Abstract
We investigated the relationship between plasma and yolk oestrogens in laying female zebra finches (Taeniopygia guttata) by manipulating plasma oestradiol (E2) levels, via injection of oestradiol-17β, in a sequence-specific manner to maintain chronically high plasma levels for later-developing eggs (contrasting with the endogenous pattern of decreasing plasma E2 concentrations during laying). We report systematic variation in yolk oestrogen concentrations, in relation to laying sequence, similar to that widely reported for androgenic steroids. In sham-manipulated females, yolk E2 concentrations decreased with laying sequence. However, in E2-treated females plasma E2 levels were higher during the period of rapid yolk development of later-laid eggs, compared with control females. As a consequence, we reversed the laying-sequence-specific pattern of yolk E2: in E2-treated females, yolk E2 concentrations increased with laying-sequence. In general therefore, yolk E2 levels were a direct reflection of plasma E2 levels. However, in control females there was some inter-individual variability in the endogenous pattern of plasma E2 levels through the laying cycle which could generate variation in sequence-specific patterns of yolk hormone levels even if these primarily reflect circulating steroid levels.
Keywords: maternal effects, plasma oestradiol-17β, Taeniopygia guttata, yolk hormones
1. Introduction
Maternal transfer of steroid hormones to egg yolk can have significant, generally positive, effects on offspring phenotype (e.g. Schwabl 1996a; Lipar & Ketterson 2000; Eising et al. 2001; Andersson et al. 2004; but see Sockman & Schwabl 2000). Almost all studies to date have focused on the role of androgens in this context, and have therefore been divorced from an understanding of the role these hormones play in regulation of the female’s own reproductive physiology. Many aspects of egg production are oestrogen- and/or progestin-dependent (e.g. Etches 1996). Females show marked but systematic variation in plasma levels of these steroid hormones associated with the cycle of follicular development and ovulation (Williams et al. 2004), and several investigators have reported significant effects of treatment with oestradiol (E2) on offspring development (Adkins-Regan et al. 1995; Williams 1999; Millam et al. 2001; von Englehardt et al. 2004). Nevertheless, very few investigators have considered the relationship between plasma steroids essential for female reproduction and yolk steroid levels. Out of 33 studies in which yolk steroids have been measured, only seven (21%) appear to have involved yolk oestrogens (i.e. E2) and in two of these non-detectable levels of E2 were reported. Where detectable levels of yolk E2 have been reported, previous studies have failed to reveal significant patterns of yolk E2 in relation to laying sequence (cf. androgens; e.g. Schwabl 1993; French et al. 2001; but see Lipar et al. 1999; Elf & Fivizzani 2002).
Physiological mechanisms regulating yolk steroids have not been established, and the source of yolk steroids itself is an unresolved question: are these derived from the systemic circulation or directly from follicular cells surrounding the developing yolk? Hackl et al. (2003) recently suggested that more than 99% of yolk steroids come directly from the cells of the follicular wall. However, this does not explain why treatment of laying females with exogenous hormone results in elevated yolk hormone levels (e.g. Adkins-Regan et al. 1995), or why factors which are assumed to elevate plasma steroid levels (e.g. social factors) also elevate yolk levels (Schwabl 1996b; Pilz & Smith 2004). The most direct way to test this would be to compare plasma hormone levels at the time of yolk formation and yolk levels within females in the same study, but surprisingly, given the large number of studies of yolk steroids (see above), only one previous study has covered this: Schwabl (1997) reported a positive correlation between maternal faecal testosterone (assumed to reflect circulating plasma levels) and yolk testosterone.
Here, we investigate the relationship between plasma and yolk oestrogens in laying female zebra finches (Taeniopygia guttata). We assumed that the endogenous pattern of plasma E2 involved a rapid increase from the onset of yolk development to maximum levels at the one-egg stage, with a subsequent linear decrease through the later stages of follicle development before the final ovulation (see Sockman & Schwabl 2000; Williams et al. 2004). Thus, we predicted that yolk E2 levels would decrease with laying sequence in non-manipulated females. We then manipulated plasma E2 levels, via injection of oestradiol-17β, in a sequence-specific manner, to maintain chronic high plasma levels for later-developing eggs. If yolk E2 levels are associated with circulating levels we therefore predicted that in E2-treated females we would abolish, or even reverse, the sequence-specific pattern of yolk E2 concentrations.
2. Material and methods
2.1 Animal care and breeding protocol
Zebra finches were maintained in controlled environmental conditions (temperature 19–23°C; humidity 35–55%; constant light schedule, 14 L:10D, lights on at 07.00). All birds were provided with a mixed seed diet (Panicum and white millet, 1:3, 11.7% protein, 0.6% lipid and 84.3% carbohydrate by dry mass), water, grit and cuttlefish bone (calcium) ad libitum, and received a multivitamin supplement in the drinking water once per week. Breeding pairs were also provided with 6 g/pair per day of egg food supplement (20.3% protein, 6.6% lipid) between pairing and clutch completion. All birds used in this experiment were of the ‘wild type’ plumage morph and were aged 6 months or older. Breeding pairs were housed individually in cages (61 cm×46 cm×41 cm), each with an external nest-box (11.5 cm×11.5 cm×11.5 cm). Females were weighed (±0.1 g, initial mass) at the time of pairing, at the one-egg stage, and again at the five-egg stage or at clutch completion (depending on which stage the bird reached first). Nest-boxes were checked daily between 09.00 and 11.00 and all new eggs were weighed (to 0.001 g) and numbered, to obtain data on egg size, clutch size and laying interval (the time between pairing and laying of the first egg). Experiments and animal husbandry were carried out under a Simon Fraser University Animal Care Committee permit (no. 558B), in accordance with guidelines from the Canadian Committee on Animal Care (CCAC).
2.2 Experimental protocol
For any pairs that initiated egg laying, females were alternately assigned to either a control (vehicle-injected) group or an oestradiol-treated (E2) group. The first-laid egg was collected to assay the yolk for oestradiol and was replaced with a zebra finch egg of approximately the same size (to maintain clutch size). Birds that were treated with E2 were given daily intramuscular injections of 17β-estradiol at 1μgg−1 in 30μl of canola oil from the one-egg stage until they laid their fifth egg or until clutch completion (for birds that laid only four eggs). Control females received daily injections of 30μl of canola oil only. The third, fourth and fifth eggs were also collected if laid for oestradiol analysis of yolks, and were again replaced with dummy eggs. At the five-egg stage, or at clutch completion for birds laying four eggs, birds were killed via exsanguination (using the anaesthetic Rompun/Ketamine, 1:1 v/v) and blood samples obtained. We dissected out the oviducts, ovaries and liver, which were dried to constant weight (dry mass, ±0.001 g) in a 60°C drying oven. Clutch size was recorded as all laid eggs plus the oviductal egg if present (no birds in either group had any remaining pre-ovulatory, yolky follicles in the ovary at the time of collection). All blood samples were centrifuged at 1800g for 10 min and the plasma was separated and frozen at −20°C until assayed for plasma vitellogenin (VTG) or oestradiol. We therefore obtained plasma samples from birds at the one- and four- or five-egg stages, and yolk samples from eggs 1, 3, 4 and 5 in the laying sequence.
2.3 Hormone determination
The concentration of oestradiol in yolk and in plasma was determined using an ultra-sensitive 17β-oestradiol enzyme-immunoassay (EIA) (Ecologiena/Japan EnviroChemicals Ltd., Abraxis LLC, Warminster, PA), following C18 solid-phase extraction (SPE). The yolk extraction method was a combination of the suggested SPE method for treated sewage effluent water (the design specifications for the EIA) and established protocols for yolk steroid extraction (e.g. Möstl et al. 2001). Specifically, a stainless steel spatula cleansed with ethanol was used to transfer a portion of each previously frozen yolk (0.11–0.28 g wet weight) to a 22 ml borosilicate glass vial (03-337-15, Fisher Scientific). The sample was then brought up to a total volume of 2 ml with distilled/deionized (dd) water and allowed to equilibrate to room temperature. Samples were sonicated for 15 min (Aquasonic Model 75D, VWR Scientific), shaken for an additional 60 min (Mistral Multi-Mixer) and then diluted with 8 ml of distilled-in-glass (DIG) methanol (Fisher Scientific). They were allowed to settle in a refrigerator overnight and the next day were vigorously mixed for 90 min. Following mixing, samples were transferred to 15 ml conical centrifuge tubes (Sarstedt 62.554.002 PP) and centrifuged at 1500g for 7 min under refrigeration (2±1.5°C). The supernatant was transferred to another 15 ml centrifuge tube and the volume adjusted to 10 ml. Half of the sample, 5 ml, was transferred to a 12 mm×75 mm disposable borosilicate culture tube. This aliquot was evaporated to dryness under vacuum (15°C; Vortex Evaporator, Buchler Instruments) and reconstituted in 5 ml of 10% methanol. The following day, each sample was passed through a C18 column (IS2200050C Isolute® SPE columns, Chromatographic Specialties, Inc., Brockville, Ontario). Using vacuum filtration, each column was primed with 3 ml of DIG methanol, followed by 10 ml of dd water, followed by the entire 5 ml sample volume, and then washed with 10 ml of dd water. Oestradiol was eluted with 5 ml of 80% methanol into 7 ml borosilicate vials (03-337-26, Fisher Scientific). Each sample was evaporated to dryness and reconstituted in 3 ml of assay buffer (10% methanol). This volume was chosen on the basis of parallelism in serial dilution during preliminary validation of the assay and it placed the yolk samples near the middle of the standard curve. To quantify recovery of oestradiol during this SPE procedure, a sample of yolk was stripped of steroid during 90 min of stirring with dextran-coated charcoal (10% dextran-charcoal in 0.1 M phosphate-buffered saline with 0.1% gelatin) at 4°C. After centrifugation, the supernatant was spiked with 3H-oestradiol (Dupont NET 317), equilibrated overnight, diluted with methanol and used in the SPE procedure. Recovery was 95% or more over five replicates. For plasma, the SPE procedure was the same except that a 50μl (or 25μl if sample volume was limited) plasma aliquot was passed through the primed C18 column, eluted with 5 ml of 80% methanol, evaporated to dryness and reconstituted in 1 ml of assay buffer.
For both the yolk and the plasma EIA, instructions supplied with the Ecologiena kit were followed exactly and a volume of 100μl was used in the assay well. For yolk samples, this represented 1/30 th of the final sample volume and 1/60 th of the original yolk sample. For plasma samples, this was 10% of the final sample volume, and represented 5μl (or 2.5μl) of plasma. Results are reported as pg oestradiol/gram of wet yolk weight and ng oestradiol/ml of plasma. Sixty-two yolk samples were run in duplicate on two plates, with a CV of 3.8±0.3% (s.e.). Out of 67 blood samples, only 14 were run in duplicate, to keep all samples on the same plate. The 14 duplicate samples showed a CV of 6.1±1.8%. Four supplied standards covered the range from 50 to 1000 pg/ml (5–100 pg per well) and were used to define the limits of sensitivity for the assay. None of the samples fell outside the range of sensitivity. All plates included duplicate high and low controls that fell within the predicted range (intra-assay CV=4.7%, inter-assay CV=10.3%). Antibody cross-reactivity to testosterone was less than 0.03% and to cholesterol 0.46%.
3. Results
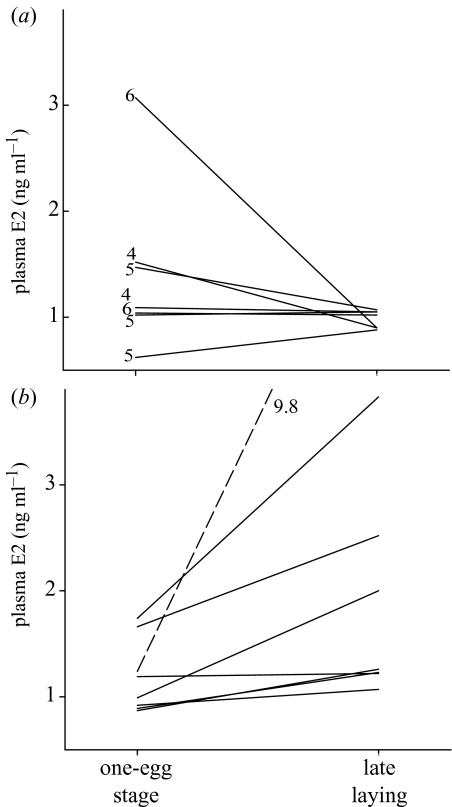
There was marked inter-individual variation in plasma E2 levels in females sampled at the one-egg stage (figure 1a,b; range 0.62–3.07 ng ml−1; no effect of body mass, p>0.5), but there was no difference between birds subsequently assigned to control or E2 treatment: control, 1.41±0.30 ng ml−1 (s.e.), n=7, E2-treated, 1.37±0.21 ng ml−1 (Wilcoxon rank test, Z=0.1, p>0.90). There was also marked individual variation in the pattern of change in plasma E2 levels with laying sequence in both treatment groups (this was not related to reproductive state at the time of the last sample: clutch completion, presence or absence of oviductal egg; see figure 1a,b). Consequently, the average change in plasma E2 concentrations in early and late-laying control birds was not significant (paired t-test, t7=1.36, p>0.20). However, in E2-treated females plasma E2 increased from early to late laying (t7=2.58, p<0.05), and during late laying there was a significant treatment effect for plasma E2: control, 0.98±0.03 ng ml−1, n=7, E2-treated, 1.78±0.34 ng ml−1, n=8 (Z=3.18, p<0.01; the E2-treated female with a late-laying E2 value of 9.78 ng ml−1 was excluded from these analyses).
Figure 1.
Individual variation in the pattern of change in plasma oestradiol-17β concentrations during laying. For (a) control females, numbers by each line indicate final clutch size for each bird. For (b) E2-treated females, note that one individual, indicated by the dashed line, is plotted on a different scale.
Although E2-treated females had higher plasma E2 levels late in the laying cycle compared with control females, there was no significant difference in the mass of the reproductive organs at this stage: dry oviduct mass; control, 84.9±1.5 mg (s.e.) versus E2 treatment, 100.1±1.3 mg (p>0.40) or wet ovary mass; control, 39.9±0.4 mg versus E2 treatment, 36.4±2.4 mg (p>0.40). Similarly, there was no treatment effect on mean egg mass (control, 1.096±0.041 g versus E2 treatment, 1.082±0.031 g, p>0.60) or clutch size (control, 5.2±0.3 versus E2 treatment, 5.0±0.3, p>0.60).
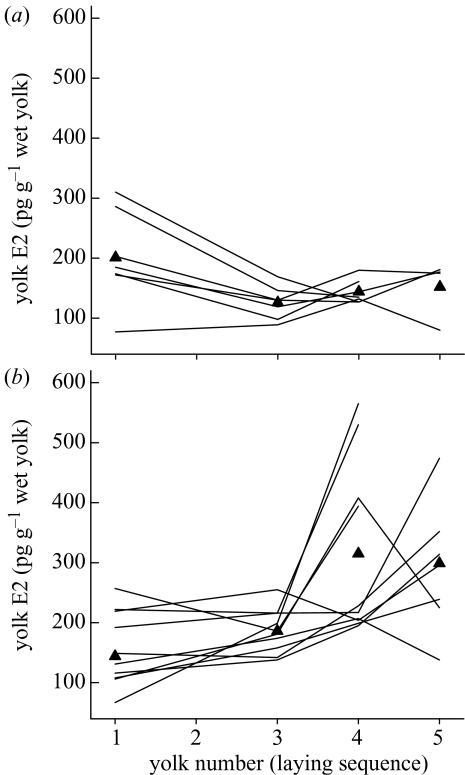
There was also marked individual variation in yolk E2 levels in relation to laying sequence (figure 2a,b), but overall there was a significant treatment effect on the sequence-specific pattern of yolk E2 (interaction term, egg number × treatment, F3,31=6.26, p<0.002). In control females, yolk E2 levels decreased with laying sequence (F3,12.6=5.45, p<0.025). Yolk E2 levels differed significantly between eggs 1 and 3 (p=0.009), but not between eggs 3, 4 and 5 (p>0.10, Bonferroni adjusted pairwise contrasts). By contrast, in E2-treated females yolk E2 levels increased with laying sequence (F3,18.6=7.06, p<0.005; figure 2b). Here, there was no significant difference in yolk E2 levels in eggs 1 and 3 (p>0.15), but eggs 4 and 5 had significantly higher yolk levels than egg 1 (p<0.025 in both cases). Yolk E2 levels did not differ among treatments as regards first-laid eggs (p>0.05), but they were significantly higher in eggs from E2 treated females at all other egg stages (egg 3, F1,16=13.7, p<0.01; egg 4, F1,16=9.27, p<0.01; egg 5, F1,16=5.79, p<0.05).
Figure 2.
Individual variation in yolk E2 levels in relation to laying sequence among (a) control females and (b) E2-treated females. Triangles indicate mean values for each laying sequence.
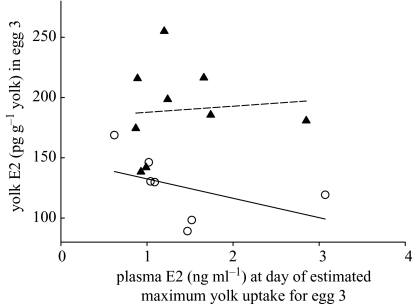
Given the timing and pattern of yolk formation in zebra finches (Haywood 1993), most of the yolk for the third-laid egg (ca. 60%) would have been deposited during the day and night after the female laid her first egg (i.e. within 24 h of the first E2 treatment; note that E2 treatment already affected yolk E2 in the third-laid egg; see above and figure 3). Comparing plasma E2 levels at the one-egg stage with E2 levels in yolk from the third-laid egg, there was a significant negative relationship between these variables in control females (rs=−0.87, n=7, p<0.025). However, in E2-treated females yolk E2 levels were independent of plasma E2 concentrations (figure 3).
Figure 3.
Relationship between plasma E2 levels at the one-egg stage of laying and yolk E2 levels in egg 3 in control females (open circles) and E2-treated females (filled triangles).
4. Discussion
We have shown that there can be systematic variation in yolk oestrogen concentrations, in relation to laying sequence, similar to that widely reported for androgenic steroids: in sham-manipulated females, yolk E2 levels decreased with laying sequence. By injecting females with oestradiol-17β from the one-egg stage, we altered the endogenous pattern of plasma E2, such that this was higher during the period of rapid yolk development of later-laid eggs. As a consequence, we reversed the laying-sequence-specific pattern of yolk E2 concentrations: in E2-treated females, yolk E2 concentrations increased with laying sequence. This confirms that the yolks of late-laid eggs developed in a very different maternal hormonal milieu. Overall, yolk E2 levels were a direct reflection of plasma E2 levels. However, at the individual level we found a negative relationship between plasma E2 levels at the egg 1 stage and yolk E2 levels in egg 3. Furthermore, among sham-manipulated females there was considerable inter-individual variation in plasma E2 levels, comparing early- and late-laying birds, with plasma levels decreasing, remaining more or less constant, or even increasing over this period in different individuals.
There have been very few detailed studies of day-to-day, or laying-sequence-specific, patterns of changes in plasma hormone levels during the laying cycle, other than for poultry species (Etches 1996). In free-living female starlings (Sturnus vulgaris) plasma E2 levels increase rapidly from the onset of rapid yolk development to reach maximum levels in birds with a complete follicle hierarchy (four or more yolky follicles). However, levels decrease linearly throughout the later stages of follicle development, returning to pre-breeding values before the final yolky follicle is ovulated (Williams et al. 2004). This pattern is very similar to that reported by Sockman & Schwabl (2000) using faecal oestradiol-17β measurements in captive canaries (Serinus canaria) during the laying cycle. These data suggest that early- and late-developing yolks are exposed to very different hormonal environments during development and, in the present study, we have shown that this can result in predictable sequence-specific variation in yolk E2 concentrations. Consistent with our results, Elf & Fivizzani (2002) reported a marginally significant (p=0.06) decrease in yolk oestradiol levels in six sequentially laid eggs in the domestic hen (Gallus domesticus).
Maintenance of chronically high plasma E2 levels through to the end of laying had no effect on egg or clutch size, even though this manipulation started before clutch size had been determined (day 3 of laying in this species; Haywood 1993). This confirms the results of previous studies where oestradiol treatment of laying females had no effect on primary reproductive output (Christians & Williams 1999; Williams 1999). Moreover, elevated plasma oestradiol levels had no effect on ovarian or oviduct development or the timing of oviduct regression. Although oestradiol is clearly required for initiation of development of the reproductive tract, these results suggest that subsequent maintenance and regression of the reproductive system are insensitive to marked changes in plasma oestradiol concentrations, perhaps as a result of temporal changes (downregulation) of oestrogen-receptor expression in these tissues (see also Hatle et al. 2001).
There was marked inter-individual variation in plasma E2 concentrations in non-manipulated females at the one-egg stage and in the pattern of change in plasma E2 levels between early and late egg development. However, there was also marked individual variation in plasma E2 levels of E2-treated females, even though all birds received a standard hormone dose. This suggests that there might be individual variation in sensitivity to exogenous E2, or perhaps in rates of metabolism of exogenous E2. In preliminary experiments we validated the E2 stock solution used in the present study by injecting non-breeding females and measuring plasma levels of the E2-dependent yolk precursor VTG (e.g. Williams & Martyniuk 2000). We found that plasma VTG levels produced in response to a standard dose of exogenous E2 were highly variable but that this response was repeatable (r22=0.58, p<0.01; T. D. Williams and C. E. Ames, unpublished data). This suggests another level of complexity in interpreting the physiological consequences of differences in plasma hormone levels. Future work should concern this individual variation in tissue sensitivity to a given hormone level, since this might in turn explain individual variation in hormone-mediated traits.
If development and function of the female reproductive tract dictated a fixed or constrained hormonal milieu, reflected in the females’ own plasma hormone levels, then this might limit a female’s ability to adjust hormone levels in yolks independently of variation in plasma levels. For example, production of E2 is highest in small early-stage follicles (Bahr et al. 1983) such that on average total ovarian output of E2, and hence plasma levels, should always peak at the onset of ovulation and decline through later stages of laying. Thus, if yolk steroid levels reflect plasma steroid levels, females would be unable to produce yolks in which E2 levels increase through the laying sequence. Data available so far support this scenario for oestrogens: the concentrations of both plasma and yolk oestrogens decrease with egg-laying sequence (Sockman & Schwabl 2000; Elf & Fivizzani 2002; Williams et al. 2004) and experimental elevation of plasma E2 levels increases yolk E2 levels (Adkins-Regan et al. 1995; this study). Thus, female birds appear to have a limited capacity to uncouple yolk E2 levels from variation in circulating levels (cf. studies in lizards (Painter et al. 2002; Lovern & Wade 2003), though these might not be strictly comparable owing to differences in timing and duration of follicle development). However, our results suggest that at the individual level there is some flexibility or variability among females in the endogenous pattern of plasma E2 levels through the laying cycle (see figure 1a) that could generate inter-individual variability in yolk hormone levels even if these primarily reflect circulating steroid levels. Given the evidence suggesting that E2 treatment can affect offspring development (Adkins-Regan et al. 1995; Millam et al. 2001; von Englehardt et al. 2004), and considering concerns about environmental contaminants that have oestrogenic effects in wildlife (Ankley et al. 1998), individual and ecological factors influencing yolk oestradiol-17β are worthy of future attention.
References
- Adkins-Regan E., Ottinger M.A., Park J. Maternal transfer of estradiol to egg yolks alters sexual differentiation of avian offspring. J. Exp. Zool. 1995;271:466–470. [Google Scholar]
- Andersson S., Uller T., Lohmus M., Sundstrom F. Effects of egg yolk testosterone on growth and immunity in a precocial bird. J. Evol. Biol. 2004;17:501–505. doi: 10.1111/j.1420-9101.2004.00706.x. [DOI] [PubMed] [Google Scholar]
- Ankley G. Overview of a workshop on screening methods for detecting potential (anti-) estrogenic/androgenic chemicals in wildlife. Environ. Toxicol. Chem. 1998;17:68–87. (and 29 others) [Google Scholar]
- Bahr J.M., Wang S.-C., Huang M.Y., Calvo F.O. Steroid concentrations in isolated theca and granulosa layers of preovulatory follicles during the ovulatory cycle of the domestic hen. Biol. Reprod. 1983;29:326–334. doi: 10.1095/biolreprod29.2.326. [DOI] [PubMed] [Google Scholar]
- Christians J.K., Williams T.D. Effects of exogenous 17-β-estradiol on the reproductive physiology and reproductive performance of European starlings (Sturnus vulgaris) J. Exp. Biol. 1999;202:2679–2685. doi: 10.1242/jeb.202.19.2679. [DOI] [PubMed] [Google Scholar]
- Eising C.M., Eikenaar C., Schwabl H., Groothuis T.G.G. Maternal androgens in black-headed gull (Larus ridibundus) eggs: consequences for chick development. Proc. R. Soc. B. 2001;268:839–846. doi: 10.1098/rspb.2001.1594. doi: 10.1098/rspb.2001.1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elf P.K., Fivizzani A. Changes in sex steroid levels in yolks of the leghorn chicken, Gallus domesticus, during embryonic development. J. Exp. Zool. 2002;293:594–600. doi: 10.1002/jez.10169. [DOI] [PubMed] [Google Scholar]
- Etches R.J. Reproduction in poultry. CAB International; Oxford: 1996. [Google Scholar]
- French J.B., Nisbet I.C.T., Schwabl H. Maternal steroids and contaminants in common tern eggs: a mechanism of endocrine disruption? Comp. Biochem. Physiol. C. 2001;128:91–98. doi: 10.1016/s1532-0456(00)00181-2. [DOI] [PubMed] [Google Scholar]
- Hackl R., Bromundt V., Daisley J., Kotrschal K. Distribution and origin of steroid hormones in the yolk of Japanese quail (Coturnix coturnix japonica) J. Comp. Physiol. B. 2003;173:327–331. doi: 10.1007/s00360-003-0339-7. [DOI] [PubMed] [Google Scholar]
- Hatle J.D., Borst D.W., Eskew M.R., Juliano S.A. Maximum titers of vitellogenin and total hemolymph protein occur during the canalised phase of grasshopper egg production. Physiol. Biochem. Zool. 2001;74:885–893. doi: 10.1086/324475. [DOI] [PubMed] [Google Scholar]
- Haywood S. Sensory control of clutch size in the zebra finch (Taeniopygia guttata) Auk. 1993;110:778–786. [Google Scholar]
- Lipar J.L., Ketterson E.D. Maternally derived yolk testosterone enhances the development of the hatching muscle in the red-winged blackbird Aegelaius phoeniceus. Proc. R. Soc. B. 2000;267:2005–2010. doi: 10.1098/rspb.2000.1242. doi: 10.1098/rspb.2000.1242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipar J.L., Ketterson E.D., Nolan V., Jr Intraclutch variation in testosterone content of red-winged blackbird eggs. Auk. 1999;116:231–235. [Google Scholar]
- Lovern M.B., Wade J. Sex steroids in green anoles (Anolis carolinensis): uncoupled maternal plasma and yolking follicle concentrations, potential embryonic steroidogenesis, and evolutionary implications. Gen. Comp. Endocrinol. 2003;134:109–115. doi: 10.1016/s0016-6480(03)00240-5. [DOI] [PubMed] [Google Scholar]
- Millam J.R., Craig-Velt C.B., Quaglino A.E., Erichsen A.L., Famula T.R., Fry D.M. Post-hatch oral estrogen exposure impairs adult reproductive performance of zebra finches in a sex-specific manner. Horm. Behav. 2001;40:542–549. doi: 10.1006/hbeh.2001.1724. [DOI] [PubMed] [Google Scholar]
- Möstl E., Spendier H., Kotrschal K. Concentration of immunoreactive progesterone and androgens in the yolk of hens’ eggs (Gallus domesticus) Weiner Tierärztliche Monatsschrift. 2001;88:62–65. [Google Scholar]
- Painter D., Jennings D.H., Moore M.C. Placental buffering of maternal steroid hormone effects on fetal and yolk hormone levels: a comparative study of a viviparous lizard, Sceloporus jarrovi, and an oviparous lizard, Sceloporus graciosus. Gen. Comp. Endocrinol. 2002;127:105–116. doi: 10.1016/s0016-6480(02)00075-8. [DOI] [PubMed] [Google Scholar]
- Pilz K.M., Smith H.G. Egg yolk levels increase with breeding density in the european starling, Sturnus vulgaris. Funct. Ecol. 2004;18:58–66. [Google Scholar]
- Schwabl H. Yolk is a source of maternal testosterone for developing birds. Proc. Natl Acad. Sci USA. 1993;90:11446–11450. doi: 10.1073/pnas.90.24.11446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabl H. Maternal testosterone in the avian egg enhances postnatal growth. Comp. Biochem. Physiol. 1996a;114A:271–276. doi: 10.1016/0300-9629(96)00009-6. [DOI] [PubMed] [Google Scholar]
- Schwabl H. Environment modifies the testosterone levels of a female bird and its eggs. J. Exp. Zool. 1996b;276:157–163. doi: 10.1002/(SICI)1097-010X(19961001)276:2<157::AID-JEZ9>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Schwabl H. Maternal steroid hormones in the egg. In: Harvey S., Etches R.J., editors. Perspectives in avian endocrinology. Journal of Endocrinology Ltd; Bristol: 1997. pp. 3–13. [Google Scholar]
- Sockman K.W., Schwabl H. Yolk androgens reduce offspring survival. Proc. R. Soc. B. 2000;267:1451–1456. doi: 10.1098/rspb.2000.1163. doi: 10.1098/rspb.2000.1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Englehardt N., Dijkstra C., Daan S., Groothuis T.G.G. Effects of 17-β-estradiol treatment of female zebra finches on offspring sex ratio and survival. Horm. Behav. 2004;45:306–313. doi: 10.1016/j.yhbeh.2003.12.009. [DOI] [PubMed] [Google Scholar]
- Williams T.D. Parental and first generation effects of exogenous 17β-estradiol on reproductive performance of female zebra finches (Taeniopygia guttata) Horm. Behav. 1999;35:135–143. doi: 10.1006/hbeh.1998.1506. [DOI] [PubMed] [Google Scholar]
- Williams T.D., Martyniuk C.J. Organ mass dynamics during egg-production in female zebra finches (Taeniopygia guttata): dietary and hormonal manipulations. J. Avian Biol. 2000;31:87–95. [Google Scholar]
- Williams T.D., Kitaysky A.S., Vézina F. Individual variation in plasma estradiol-17β and androgen levels during egg formation in the European starling Sturnus vulgaris: implications for regulation of yolk steroids. Gen. Comp. Endocrinol. 2004;136:346–352. doi: 10.1016/j.ygcen.2004.01.010. [DOI] [PubMed] [Google Scholar]