Abstract
Comprehensive analyses of long-term (1977–2003) small-mammal abundance data from western Finland showed that populations of Microtus voles (field voles M. agrestis and sibling voles M. rossiaemeridionalis) voles, bank (Clethrionomys glareolus) and common shrews (Sorex araneus) fluctuated synchronously in 3 year population cycles. Time-series analyses indicated that interspecific synchrony is influenced strongly by density-dependent processes. Synchrony among Microtus and bank voles appeared additionally to be influenced by density-independent processes. To test whether interspecific synchronization through density-dependent processes is caused by predation, we experimentally reduced the densities of the main predators of small mammals in four large agricultural areas, and compared small mammal abundances in these to those in four control areas (2.5–3 km2) through a 3 year small-mammal population cycle. Predator reduction increased densities of the main prey species, Microtus voles, in all phases of the population cycle, while bank voles, the most important alternative prey of predators, responded positively only in the low and the increase phase. Manipulation also increased the autumn densities of water voles (Arvicola terrestris) in the increase phase of the cycle. No treatment effects were detected for common shrews or mice. Our results are in accordance with the alternative prey hypothesis, by which predators successively reduce the densities of both main and alternative prey species after the peak phase of small-mammal population cycles, thus inducing a synchronous low phase.
Keywords: interspecific temporal synchrony, density-dependent processes, predator reduction experiment, population cycles, predator–prey interactions, small mammals
1. Introduction
Multi-annual periodic population fluctuations of northern small mammals and game animals have fascinated ecologists for at least 75 years (e.g. Elton 1924, 1942; Elton & Nicholson 1942; Lindström et al. 2001). In northern Europe and the Arctic tundra, voles and lemmings show population cycles of between 3 and 5 years, whereas in the boreal forests of North America, snowshoe hares (Lepus americanus) undergo 9–10 year cycles in density (Hansson & Henttonen 1988; Keith 1990; Stenseth & Ims 1993; Norrdahl 1995; Korpimäki & Krebs 1996; Stenseth 1999; Krebs et al. 2001; Klemola et al. 2002a). An eminent additional feature of population cycles of these mammals is that they occur temporally relatively synchronously with population cycles of some other small- and medium-sized vertebrate animals. For example, populations of voles and small game (i.e. hare and forest grouse) in Sweden and northern Finland (Angelstam et al. 1984, 1985; Hörnfeldt et al. 1986; Lindén 1988), voles and shrews in northern Europe (Hansson 1984; Henttonen 1985; Korpimäki 1986; Henttonen et al. 1989), and snowshoe hares, Arctic ground squirrels (Spermophilus parryii) and willow ptarmigans (Lagopus lagopus) in the boreal forest of Canada (Boutin et al. 1995) have been found to fluctuate synchronously.
Climatic factors, affecting populations directly or indirectly through general productivity, have traditionally been invoked as the most natural explanation for large-scale regional synchrony in the density fluctuations of many vertebrate species (e.g. Moran 1953; Sinclair et al. 1993; Ranta et al. 1995, 1997; Hudson & Cattadori 1999). Populations of species with similar intrinsic structures of density dependence may become synchronized by factors affecting populations in a density-independent manner, if such factors are correlated between focal populations (i.e. Moran effect; Moran 1953; Royama 1992; Ranta et al. 1995). Natural enemies, in particular abundant generalist predators, may also have synchronizing effects on a large array of prey populations (Hansson & Henttonen 1988; Korpimäki & Norrdahl 1989a,b, 1991a, 1998; Korpimäki et al. 1991). By contrast, food supply as such is an unlikely proximate factor behind temporal synchrony at a vertebrate community level, because the diet composition of synchronously fluctuating species often varies from herbivorous to insectivorous (Hansson 1984; Henttonen 1985; Korpimäki 1986).
Predators may synchronize population fluctuations of coexisting prey species by killing prey selectively (the alternative prey hypothesis or APH; Hagen 1952; Lack 1954; Angelstam et al. 1984; Stuart-Smith 1992) or unselectively (the shared predation hypothesis or SPH; Norrdahl & Korpimäki 2000). APH states that predators hunt mainly individuals of their main prey until their densities profoundly decrease, after which they switch to hunting alternative prey, also causing a subsequent reduction in their densities. SPH states that all prey species in a community suffer proportionately equal losses to predators during all phases of a population cycle. Although the proportion of alternative prey in the diet of predators is lower in years with high densities of main prey (e.g. Korpimäki & Norrdahl 1989b, 1991b; Korpimäki et al. 1990, 1991; Reif et al. 2001), predators eat alternative prey in all phases of a population cycle. Owing to a rapid numerical response of predator populations to density changes of main prey (Korpimäki 1985, 1994; Norrdahl & Korpimäki 2002c), the actual number of alternative prey killed by predators may be higher in years with high rather than low densities of main prey. In the APH, the impact of predators on alternative prey depends more on the density of the main prey than on the ratio of predators to alternative prey. Accordingly, the APH predicts that predation may have a limiting or regulating (sensu ‘mechanistic paradigm’ i.e. experimentally seeking for mechanisms by which population effects are achieved; see Krebs (1995)) impact on alternative prey less often or during shorter periods than predicted by the SPH. According to both hypotheses, a prerequisite of a synchronizing effect is that the impact of predators on alternative prey is at least periodically strong enough to decrease population densities.
We have studied a small mammal assemblage in western Finland where small mustelids (the least weasel (Mustela nivalis) and the stoat (M. erminea)) and birds of prey (the European kestrel (Falco tinnunculus), the short-eared owl (Asio flammeus), the long-eared owl (A. otus) and Tengmalm’s owl (Aegolius funereus)) are the main predators. The following small mammal species coexist in agricultural fields, small forest patches and forest edges (Norrdahl & Korpimäki 2002a,b): two Microtus voles (field vole M. agrestis and sibling vole M. rossiaemeridionalis, both ca. 30 g) are the main prey of all small- and medium-sized avian and mammalian predators, and bank voles (Clethrionomys glareolus, ca. 20 g) are the most important alternative prey. In addition, water voles (Arvicola terrestris, young ca. 100 g, adults ca. 200 g), mice (harvest mouse (Micromys minutus, less than 10 g)) and house mouse (Mus musculus, less than 20 g) and common shrews (Sorex araneus, less than 10 g) are used by most predators as alternative prey together with small birds (Korpimäki et al. 1991; Korpimäki & Norrdahl 1991b; Korpimäki 1992, 1993; Norrdahl & Korpimäki 1995b; Reif et al. 2001). However, adult water voles are not customarily killed by the smallest predators, least weasels (mean body mass: males 48 g, females 35 g; Korpimäki et al. 1991) and Tengmalm’s owls (males 112 g and females 163 g; Korpimäki 1990).
In our study area and elsewhere in northern Europe, Microtus voles fluctuate in temporal synchrony with bank voles (Henttonen et al. 1987; Hanski & Henttonen 1996; Norrdahl & Korpimäki 2002b), and even with insectivorous shrews (Sorex spp.), all showing their lowest densities simultaneously (Henttonen et al. 1987, 1989; Hanski & Henttonen 1996). The patterns of cyclicity and temporal synchrony of population oscillations of many small mammal species, in particular common shrews, water voles and mice with Microtus and bank voles, have largely remained unstudied.
By a novel series of large-scale replicated field experiments where densities of predators were manipulated, we have provided experimental evidence for the hypothesis that synergetic impacts of all main predators drive the population cycles of Microtus voles in our study area (Korpimäki & Norrdahl 1998; Klemola et al. 2000a,b, 2002b; Korpimäki et al. 2002; Huitu et al. 2003). In addition, the reduction of least weasels only increased the abundance of small ground-nesting birds, but did not affect the abundance of common shrews (Norrdahl & Korpimäki 2000). The reduction of only avian predators increased the abundance of common shrews and small birds, but only in years when populations of main prey declined (Norrdahl & Korpimäki 1995a, 2000). In addition, we reduced the densities of main mammalian and avian predators through a 3 year vole cycle and compared vole abundances between four large reduction and four comparable control areas. The reduction of predator densities increased the autumn density of Microtus voles by two- to fourfold in the low, increase and peak phases, retarded the initiation of a decline, and prevented a summer decline of the Microtus vole cycle (Korpimäki & Norrdahl 1998; Korpimäki et al. 2002).
Here, for the first time to our knowledge, we analyse statistically long-term (more than 20 years) time-series data on densities of various coexisting small mammal species in the same location to detect patterns of cyclicity and interspecific temporal synchrony. Most earlier analyses only cover Microtus voles or other most abundant small rodent species in an area (summarized by Turchin (2003)), but do not include analyses of temporal synchrony of population oscillations of many small mammal species. Thereafter, we use data on small mammal species sympatric to Microtus voles, collected during the 3 year predator reduction experiment (Korpimäki et al. 2002), to study whether predators whose main prey are Microtus voles can also regulate or limit the abundance of alternative prey. Finally, we ask which of the two hypotheses, the SPH or the APH, is better able to predict the impacts of predators on different alternative prey species. The SPH predicts that when the densities of predators are manipulated, the abundances of both main and alternative prey types are affected during both high and low densities of main prey, whereas the APH predicts that the abundances of alternative prey types are only affected at low densities of main prey.
2. Material and methods
2.1 Long-term estimation of small mammal abundance
The study area in the vicinity of Kauhava and Lapua, western Finland (63° N, 23° E), comprises mainly agricultural fields (Korpimäki & Norrdahl 1998; Korpimäki et al. 2002). To estimate small-mammal abundance in the area, we have conducted snap trappings in early May and in mid-September at two sites (Ruotsala in Kauhava and Alajoki in Lapua), separated by ca. 14 km, during 1977–2003. We sampled the main habitat types (a cultivated field, an abandoned field, a spruce forest and a pine forest) in both sites (Korpimäki & Norrdahl 1991a,b) by means of 50–100 Finnish metal mouse snap-traps (suitable for Microtus voles, bank voles, mice and shrews), which were set at intervals of 10 m in runways of small mammals and checked once a day for 4 days. The mouse snap-traps were baited with mixed-grain bread (i.e. bread made of a mixture of rye and wheat flours), which has been shown to be an appropriate bait for voles, mice and shrews (e.g. Koivunen et al. 1996). In addition, 20–50 Finnish metal rat snap-traps, which are suitable for trapping larger water voles (Korpimäki et al. 1991), were used both at Alajoki and at Ruotsala during 1981–2002. The rat snap-traps were baited with dried apple and mixed-flour bread, both appropriate bait for water voles (Myllymäki et al. 1971). We pooled the results from the four-night trapping periods for each species separately and standardized them to the number of animals caught per 100 rattrap nights for water voles and brown rats and per 100 mousetrap nights for all other small mammal species. As the synchrony of trap indices between Ruotsala and Alajoki is high (cross correlation coefficient with lag 0 for Microtus voles = 0.68, for bank voles = 0.62, for other species 0.38–0.45), the species-wise density indices used in all analyses in this study are means of the two sites of the study area. For all analyses, only autumn trapping indices are used.
2.2 Predator reduction experiment
Eight large (2.5–3 km2 each) agricultural field areas (15–23% forest in each area) were used for the predator reduction experiment; four of these areas were randomly assigned to the predator reduction treatment, while the remaining four served as unmanipulated control areas. All experimental areas were within 180 km2 (12 km×15 km), with an inter-area distance of at least 5 km. This effectively precluded small mustelids and breeding avian predators from dispersing between control and reduction areas during the yearly experimental periods of between six and seven summer months (Korpimäki & Norrdahl 1998; Norrdahl & Korpimäki 1996). However, predators were free to move into and out of each area. During the 3 year experiment (1997–1999), vole populations underwent a 3 year population cycle (figure 1a). In the year preceding the initiation of the experiment (1996), densities of vole populations were moderate and declined to low numbers by spring 1997.
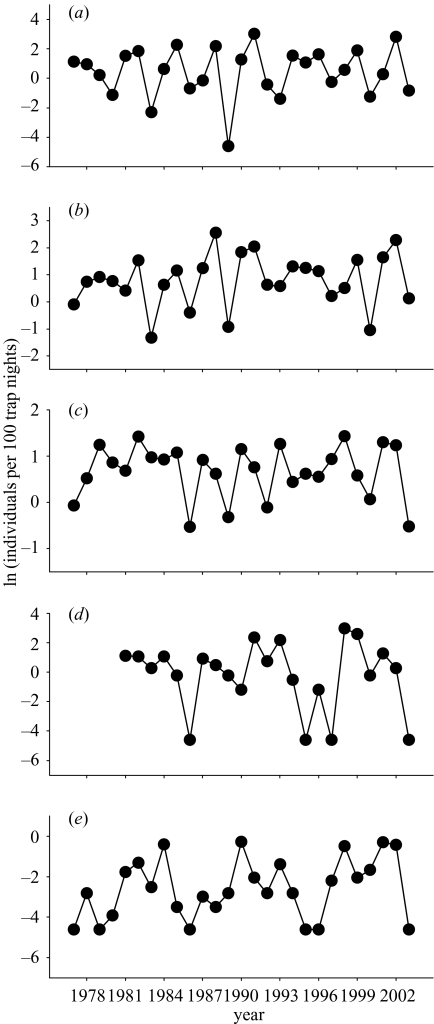
Figure 1.
Long-term population fluctuations of (a) Microtus voles, (b) bank voles, (c) common shrews, (d) water voles and (e) pooled harvest and house mice in western Finland. Symbols denote ln-transformed values of autumn trapping indices (number of individuals caught per 100 trap nights) during 1977–2003 (1981–2003 for water voles). Original trapping data for common shrews were detrended by fitting a second-order polynomial curve.
In each of the four predator reduction areas, stoats and least weasels were removed by live-trapping from April–May to October each year (for details of trapping programme, see Korpimäki et al. (1994) and Korpimäki & Norrdahl (1998)). In mid-May 1997, we distributed 50–65 least weasel traps and 20–30 stoat traps evenly in one reduction area for 5 days and checked the traps once a day. In the following week, the traps were transferred to the next reduction area. A trapping session in all four reduction areas took about one month, after which identical live-trapping sessions were repeated in mid-June to mid-July, and again in August, September and October. In 1998 and 1999, trapping followed the same protocol except that trapping was started in early April and performed six times a year between the periods April–June and August–October. The pooled number of small mustelids removed from each of the four reduction areas were 11 (1997: 5, 1998: 2, 1999: 4), 15 (1997: 2, 1998: 1, 1999: 12), 23 (1997: 10, 1998: 3, 1999: 10) and 32 (1997: 11, 1998: 6, 1999: 15). Trapped mustelids were transferred and released at least 30 km from our study sites. Stoats were trapped and transferred with the permission of the Finnish Ministry of Agriculture and Forestry, and least weasels with the permission of the Finnish Ministry of Environment.
From late February to March in 1997, 1998 and 1999 (before predator reduction) and from late November to December 1997, 1998 and 1999 (after predator reduction), density indices of mammalian predators were obtained by snow-tracking soon after a snowfall, so that tracks made by animals only during the previous 1–2 nights were visible. Six lines per area, each ca. 1 km in length, were skied. Identification of small mustelids was based on track dimensions, and density indices were calculated as the mean number of individuals crossing track lines per kilometre (see Korpimäki et al. 1991). The initial density of small mustelids did not differ between the reduction and control areas at the onset of the experiment in spring 1997 (table 1; t6 = 0.31, two-tailed p=0.77), whereas the density of small mustelids was two- to fivefold higher in control areas than in reduction areas in each autumn of the experiment (table 1). Because of suddenly changing weather, we were not able to ski snow-track lines in two reduction and three control areas in autumn 1997, in one reduction and control area in spring 1998, and in one control area in spring 1999. However, the number of small mustelids present in each study area was estimated by intensive 1 day censuses and mapping of snow-tracks (for methods, see Korpimäki & Norrdahl 1998) in late December 1997–January 1998. Again, the density of small mustelids was significantly lower in reduction than in control areas (mean (±s.e.m.) index value 0.50 (±0.17) versus 1.83 (±0.42), t6 = 2.95, two-tailed p=0.03).
Table 1.
Mean (±s.e.m.) indices of density for small mustelids in early spring (S) and late autumn (A) in reduction and control areas, and pooled breeding numbers of vole-eating avian predators in reduction and control areas during 1997–1999.
| small mustelidsa |
avian predatorsb |
||||
| year | reduction | control | reduction | control | |
| 1997 | S | 0.36±0.16 | 0.43±0.15 | 0.5±0.5 | 2.5±0.6 |
| A | 0.12±0.03 | 0.99 | |||
| 1998 | S | 0.04±0.04 | 0.06±0.06 | 0.3±0.3 | 1.3±0.3 |
| A | 0.14±0.06 | 1.00±0.27 | |||
| 1999 | S | 0.24±0.10 | 0.22±0.15 | 2.0±0.9 | 3.5±0.9 |
| A | 0.70±0.07 | 1.54±0.34 | |||
a Number of individuals crossing track lines per kilometre per area (number of reduction and control areas studied was four, except in autumn 1997 (two reduction areas and one control area), in spring 1998 (three reduction and three control areas), and in spring 1999 (three control areas)). Repeated-measures analysis of variance (ANOVA) showed significant effects of treatment (F1,6 = 7.31, p = 0.035), season (F1,22 = 35.37, p < 0.001), treatment×season (F1,22 = 23.02, p < 0.001) and study year (F2,22 = 8.11, p = 0.002) on density estimates of small mustelids.
b Pooled number of nests and breeding territories of vole-eating birds of prey (Eurasian kestrels, Tengmalm’s owls, short-eared owls, long-eared owls and common buzzards (Buteo buteo)). Two-way ANOVA showed significant effects of treatment and study year on breeding densities of avian predators (treatment: F1,18 = 8.53, p = 0.009; year: F2,18 = 5.16, p = 0.017; treatment×year: F2,18 = 0.32, p = 0.73).
In each spring of 1997–1999, before the breeding season of avian predators, we removed all known stick-nests, natural cavities and nest-boxes from the manipulation areas (for methods, see Norrdahl & Korpimäki (1996) and Korpimäki & Norrdahl (1998)). This procedure significantly reduced the number of breeding territories of main vole-eating avian predators in the manipulation areas relative to the control areas (table 1).
Small mammal abundance in the eight experimental areas was monitored using a random subset of eight ditch lines and two forest lines, chosen separately for each trapping session from 32 numbered ditches in agricultural fields and from eight numbered forest plots (i.e. the short line method; Korpimäki et al. 1994; Korpimäki & Norrdahl 1998). Because one ditch or forest plot was only snap-trapped once per year, possible deleterious effects of snap trapping on small mammal populations were probably diminutive and rather similar in the predator reduction and control areas. Ten mouse snap-traps and one rat snap-trap were set 10 m apart in each selected line and checked once a day for 2 days. Snap-trapping was performed simultaneously in all experimental areas from late March to early April (before predator reduction), and again in late June, August and October each year in 1997–1999.
Small-mammal density indices for each area and trapping session were derived from the trapping data as the mean number of individuals caught per trap line per area (n=4 for both predator reduction and control areas). Abundances of main prey (Microtus voles) did not differ between reduction and control areas at the onset of the experiment in April 1997 (figure 3a; one-way ANOVA: F16 = 0.1, p = 0.82).
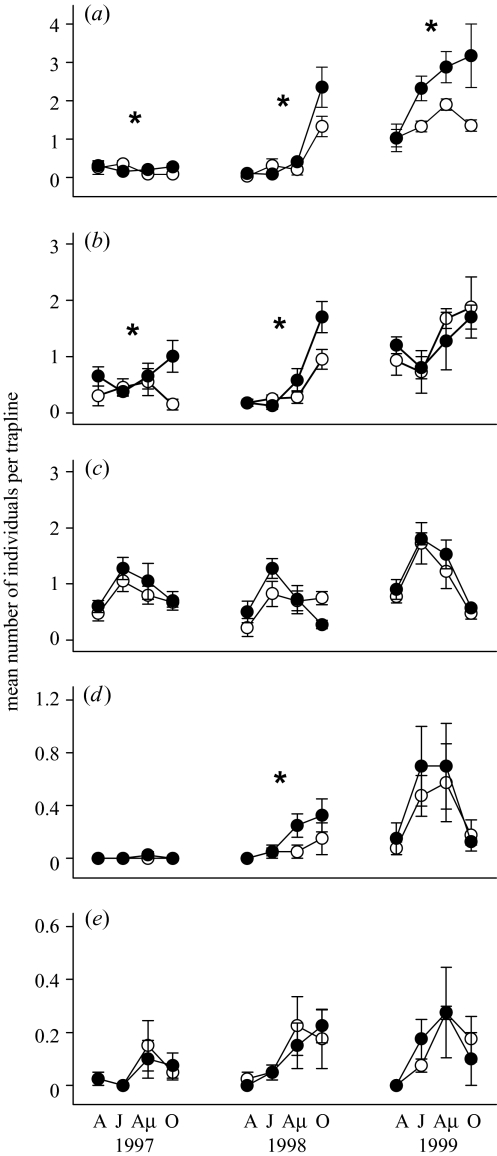
Figure 3.
The density index (measured as the mean number of individuals per trap line per area ±s.e.m., n=4) of (a) Microtus voles, (b) bank voles, (c) common shrews, (d) water voles and (e) pooled harvest and house mice from April to October (A, April; J, June; Au, August; O, October) 1997–1999 in predator reduction (filled symbols) and control areas (open symbols). Note the different scale of the y-axis in the different panels. Stars above the plots indicate significant interactions (p<0.05) between time and treatment (see table 3; statistics for Microtus are in Korpimäki et al. (2002)).
2.3 Statistical analyses
We analysed the autocorrelation structure and the degree of cyclicity and interspecific synchrony in long-term (1981–2003 for water voles, 1977–2003 for the other species) population fluctuations of the small mammal species. Before analysis, all zero trapping index values were replaced with 0.01, corresponding to the smallest potentially observable value as recommended by Turchin (2003). The time-series for common shrews were clearly non-stationary, exhibiting a declining trend, which we removed by fitting a second-order polynomial curve, subtracting this from the data and adding the mean index value of the original series to each residual value (Turchin 2003). All subsequent procedures and analyses on common shrew time-series use this detrended data. As a final pre-analysis procedure, all time-series were ln-transformed.
Autocorrelation functions (ACFs; Chatfield 2003) for various time lags were estimated for the species using ln-transformed data with PROC ARIMA in SAS statistical software. The dominant cycle period indicated by the ACFs (i.e. the lag at which the ACF reaches its first maximum) was pronounced statistically significant with Bartlett’s test, i.e. if ACF>2/√n, where n is the number of data points in the series (Chatfield 2003; Turchin 2003). Weak evidence for cyclicity in the time-series was inferred when the ACF was not significantly different from zero at its first positive maximum, but the ACF at the time lag approximating half of the dominant period (i.e. the first positive maximum) was significantly negative (Turchin 2003).
When modelling population fluctuations, we estimated partial rate correlation functions (PRCFs; Berryman & Turchin 2001; Turchin 2003) to aid in determining process order, i.e. the number of lags to be included as explanatory variables in autoregressive (AR) models. Process order was firstly defined as the number of consecutive time lags, which produced significant PRCFs, according to Bartlett’s test (Chatfield 2003; Turchin 2003). This was verified thereafter by first fitting the time-series with AR models with the number of lags specified by the PRCF (PROC AUTOREG in SAS, with the maximum-likelihood method), and then analysing whether the residuals from this model either retained an autocorrelative structure, or were reduced to white noise (often termed ‘pre-whitening’; Chatfield 2003). Process order was ultimately determined on the basis of combined information from these two procedures as the number of lags necessary to be included in the AR model to remove autocorrelation from the residuals.
To assess the degree of interspecific temporal synchrony in long-term population fluctuations of small mammal species, we calculated cross-correlation coefficients with time lags of −1 to 1 years (large positive values at time lag=0 indicate synchronously fluctuating populations) with PROC ARIMA in SAS (e.g. Ranta et al. 1995; Chatfield 2003). To further analyse whether the observed synchrony in population fluctuations is due solely to factors operating through density-dependent processes (e.g. predation), or also through density-independent processes (e.g. weather), we also calculated cross-correlation coefficients for the residuals of the AR models fitted to each time-series (see previous paragraph). The presence of correlation in two series of white noise would, with relative confidence, be indicative of density-independent processes affecting the pertinent species similarly, in a synchronizing manner akin to the Moran effect (Moran 1953). The statistical significance of the cross-correlation coefficients was determined using Bartlett’s formula, as for the ACFs.
In the predator reduction experiment, yearly effects of treatment (predator reduction versus control) and trapping month on the density indices of the small mammal species, and the effectiveness of predator reduction on small mustelid abundances were analysed using repeated-measures ANOVAs with treatment and trapping month as explanatory variables in the MIXED procedure of SAS statistical software (Littell et al. 1996). Owing to small sample size (n=4), traded off for spatial scale, we were unable to include year as a third variable in the ANOVAs; each year was therefore analysed separately. All p-values are given for two-tailed tests.
3. Results
3.1 Cyclicity and synchrony
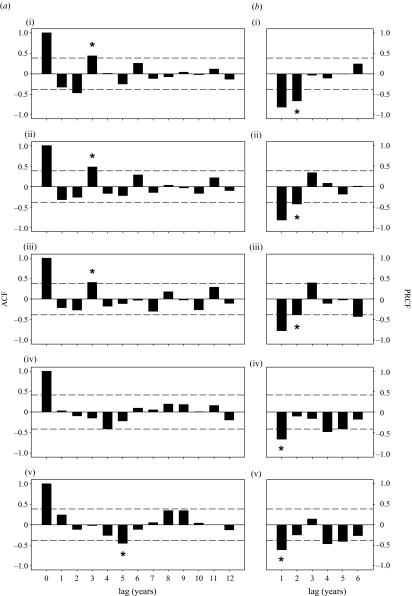
Time-series analyses of long-term snap-trapping data showed that population density indices of the main prey of predators, Microtus voles, as well as those of the most important alternative prey species, bank voles and common shrews, fluctuated in 3 year cycles (figure 1a–c, 2a–c). Weak evidence for water vole and mice populations fluctuating in 8–10 year cycles can be inferred from strong negative ACFs at time lags of 4 and 5 years, respectively (figures 1d,e, 2d,e; the ACF for water voles fell 0.01 units short of statistical significance; that for mice was clearly significant). The population fluctuations of Microtus voles, bank voles and common shrews were best described as second-order processes (figure 2a–c; a second-order process was deemed also for common shrews, as their PRCF fell only 0.01 units short of statistical significance), whereas those of water voles and mice as first-order processes (figure 2d,e).
Figure 2.
(a) Autocorrelation functions (ACFs) for population time series of (i) Microtus voles, (ii) bank voles, (iii) common shrews, (iv) water voles and (v) pooled harvest and house mice in western Finland at time lags of 0–12 years. Stars above positive value bars indicate strong evidence for cyclic population fluctuations with a period length equal to the number of time lags on the x-axis. Stars below negative value bars indicate weak evidence for cyclic population fluctuations with a period length equal to approximately twice the number of time lags on the x-axis. (b) Partial rate correlation functions (PRCFs) for the same time-series at time lags of 1–6 years. Stars below the bars indicate the number of lags included as explanatory variables into autoregressive models for each species. Dashed horizontal lines in all plots indicate critical levels of significance for the correlations, obtained with Bartlett’s test.
Microtus voles, bank voles and common shrews fluctuated synchronously with one another (figure 1a–c, table 2; cross-correlation at lag 0 between bank voles and common shrews=0.49). Density variations of water voles did not exhibit 3 year cyclicity, and they were therefore not synchronous with Microtus voles (figure 1d; table 2) or bank voles (cross-correlation at lag 0=0.15). Populations of common shrews, water voles and mice all fluctuated relatively synchronously (cross-correlation at lag 0 for common shrews and water voles=0.50, common shrews and mice=0.55, water voles and mice=0.61). No species appeared to fluctuate with a 1 year delay relative to the other species, as inferred from a lack of significant positive cross-correlations at lags + 1 or −1 years (table 2).
Table 2.
Cross-correlation coefficients with time lags of −1, 0 and 1 years calculated between Microtus voles (main prey of predators), and bank voles, water voles, pooled harvest and house mice, and common shrews.
(Coefficients in columns labelled ‘data’ are calculated from ln-transformed trapping index series of the respective species. Coefficients in columns labelled ‘residuals’ are calculated from residuals of autoregressive models fitted to the ln-transformed trapping series (AR(2) for Microtus voles, bank voles and common shrews, AR(1) for water voles and mice; see figure 2). Bold values indicate significant correlations according to Bartlett’s test. For the ‘data’ coefficients, significant positive values at time lag (0) indicate synchronously fluctuating populations. For the ‘residuals’ coefficients, significant positive values at time lag (0) indicate synchrony in population fluctuations independent of autocorrelation structure.)
|
Microtus voles with time lag (year) |
|||||||
| −1 |
0 |
1 |
|||||
| alternative prey species | n | data | residuals | data | residuals | data | residuals |
| bank vole | 27 | −0.44 | −0.34 | 0.79 | 0.42 | −0.28 | −0.21 |
| water vole | 23 | −0.31 | −0.13 | 0.19 | 0.05 | −0.05 | −0.16 |
| mice | 27 | −0.52 | −0.33 | 0.13 | −0.09 | 0.29 | 0.10 |
| common shrewa | 27 | −0.51 | −0.12 | 0.37b | 0.22 | 0.11 | 0.15 |
a Original trapping data detrended by fitting a second-order polynomial curve.
b The cross-correlation coefficient fell 0.01 units short of statistical significance.
The series of residuals from autoregressive models fitted for the time-series of Microtus and bank voles exhibited significant positive correlation at lag 0, despite the lack of serial autocorrelation, and hence cyclicity, in the series. No other correlations were found between the series of residuals among the species (table 2).
3.2 Predator reduction and small mammal densities
The reduction of mustelid and avian predators had profound positive effects on the densities of main prey, Microtus voles, in each phase of the 3 year population cycle (figure 3a; see Korpimäki et al. (2002) for further details). Besides the effects on the main prey, the reduction of predators increased densities of most important alternative prey of predators, the bank vole, in the low (1997) and increase (1998) phases but not in the peak (1999) phase of the 3 year population cycle of voles (figure 3b). This is indicated by a significant effect of the interaction between treatment and month in repeated-measures ANOVAs on rodent abundances in 1997 and 1998 but not in 1999 (table 3). The reduction of predators prevented the density decline of bank voles during the summer 1997, thereby increasing their autumn density over fivefold (figure 3b). During the following winter (November 1997–March 1998) without predator reduction, densities of bank voles in treatment areas decreased to the level of control areas. The reduction of predators in 1998 induced an earlier initiation of increase in bank vole densities resulting in twofold higher abundances in reduction than in control areas in autumn 1998. During the following winter (November 1998–March 1999), densities of bank voles in reduction areas again declined to the level of control areas.
Table 3.
Repeated-measures ANOVA (restricted maximum-likelihood method with compound symmetry as covariance structure) table for the yearly effects of treatment (Trt: reduction of predators versus control without reduction) and trapping month (Mo: April, June, August and October) on the density indices of bank voles and water voles.
(Note that significant interactions between treatment and trapping month reveal differences in small mammal densities as a result of treatment. NDF, numerator degrees pf freedom; DDF, denominator degrees of freedom.)
| 1997 |
1998 |
1999 |
|||||||
| species | source | NDF | DDF | F | p | F | p | F | p |
| bank vole | Trt | 1 | 6 | 2.00 | 0.207 | 5.04 | 0.066 | 0.03 | 0.862 |
| Mo | 3 | 18 | 0.90 | 0.461 | 26.46 | <0.001 | 4.68 | 0.014 | |
| Trt×Mo | 3 | 18 | 4.76 | 0.013 | 3.39 | 0.041 | 0.50 | 0.689 | |
| water volea | Trt | 1 | 6 | 1.00 | 0.356 | 2.00 | 0.207 | 0.38 | 0.562 |
| Mo | 2 | 12 | 1.00 | 0.397 | 7.00 | 0.010 | 4.08 | 0.044 | |
| Trt×Mo | 2 | 12 | 1.00 | 0.397 | 4.00 | 0.047 | 0.07 | 0.932 | |
a Only April–August analysed, owing to unreliable trapping data in October caused by seasonal alterations in behaviour, for example increased burrowing activity leading to reduced trappability of water voles (Myllymäki 1972; Jeppson 1990).
Predator reduction increased the autumn densities of water voles only in the increase (1998) phase of the population cycle of the main prey (figure 3d; table 3). No obvious effects of predator density manipulation on density indices of mice and common shrews were detected during the experiment (figure 3c,e).
4. Discussion
4.1 Interspecific temporal synchrony
We found that populations of Microtus voles, bank voles and common shrews fluctuated in significant 3 year period cycles and in temporal synchrony with each other, which suggests that a common factor is involved in synchronizing these population cycles. According to autoregression analyses, the degree of synchrony between Microtus voles and bank voles is influenced by both density-dependent and density-independent factors, whereas the degree of synchrony between these voles and common shrews is influenced only by density-dependent factors. An experimental reduction of predators had positive effects on densities of the most important alternative prey species, the bank vole, in the low and increase phases but not in the peak phase of the 3 year population cycle. In addition, predator reduction increased water vole densities in the increase phase of the population cycle of Microtus and bank voles. Accordingly, the APH appeared to better predict the impacts of predators on alternative prey species than the SPH.
Both our time-series analyses and experimental results indicate that the degree of interspecific temporal synchrony among Microtus voles, bank voles and common shrews is heavily influenced in a density-dependent manner by predation. However, population fluctuations of Microtus voles and bank voles appear also to be affected by density-independent factors, such as climate, as judged by the presence of significant interspecific correlation in the residuals of autoregressive models. These findings are not entirely unexpected, because populations of these species are expected to exhibit similar basic intrinsic structures of density dependence (e.g. Klemola et al. 2003; Huitu et al. 2004). This leads to the fact that focal populations are predisposed to the synchronizing action of spatially correlated environmental factors operating in a density-independent manner (Moran 1953). In an ecological context, this may imply that weather conditions may be similarly affecting both Microtus voles and bank voles through, for example, their common food plants in agricultural environments. Insectivorous shrews, on the other hand, differ ecologically from voles and, as suggested by our results, by contrast, synchronized with them merely by the common predator assemblage in the community. This idea fits well with earlier studies where especially the deepest lows of shrew populations have been found to occur simultaneously with the lowest densities of voles (Henttonen et al. 1989; Norrdahl & Korpimäki 2000).
We also found some evidence for long, 8–10 year cycles in populations of water voles and mice. Furthermore, it appears from the autocorrelation functions (figure 2d,e) that these ‘population cycles’ are at least partly independent from, and not merely reflections of, the dominant 3 year Microtus/bank vole cycle in the area. This is indicated by the essential absence of positive peaks in the autocorrelograms at lags of 3 and 6 years (figure 2d,e). Nonetheless, visual inspection of the time series indicates that low phases do occasionally coincide among all species (figure 1). The actual validity of these interesting observations, as well as their ultimate and proximate reasons, unfortunately cannot be verified with the current amount of data.
4.2 Predator reduction and densities of small mammals
Bank voles are the most important alternative prey for most of the predators whose densities were manipulated in this study (see table 1 in Oksanen et al. (2000) for a summary of the diet data). Consequently, it is not unexpected that population oscillations of bank voles exhibited the strongest degree of temporal synchrony with the main prey (figure 1b). Our experimental results thus support the hypothesis that in boreal areas cyclicity is externally imposed upon bank voles (Hanski & Henttonen 1996). That is, when densities of main prey decrease, predators shift to bank voles and also induce a density decline of bank voles. This dietary shift is also predicted by the APH and conventional models of optimal diet theory (ODT; e.g. Pyke 1984; Stephens & Krebs 1986), which predict that predators should feed on the energetically most profitable prey type in years of prey abundance (in this case in peak years of Microtus voles), and switch to alternative, energetically poorer, food types only when densities of the main prey have become significantly reduced. It is worth noting that the observed dietary shift of predators from Microtus to bank voles is not explainable in this study by predators moving from agricultural fields (preferred habitat of Microtus voles) to forest habitats (main habitat of bank voles) (see Oksanen et al. (2000) for details of this hypothesis), as our experiment was performed mainly in agricultural fields. To summarize, cyclicity in bank vole populations per se appears to be caused by predation, whereas the degree of interspecific synchrony between bank voles and Microtus voles by both predation and common, density-independent environmental factors, such as climate.
The cessation of predator reduction during two winters (1997–98 and 1998–99) brought densities of Microtus voles to the level of control areas by early spring (figure 2a). This was probably because of an influx of new predators to the reduction areas during the winter, as there were no obvious differences in the densities of small mustelids in early spring, before the initiation of predator reduction. By contrast, there were significantly fewer small mustelids in the reduction than in the control areas in autumn, after the small mustelid reduction effort (table 1). This suggests that small mustelid predators rapidly respond to small-scale changes in the density of main prey and subsequently bring these out-of-phase patches to the same density level as in the surrounding areas (Korpimäki et al. 2002). The same was observable for most important alternative prey, the bank vole, during the five winter months without predator removal (figure 2b). This shows that predators can have profound impacts on the population dynamics of their important alternative prey along with their main prey.
Densities of common shrews fluctuated in synchrony with bank voles (figure 1c). However, no obvious effects of predator reduction on common shrews could be observed. Similarly, no effects of least weasel reduction on common shrew abundances were detected in earlier large-scale experiments (Norrdahl & Korpimäki 2000). However, earlier experiments in which either avian or both avian and mammalian predators were reduced revealed a positive treatment effect on common shrews in the decline, but not in the increase phase of the main prey cycle (Norrdahl & Korpimäki 2000). Because least weasels and small mustelids in general dislike shrews, possibly because of their unpleasant odour (Korpimäki & Norrdahl 1987), we suggest that avian predators are important in synchronizing the decline phases of insectivorous shrews and herbivorous main prey species. The probable reason for undetected treatment effects on common shrews in this study was that the present predator reduction experiment was not done during the steep summer decline of the main prey. Furthermore, as the density of water voles was unusually high in two of the three years of the experiment, avian predators were most probably able to satisfy their dietary requirements with these instead of the small shrews.
Densities of water voles did not oscillate in obvious temporal synchrony with Microtus or bank voles. This may be partly explained by the weak evidence we obtained from time-series analyses that water voles may be fluctuating in ca. 8–9 year cycles, independent of the smaller voles but synchronously with mice (figure 1d,e). In an earlier long-term study on water voles (a smaller-sized fossorial form, in Switzerland), a significant periodicity of 5–7 years was detected for their density indices (Saucy 1994). In our experiment, predator reduction increased the autumn densities of water voles in the increase phase of the population cycle of the small voles. Among the predators reduced, large water voles are important alternative prey only for male stoats (Korpimäki et al. 1991) and larger birds of prey (the kestrel, the short-eared owl and the long-eared owl; Korpimäki & Norrdahl 1991b; Korpimäki 1992). It is likely that some of these predators shifted to water voles, in particular to younger individuals, already in the increase phase of the smaller vole population cycle. This dietary shift is also predicted by optimal diet theory, as even young water voles are more than twice as large as Microtus voles. Therefore, water voles may become the most profitable prey for all predators that are able to kill and carry them to their nests. The density peak of water voles in 1998–1999 was also the highest recorded in our study area during 1981–2002 (figure 1d), which may have substantially contributed to their profitability to predators during these years.
The densities of mice in our study areas (figure 1e) and their proportions in the diet of predators (Korpimäki et al. 1991; Korpimäki & Norrdahl 1991b; Korpimäki 1992, 1993; Norrdahl & Korpimäki 1995b) were so low that it was not unexpected that the manipulation did not have obvious effects on their densities. Again, reasons behind the intriguingly strong correlation between water vole and mice numbers remain elusive, requiring further studies.
5. Conclusions
We have demonstrated here that predators appear to have at least temporary detrimental effects on small rodent densities at the community level. Predators were shown to be sufficient to limit the population densities of the most important alternative prey, the bank vole, together with other alternative prey (in this case water voles and in an earlier experiment common shrews; Norrdahl & Korpimäki 2000), as well as their main prey, Microtus voles. The effects of predation resulted in synchronization of the low phases of population cycles of all important small mammal species in the community. Therefore, it is not plausible to consider the Moran effect as an important determinant of interspecific temporal synchrony in small mammal communities. More likely, predation mortality and environmental perturbations (e.g. detrimental weather conditions or seasonality) act in concert to induce such patterns of temporal dynamics; increasingly so when moving from local to regional scales at northern latitudes. At the much larger continent-wide scales, the Moran effect is likely to be a more important factor than natural enemies in causing both spatial and interspecific temporal synchrony in population fluctuations (see Stenseth et al. 1999; Haydon et al. 2001). According to our results, however, Moran-type density-independent processes are not altogether absent from local-scale processes either. The dynamics of ecologically similar species appear to be affected by density-independent processes through, for example, plant productivity. This is supported by the fact that density-independent relationships were not observed between ecologically dissimilar groups, herbivores and insectivores. Nonetheless, the biological mechanism behind the Moran effect still remains largely unidentified and its importance relative to other extrinsic factors difficult to test experimentally.
Acknowledgments
The authors thank Ossi Hemminki, Sakari Ikola and Jukka Koivisto for help with the fieldwork, and Jan Lindström and Esa Ranta for comments on the manuscript. The study was financially supported by the Academy of Finland (grant numbers 69014, 71110, 74131, 8202013, 880696 and 8206140 to E.K.).
References
- Angelstam P., Lindström E., Widén P. Role of predation in short-term population fluctuations of some birds and mammals in Fennoscandia. Oecologia. 1984;62:199–208. doi: 10.1007/BF00379014. [DOI] [PubMed] [Google Scholar]
- Angelstam P., Lindström E., Widén P. Synchronous short-term population fluctuctions of some birds and mammals in Fennoscandia—occurrence and distribution. Holarct. Ecol. 1985;8:285–298. [Google Scholar]
- Berryman A.A., Turchin P. Identifying the density-dependent structure underlying ecological time series. Oikos. 2001;92:265–270. [Google Scholar]
- Boutin S. Population changes of the vertebrate community during a snowshoe hare cycle in Canada’s boreal forest. Oikos. 1995;74:69–80. (and 21 others) [Google Scholar]
- Chatfield C. The analysis of time series: an introduction. Chapman & Hall/CRC; London: 2003. [Google Scholar]
- Elton C.S. Periodic fluctuations in the numbers of animals: their causes and effects. Br. J. Exp. Biol. 1924;2:119–163. [Google Scholar]
- Elton C.S. Voles, mice and lemmings: problems in population dynamics. Oxford University Press; 1942. [Google Scholar]
- Elton C.S., Nicholson M. The ten-year cycle in numbers of the lynx in Canada. J. Anim. Ecol. 1942;11:215–244. [Google Scholar]
- Hagen Y. Rovfuglene og viltpleien. Gyldendal Norsk Forlag; Oslo: 1952. [Google Scholar]
- Hanski I., Henttonen H. Predation on competing rodent species: a simple explanation of complex patterns. J. Anim. Ecol. 1996;65:220–232. [Google Scholar]
- Hansson L. Predation as the factor causing extended low densities in microtine cycles. Oikos. 1984;43:255–256. [Google Scholar]
- Hansson L., Henttonen H. Rodent dynamics as community processes. Trends Ecol. Evol. 1988;3:195–200. doi: 10.1016/0169-5347(88)90006-7. [DOI] [PubMed] [Google Scholar]
- Haydon D.T., Stenseth N.C., Boyce M.S., Greenwood P.E. Phase coupling and synchrony in the spatiotemporal dynamics of muskrat and mink populations across Canada. Proc. Natl Acad. Sci. USA. 2001;98:13 149–13 154. doi: 10.1073/pnas.221275198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henttonen H. Predation causing extended low densities in microtine cycles: further evidence from shrew dynamics. Oikos. 1985;44:156–157. [Google Scholar]
- Henttonen H., Oksanen T., Jortikka A., Haukisalmi V. How much do weasels shape microtine cycles in the northern Fennoscandian taiga? Oikos. 1987;50:353–365. [Google Scholar]
- Henttonen H., Haukisalmi V., Kaikusalo A., Korpimäki E., Norrdahl K., Skarén U.A.P. Long-term dynamics of the common shrew Sorex araneus in Finland. Ann. Zool. Fenn. 1989;26:349–355. [Google Scholar]
- Hörnfeldt B., Löfgren O., Carlsson B.-G. Cycles in voles and small game in relation to variations in plant production indices in northern Sweden. Oecologia. 1986;68:496–502. doi: 10.1007/BF00378761. [DOI] [PubMed] [Google Scholar]
- Hudson P.J., Cattadori I.M. The Moran effect: a cause of population synchrony. Trends Ecol. Evol. 1999;14:1–2. doi: 10.1016/s0169-5347(98)01498-0. [DOI] [PubMed] [Google Scholar]
- Huitu O., Koivula M., Korpimäki E., Klemola T., Norrdahl K. Winter food supply limits growth of northern vole populations in the absence of predation. Ecology. 2003;84:2108–2118. [Google Scholar]
- Huitu O., Norrdahl K., Korpimäki E. Competition, predation and interspecific synchrony in cyclic small mammal communities. Ecography. 2004;27:197–206. [Google Scholar]
- Jeppson B. Effects of density and resources on the social system of water voles. In: Tamarin R.H., Ostfeld R.S., Pugh S.R., Bujalska G., editors. Social systems and population cycles of voles. Birkhäuser; Basel: 1990. pp. 213–226. [Google Scholar]
- Keith L.B. Dynamics of snowshoe hare populations. In: Genoways H.H., editor. Current mammalogy. Plenum Press; New York: 1990. pp. 119–195. [Google Scholar]
- Klemola T., Koivula M., Korpimäki E., Norrdahl K. Experimental tests of predation and food hypotheses for population cycles of voles. Proc. R. Soc. Lond. B. 2000a;267:351–356. doi: 10.1098/rspb.2000.1008. doi:10.1098/rspb.2000.1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemola T., Norrdahl K., Korpimäki E. Do delayed effects of overgrazing explain population cycles in voles? Oikos. 2000b;90:509–516. [Google Scholar]
- Klemola T., Korpimäki E., Koivula M. Rate of population change in voles from different phases of the population cycle. Oikos. 2002a;96:291–298. [Google Scholar]
- Klemola T., Tanhuanpää M., Korpimäki E., Ruohomäki K. Specialist and generalist natural enemies as an explanation for geographical gradients in population cycles of northern herbivores. Oikos. 2002b;99:83–94. [Google Scholar]
- Klemola T., Pettersen T., Stenseth N.C. Trophic interactions in population cycles of voles and lemmings: a model-based synthesis. Adv. Ecol. Res. 2003;33:75–160. [Google Scholar]
- Koivunen V., Korpimäki E., Hakkarainen H., Norrdahl K. Prey choice of Tengmalm’s owls (Aegolius funereus funereus): preference for substandard individuals? Can. J. Zool. 1996;74:816–823. [Google Scholar]
- Korpimäki E. Rapid tracking of microtine populations by their avian predators: possible evidence for stabilizing predation. Oikos. 1985;45:281–284. [Google Scholar]
- Korpimäki E. Predation causing synchronous decline phases in microtine and shrew populations in western Finland. Oikos. 1986;46:124–127. [Google Scholar]
- Korpimäki E. Body mass of breeding Tengmalm’s owls Aegolius funereus: seasonal, between-year, site and age-related variation. Ornis Scand. 1990;21:169–178. [Google Scholar]
- Korpimäki E. Diet composition, prey choice and breeding success of long-eared owls: effects of multiannual fluctuations in food abundance. Can. J. Zool. 1992;70:2373–2381. [Google Scholar]
- Korpimäki E. Regulation of multiannual vole cycles by density-dependent avian and mammalian predation. Oikos. 1993;66:359–363. [Google Scholar]
- Korpimäki E. Rapid or delayed tracking of multi-annual vole cycles by avian predators? J. Anim. Ecol. 1994;63:619–628. [Google Scholar]
- Korpimäki E., Krebs C.J. Predation and population cycles of small mammals. A reassessment of the predation hypothesis. BioScence. 1996;46:754–764. [Google Scholar]
- Korpimäki E., Norrdahl K. Low proportion of shrews in the diet of small mustelids in western Finland. Z. Säugetierkd. 1987;52:257–260. [Google Scholar]
- Korpimäki E., Norrdahl K. Avian predation on mustelids in Europe 2: impact on small mustelid and microtine dynamics—a hypothesis. Oikos. 1989a;55:273–276. [Google Scholar]
- Korpimäki E., Norrdahl K. Predation of Tengmalm’s owls: numerical responses, functional responses and dampening impact on population fluctuations of voles. Oikos. 1989b;54:154–164. [Google Scholar]
- Korpimäki E., Norrdahl K. Do breeding nomadic avian predators dampen population fluctuations of small mammals? Oikos. 1991a;62:195–208. doi: 10.1007/BF00333938. [DOI] [PubMed] [Google Scholar]
- Korpimäki E., Norrdahl K. Numerical and functional responses of kestrels, short-eared owls, and long-eared owls to vole densities. Ecology. 1991b;72:814–826. [Google Scholar]
- Korpimäki E., Norrdahl K. Experimental reduction of predators reverses the crash phase of small-rodent cycles. Ecology. 1998;76:2448–2455. [Google Scholar]
- Korpimäki E., Huhtala K., Sulkava S. Does the year-to-year variation in the diet of eagle and Ural owls support the alternative prey hypothesis? Oikos. 1990;58:47–54. [Google Scholar]
- Korpimäki E., Norrdahl K., Rinta-Jaskari T. Responses of stoats and least weasels to fluctuating vole abundances: is the low phase of the vole cycle due to mustelid predation? Oecologia. 1991;88:552–561. doi: 10.1007/BF00317719. [DOI] [PubMed] [Google Scholar]
- Korpimäki E., Norrdahl K., Valkama J. Reproductive investment under fluctuating predation risk: microtine rodents and small mustelids. Evol. Ecol. 1994;8:357–368. [Google Scholar]
- Korpimäki E., Norrdahl K., Klemola T., Pettersen T., Stenseth N.C. Dynamic effects of predators on cyclic voles: field experimentation and model extrapolation. Proc. R. Soc. Lond. B. 2002;269:991–997. doi: 10.1098/rspb.2002.1972. doi:10.1098/rspb.2002.1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs C.J. Two paradigms of population regulation. Wildl. Res. 1995;22:1–10. [Google Scholar]
- Krebs C.J., Boonstra R., Boutin S., Sinclair A.R.E. What drives the 10-year cycle of snowshoe hares? BioScience. 2001;51:25–35. [Google Scholar]
- Lack D. The natural regulation of animal numbers. Oxford University Press; London: 1954. [Google Scholar]
- Lindén H. Latitudinal gradients in predator–prey interactions, cyclicity and synchronism in voles and small game populations in Finland. Oikos. 1988;52:341–349. [Google Scholar]
- Lindström J., Ranta E., Kokko H., Lundberg P., Kaitala V. From arctic lemmings to adaptive dynamics: Charles Elton’s legacy in population ecology. Biol. Rev. 2001;76:129–158. doi: 10.1017/s1464793100005637. [DOI] [PubMed] [Google Scholar]
- Littell R.C., Milliken G.A., Stroup W.W., Wolfinger R.D. SAS system for mixed models. SAS Institute Inc.; Cary, NC: 1996. [Google Scholar]
- Moran P.A.P. The statistical analysis of the Canadian lynx cycle. II. Synchronization and meteorology. Aust. J. Zool. 1953;1:291–298. [Google Scholar]
- Myllymäki A. Vesimyyrä. In: Siivonen L., editor. Suomen nisäkkäät 1. Otava; Helsinki: 1972. pp. 350–363. [Google Scholar]
- Myllymäki A., Paasikallio A., Häkkinen U. Analysis of a ‘standard trapping’ of Microtus agrestis (L.) with triple isotope marking outside the quadrat. Ann. Zool. Fenn. 1971;8:22–34. [Google Scholar]
- Norrdahl K. Population cycles in northern small mammals. Biol. Rev. 1995;70:621–637. doi: 10.1111/j.1469-185x.1995.tb01654.x. [DOI] [PubMed] [Google Scholar]
- Norrdahl K., Korpimäki E. Effects of predator removal on vertebrate prey populations: birds of prey and small mammals. Oecologia. 1995a;103:241–248. doi: 10.1007/BF00329086. [DOI] [PubMed] [Google Scholar]
- Norrdahl K., Korpimäki E. Mortality factors in a cyclic vole population. Proc. R. Soc. Lond. B. 1995b;261:49–53. doi: 10.1098/rspb.1995.0116. [DOI] [PubMed] [Google Scholar]
- Norrdahl K., Korpimäki E. Do nomadic avian predators synchronize population fluctuations of small mammals? A field experiment. Oecologia. 1996;107:478–483. doi: 10.1007/BF00333938. [DOI] [PubMed] [Google Scholar]
- Norrdahl K., Korpimäki E. Do predators limit the abundance of alternative prey? Experiments with vole-eating avian and mammalian predators. Oikos. 2000;91:528–540. [Google Scholar]
- Norrdahl K., Korpimäki E. Changes in individual quality during a 3-year population cycle of voles. Oecologia. 2002a;130:239–249. doi: 10.1007/s004420100795. [DOI] [PubMed] [Google Scholar]
- Norrdahl K., Korpimäki E. Changes in population structure and reproduction during a 3-year population cycle of voles. Oikos. 2002b;96:331–345. doi: 10.1007/s004420100795. [DOI] [PubMed] [Google Scholar]
- Norrdahl K., Korpimäki E. Seasonal changes in the numerical responses of predators to cyclic vole populations. Ecography. 2002c;25:428–438. [Google Scholar]
- Oksanen T., Oksanen L., Jedrzejewski W., Jedrzejewska B., Korpimäki E., Norrdahl K. Predation and the dynamics of the bank vole, Clethrionomys glareolus. Pol. J. Ecol. 2000;48(Suppl.):197–217. [Google Scholar]
- Pyke G.H. Optimal foraging theory: a critical review. A. Rev. Ecol. Syst. 1984;15:523–575. [Google Scholar]
- Ranta E., Lindström J., Lindén H. Synchrony in tetraonid population dynamics. J. Anim. Ecol. 1995;64:767–776. [Google Scholar]
- Ranta E., Kaitala V., Lindström J., Helle E. The Moran effect and synchrony in population dynamics. Oikos. 1997;78:136–142. [Google Scholar]
- Reif V., Tornberg R., Jungell S., Korpimäki E. Diet variation of common buzzards in Finland supports the alternative prey hypothesis. Ecography. 2001;24:267–274. [Google Scholar]
- Royama T. Analytical population dynamics. Chapman & Hall; London: 1992. [Google Scholar]
- Saucy F. Density dependence in time series of the fossorial form of the water vole, Arvicola terrestris. Oikos. 1994;71:381–392. [Google Scholar]
- Sinclair A.R.E., Gosline J.M., Holdsworth G., Krebs C.J., Boutin S., Smith J.N.M., Boonstra R., Dale M. Can the solar cycle and climate synchronize the snowshoe hare cycle in Canada? Evidence from tree rings and ice cores. Am. Nat. 1993;141:173–198. doi: 10.1086/285468. [DOI] [PubMed] [Google Scholar]
- Stenseth N.C. Population cycles in voles and lemmings: density dependence and phase dependence in a stochastic world. Oikos. 1999;87:427–461. [Google Scholar]
- Stenseth N.C., Ims R.A. Population dynamics of lemmings: temporal and spatial variation—an introduction. In: Stenseth N.C., Ims R.A., editors. The biology of lemmings. Linnean Society; London: 1993. pp. 61–96. [Google Scholar]
- Stenseth N.C. Common dynamic structure of Canada lynx populations within three climatic regions. Science. 1999;285:1071–1073. doi: 10.1126/science.285.5430.1071. (and 10 others) [DOI] [PubMed] [Google Scholar]
- Stephens D.W., Krebs J.R. Foraging theory. Princeton University Press; Princeton, NJ: 1986. [Google Scholar]
- Stuart-Smith K. Do lemming, vole, and snowshoe hare cycles affect other small birds and mammals in northern ecosystems? Musk-Ox. 1992;39:181–188. [Google Scholar]
- Turchin P. Complex population dynamics: a theoretical/empirical synthesis. Princeton Universiy Press; Princeton, NJ: 2003. [Google Scholar]