales. Because the combined effects of different selective forces on body size may often be species and sex specific, we can expect that similar analyses using other taxa will clarify how, and under which conditions, different evolutionary processes regulate the coevolution of male and female body size to promote or constrain allometry for SSD.
Acknowledgments
I thank B. Crespi, D. Fairbairn, A. Ø. Mooers and three anonymous referees for comments on earlier versions of the manuscript. This work was supported by a Natural Sciences and Engineering Research Council of Canada (NSERC) Post-Doctoral Fellowship to K.A.Y. and NSERC operating grants to B. Crespi and A. Ø. Mooers.
References
- Abouheif E., Fairbairn D.J. A comparative analysis of allometry for sexual size dimorphism: assessing Rensch's rule. Am. Nat. 1997;149:540–562. [Google Scholar]
- Andersson M. Sexual selection. Princeton University Press; 1994. [Google Scholar]
- Badyaev A.V. Growing apart: an ontogenetic perspective on the evolution of sexual size dimorphism. Trends Ecol. Evol. 2002;17:369–378. [Google Scholar]
- Beacham T.D., Murray C.B. A genetic analysis of body size in pink salmon (Oncorhynchus gorbuscha) Genome. 1988;30:31–35. doi: 10.1139/g88-006. [DOI] [PubMed] [Google Scholar]
- Butler M.A., Losos J.B. Multivariate sexual dimorphism, sexual selection, and adaptation in greater Antillean Anolis lizards. Ecol. Monogr. 2002;72:541–559. [Google Scholar]
- Chebanov N.A. Effect of spawner length and age on the viability of progeny during early ontogeny in some species of the genus Oncorhynchus (Salmonidae) J. Ichth. 1984;1984:82–93. [Google Scholar]
- Chebanov N.A. Factors controlling spawning success in pink salmon, Oncorhynchus gorbuscha. J. Ichth. 1986;1986:69–78. [Google Scholar]
- Einum S., Kinnison M.T., Hendry A.P. Evolution of egg size and number. In: Hendry A.P., Stearns S., editors. Evolution illuminated: salmon and their relatives. Oxford University Press; 2004. pp. 126–153. [Google Scholar]
- Fairbairn D.J. Allometry for sexual size dimorphism: pattern and process in the coevolution of body size in males and females. Ann. Rev. Ecol. Syst. 1997;28:659–687. [Google Scholar]
- Fairbairn D.J., Preziosi R.F. Sexual selection and the evolution of allometry for sexual size dimorphism in the water strider (Aquarius remigis) Am. Nat. 1994;144:101–118. doi: 10.1111/j.1558-5646.1996.tb03927.x. [DOI] [PubMed] [Google Scholar]
- Felsenstein J. Phylogenies and the comparative method. Am. Nat. 1985;125:1–15. doi: 10.1086/703055. [DOI] [PubMed] [Google Scholar]
- Fleming I.A., Gross M.R. Breeding competition in a Pacific salmon (coho: Oncorhynchus kisutch): measures of natural and sexual selection. Evolution. 1994;48:637–657. doi: 10.1111/j.1558-5646.1994.tb01350.x. [DOI] [PubMed] [Google Scholar]
- Fleming I.A., Reynolds J.D. Salmonid breeding systems. In: Hendry A.P., Stearns S.C., editors. Evolution illuminated: salmon and their relatives. Oxford University Press; 2004. pp. 264–294. [Google Scholar]
- Groot C., Margolis L. Pacific salmon life histories. University of British Columbia Press; Vancouver, Canada: 1991. [Google Scholar]
- Hendry A.P., Wenburg J.K., Bentzen P., Volk E.C., Quinn T.P. Rapid evolution of reproductive isolation in the wild: evidence from introduced salmon. Science. 2000;290:516–518. doi: 10.1126/science.290.5491.516. [DOI] [PubMed] [Google Scholar]
- Holtby L.B., Healey M.C. Sex-specific life history tactics and risk-taking in coho salmon. Ecology. 1990;71:678–690. [Google Scholar]
- Kraushaar U., Blanckenhorn W.U. Population variation in sexual selection and its effects on size allometry in two dung fly species with contrasting sexual size dimorphism. Evolution. 2002;56:307–321. doi: 10.1111/j.0014-3820.2002.tb01341.x. [DOI] [PubMed] [Google Scholar]
- Lande R. Sexual dimorphism, sexual selection and adaptation in polygenic characters. Evolution. 1980;34:292–307. doi: 10.1111/j.1558-5646.1980.tb04817.x. [DOI] [PubMed] [Google Scholar]
- McKay J.M., Devlin R.H., Smith M.J. Phylogeny of Pacific salmon and trout based on growth hormone type-2 and mitochondrial NADH dehydrogenase subunit 3 DNA sequences. Can. J. Fish. Aquat. Sci. 1996;53:1165–1176. [Google Scholar]
- Partridge L., Endler J.A. Life-history constraints on sexual selection. In: Bradbury J.W., Andersson M.B., editors. Sexual selection: testing the alternatives. Wiley; New York: 1987. pp. 265–277. [Google Scholar]
- Price T.D. The evolution of sexual size dimorphism in Darwin's finches. Am. Nat. 1984;123:500–518. [Google Scholar]
- Price T.D. Constraints on the effects of sexual selection. In: Bradbury J.W., Andersson M.B., editors. Sexual selection: testing the alternatives. Wiley; New York: 1987. pp. 278–294. (and 11 others) [Google Scholar]
- Purvis A., Rambaut A. Comparative analysis by independent contrasts (CAIC): an Apple Macintosh application for analysing comparative data. CABIOS. 1995;11:241–251. doi: 10.1093/bioinformatics/11.3.247. [DOI] [PubMed] [Google Scholar]
- Quinn T.P. A review of homing and straying of wild and hatchery-produced salmon. Fish. Res. 1993;18:29–44. [Google Scholar]
- Reeve J.P., Fairbairn D.J. Sexual size dimorphism as a correlated response to selection on body size: an empirical test of the quantitative genetic model. Evolution. 1996;50:1927–1938. doi: 10.1111/j.1558-5646.1996.tb03580.x. [DOI] [PubMed] [Google Scholar]
- Rensch B. Evolution above the species level. Columbia University Press; New York: 1960. [Google Scholar]
- Roni P., Quinn T.P. Geographic variation in size and age of North American chinook salmon. N. Am. J. Fish. Mngmt. 1995;15:325–345. [Google Scholar]
- Shine R. Ecological causes for the evolution of sexual size dimorphism: a review of the evidence. Q. Rev. Biol. 1989;64:419–461. doi: 10.1086/416458. [DOI] [PubMed] [Google Scholar]
- Shuster S.M., Wade M.J. Mating systems and strategies. Princeton University Press; 2003. [Google Scholar]
- Slatkin M. Ecological causes of sexual size dimorphism. Evolution. 1984;38:622–630. doi: 10.1111/j.1558-5646.1984.tb00327.x. [DOI] [PubMed] [Google Scholar]
- Sokal R.R., Rohlf F.J. Biometry. Freeman; New York: 1995. [Google Scholar]
- Van den Berghe E.P., Gross M.R. Female size and nest depth in coho salmon (Oncorhynchus kisutch) Can. J. Fish. Aquat. Sci. 1984;41:204–206. [Google Scholar]
- Van den Berghe E.P., Gross M.R. Natural selection resulting from female breeding competition in a Pacific salmon (coho: Oncorhynchus kisutch) Evolution. 1989;43:125–140. doi: 10.1111/j.1558-5646.1989.tb04212.x. [DOI] [PubMed] [Google Scholar]
- Watkins G.G. Proximate causes of sexual size dimorphism in the Iguanian lizard Microlophus occipitalis. Evolution. 1996;77:1473–1482. [Google Scholar]
- Withler R.E., Beacham T.D. Genetic variation in body weight and flesh colour of the coho salmon (Oncorhynchus kisutch) in British Columbia. Aquaculture. 1994;119:135–148. [Google Scholar]