Abstract
A twig of a cypress plant preserved for ca. 45Myr in Baltic amber was analysed by light and electron microscopy. Cross-sections of the whole plant showed an almost intact tissue of the entire stem and leaves, revealing, to our knowledge, the oldest and most highly preserved tissue from an amber inclusion reported so far. The preparations are based on a new technique of internal imbedding, whereby the hollow spaces within the inclusion are filled with synthetic resin which stabilizes the cellular structures during the sectioning procedure. Cytological stains applied to the sections reacted with cell walls and nuclei. A strong green auto-fluorescence of the cuticle and the resin canals in the leaves was observed. Transmission electron micrographs revealed highly preserved fine structures of cell walls, membranes and organelles. The results were compared with taxonomically related recent Glyptostrobus and Juniperus plants.
Keywords: Baltic amber, cypress, microscopy, cell structures, organelles, histochemistry
1. Introduction
The documentation and morphological investigation of fossil plants in amber dates back for more than 150 years. In 1853 Alexander von Humboldt introduced a lecture entitled ‘Über die Bernstein Flora’ (‘About the amber flora’), which was held at the Königliche Akademie der Wissenschaften zu Berlin (Göppert 1853). Following Humboldt’s recommendation, two volumes of Die Flora des Bernsteins were published with copper plate engravings of more than 160 plant species from inclusions of Baltic amber (Goeppert & Menge 1883). Today, a much larger number of fossil species of plants, animals and micro-organisms from Baltic and Dominican amber, and also from other deposits, have been described. (Poinar & Poinar 1990; Grimaldi et al. 1994; Weitschat & Wichard 2002).
A characteristic of amber inclusions is their high degree of structural conservation. Examining inclusions within the amber, epidermis cells of leaves can often be recognized and the preservation of tracheids and pith rays in pieces of wood has been known for a long time (Goeppert & Menge 1883; Conwentz 1890; Caspary 1907). When amber inclusions are opened mechanically, they usually break at the interface between the resin and the fossil and often expose the surface of the tissue. With this technique, scanning electron microscope images have been obtained, showing details of the structures at the exposed surface (Grimaldi et al. 1994; Kohring 1998; Weitschat & Wichard 2002; Ascaso et al. 2003).
Histological and cytological studies of amber fossils at higher resolution are much more difficult. To prepare ultrathin sections for transmission electron microscopy, amber inclusions were opened and the tissue was removed and embedded in synthetic resin. The main technical problem with these preparations is the high fragility of the material. Often most of the tissue crumbles into fine pieces when it is handled. However, fragments have been recovered and analysed; for example, the tissue from a gnat from Baltic amber, showing nuclei, mitochondrial cristae and cellular membranes (Poinar & Hess 1982). Small parts of the tissue of a bee from 15–30 Myr old Dominican amber were removed and embedded. Electron micrographs of this material show myofibrils and mitochondria of the flight muscle tissue and possibly attached collagen fibres (Grimaldi et al. 1994; Grimaldi 1995; Iturralde-Vinent & MacPhee 1996). In a leaf from Hymenaea protera, also found in Dominican amber, epidermis and parenchyma tissue, bundles of thylakoid membranes, mitochondria and chromatin-like structures were detected (Poinar et al. 1996a).
Owing to the fragility of the material, microscopical images of large cross-sections of an entire plant or animal from an amber inclusion have not, to our knowledge, been reported. Here, a new preparation technique of internal embedding is used, which stabilizes the inner structures of the fossil. Using this method, a cypress twig preserved for 40–50 Myr in Baltic amber (Ritzkowski 1997) was prepared, demonstrating, to our knowledge, the oldest and most highly preserved cellular structures of an amber inclusion reported so far. The implications of the results for questions related to the preservation, modification and decay of ancient biomolecules during diagenetic processes are discussed.
2. Material and methods
2.1 Material
A piece of Baltic amber containing an inclusion of a cypress twig was obtained from Dr Wolfgang Weitschat from the Institut für Paläontologie at the Universität Hamburg. The source of the amber is the Blue Earth in Jantarnyi near Kaliningrad in Russia. For comparison with recent species of the Cupressaceae family, shoots of Glyptostrobus pensilis (subfamily Taxodiaceae) were obtained from Dr Armin Jagel of the Botanical Garden in Bochum and Juniperus sabina from the Botanical Garden in Berlin-Dahlem.
2.2 Internal embedding of the fossil plant
The amber stone was trimmed to within ca. 2 mm of the inclusion. Two cuts were made at right angles to the axis of the stem, just inside the tissue. Thus a 1.5 mm slice of the shoot was obtained, which was open at both ends. The slice was placed on a glass slide under the preparation microscope, orientating the axis of the plant in a vertical position, and a small drop of liquid ERL resin (composed of 5 g ERL (vinylcyclohexane dioxide), 3 g diepoxide, 13 g nonenyl succinic acid anhydride and 0.2 g S1 dimethylaminoethanol (Spurr 1969)) was pipetted to the bottom of the slice. The upper surface was not submerged in liquid. The resin was sucked into the tissue by capillary forces and more liquid was applied to the bottom. After a while, air bubbles and liquid droplets appeared at the surface of the inclusion, indicating that the resin had penetrated the tissue. The slice was placed in a small resin-filled chamber and incubated at 60°C overnight. After polymerization, the internally embedded fossil tissue was cut with an ultramicrotome and handled like embedded tissue of recent plants.
2.3 Embedding of recent plants
Shoots from J.sabina were cut into sections of ca. 1–2 mm thickness with a razor blade. The tissue was fixed for 2 h with glutaraldehyde (2.5%) containing paraformaldehyde (2%) and tannic acid (0.2%) in sodium–potassium phosphate buffer (0.1M, pH 7.0). After washing with phosphate buffer, samples were incubated for 12 h in osmium tetroxide (1% in 50 mM sodium-potassium phosphate buffer, pH 7.0). After washing in phosphate buffer, the specimens were dehydrated in a graded series of ethanol, followed by propylene oxide, incubated in a mixture of propylene oxide/ERL (v/v) and pure ERL and polymerized overnight at 60°C.
2.4 Light microscopy
Preparations for light microscopy were made by cutting 0.5–1.0μm sections using a Reichert OMU3 ultramicrotome. The sections were fixed onto glass slides by heating for 10 min at 60°C. For histochemical analysis, sections were stained for 4 min at 60°C with either 0.5% w/v safranine B (Grübler) in water, or with 0.5% w/v methylene blue (Serva) in 1% w/v tetraborate, washed in de-ionized water and dried. Stains All (Serva) was dissolved at 0.5% w/v in absolute ethanol and diluted to 0.1% w/v in 25% ethanol immediately before use. The sections were stained for 1 min at 60°C, rinsed in 25% ethanol and dried. Images were obtained with a Leitz Ortholux microscope using bright field and photographed with a Nikon Digital Camera Cool PIX 990. Fluorescence images were obtained by irradiating the sections with a mercury super pressure lamp HBO using a 450–490 nm filter.
2.5 Transmission electron microscopy
Ultrathin sections of ca. 100–120 nm were prepared and mounted on carbon films on 300 mesh gold microscope grids. The samples were contrasted with 2% w/v uranyl acetate for 5 min and with 0.2% w/v lead citrate in 0.2M NaOH for 20 s. Electron micrographs were obtained with a Zeiss EM 10 microscope using Scientia negative films. The negatives were scanned with an Epson 1680 Pro scanner at a resolution of 1200 dpi.
3. Results
A piece of Baltic amber with an inclusion of a 6 mm long twig of a cypress plant from the Blue Earth in Jantarnyi, Russia was used for this study (figure 1a). To identify the genus within the family of Cupressaceae, the shape and position of the leaves were compared with those of recent and fossil genera. The shape and the 5/13 right-hand spiral arrangement of the scale leaves of the amber plant are almost identical to those of a twig of the recent species G.pensilis (figure 1c). They also compare well with the description of the genus Glyptostrobus in the Flora des Bernsteins (Caspary 1907). Although the genus cannot be determined with certainty from a small twig without cones, the amber plant is most probably related to the genus Glyptostrobus, which belongs to the subfamily Taxodiaceae (swamp cypresses).
Figure 1.
(a) The cypress twig inside the amber piece with scale leaves in a spiral arrangement. The arrows indicate the two cuts that were made to prepare the slice for internal embedding. (b) The surface of the cypress twig after the cut. (c) A young twig of the recent species Glyptostrobus pensilis with a similar arrangement of leaves. Size bars: 1 mm.
To study the inner structure of the fossil, a cross-cut was made near the bottom of the plant (lower arrow, figure 1a). The tissue, as seen in the cross-section, has a dark brown colour and the stem and leaves can be distinguished (figure 1b). In a first attempt to prepare thin sections for light microscopy, the amber was trimmed and directly cut with an ultramicrotome. Well-defined sections were obtained from the amber matrix, but the fossil tissue crumbled and was lost completely during the procedure.
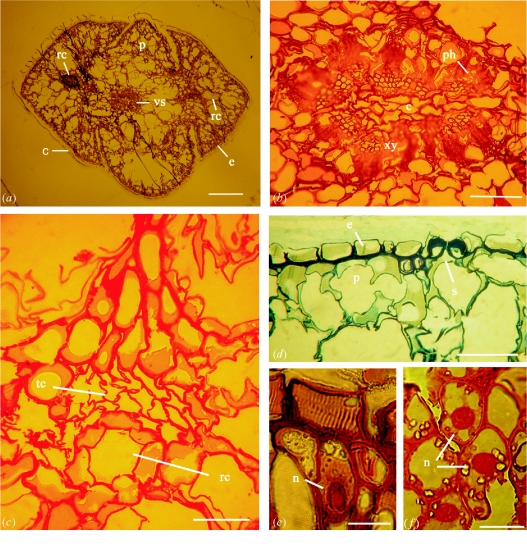
To preserve the plant structures in situ, a technique was developed of internal imbedding of the fossil tissue. Without breaking the inclusion, a slice was cut, as indicated by the two arrows in figure 1a, and liquid resin was allowed to rise in the plant tissue by capillary forces. Thin sections were prepared, resulting in images of highly preserved tissue of the entire stem and leaves of the cypress twig (figure 2). Depending on the thickness of the sections, the tissue has a light to dark brown colour and could be viewed in the light microscope without staining. Figure 2a shows a cross-section of the unstained shoot seen in a bright field light microscope. The four leaves are arranged around the central shoot; the cuticle, epidermis, parenchyma and vascular bundles are well preserved. Resin canals can be seen in the centre of the two larger leaves. The phloem appears to be partly unfocused, which is a result of uneven stretching and incomplete attachment to the glass slide, probably caused by the large size and variations in the flexibility of the tissue. Further stretching of the sections resulted in breakage of the tissue.
Figure 2.
Light microscope images of thin sections of the amber cypress (a–e) and of a recent Juniperus leaf (f). (a) Unstained cross-section of the entire stem and leaves, showing the cuticle (c) the epidermis (e), the parenchyma (p), the vascular system (vs) and the resin canals (rc). (b) Safranine stained vascular system with phloem (ph), xylem (xy) and core tissue (c). (c) Safranine stained tissue of a small resin canal (rc) formed by a layer of glandular cells adjacent to a layer of tracheid-like cells (tc) with double bordered pits. (d) Methylene blue stained tissue of the epidermis (e) with a stomatal pore (s) and the parenchyma (p). (e) A parenchyma cell stained with Stains All displaying a nucleus (n) and intensively stained cell walls. (f) Parenchyma cells from a leaf of a recent Juniperus sabina with nuclei (n) stained with Stains All. Size bars: (a) 300μm; (b–d) 50μm; (e, f) 20μm.
The sections were stained with three basic histological stains, commonly used for recent material. Safranin (figure 2b,c) stained the cell walls of the vascular system of the amber tissue bright red. Xylem, phloem and the central core can be distinguished in the cross-section. In the leaf, a small resin canal formed by glandular cells, adjacent to a layer of supporting tissue of tracheid-like cells with double bordered pits, was detected. The parenchyma and glandular cells contain light-coloured regions which can be interpreted as cytoplasm. With methylene blue (figure 2d), cell walls of the mesophyll and the epidermis were stained. A stomatal pore with characteristic secondary walls is seen. The cells of the palisade parenchyma are partly distorted and seem to have shrunk. Incubation with Stains All (figure 2e) resulted in differential staining of the nucleus, cell walls and the middle lamellae. Stained nuclei were also seen after incubation with safranin and methylene blue (not shown). To compare the amber tissue with a closely related extant species, a section of an embedded leave from a recent Juniperus plant was incubated with Stains All (figure 2f) The purple stained nuclei, cell walls and the dark middle lamellae in the parenchyma cells have almost the same appearance as in the fossil tissue.
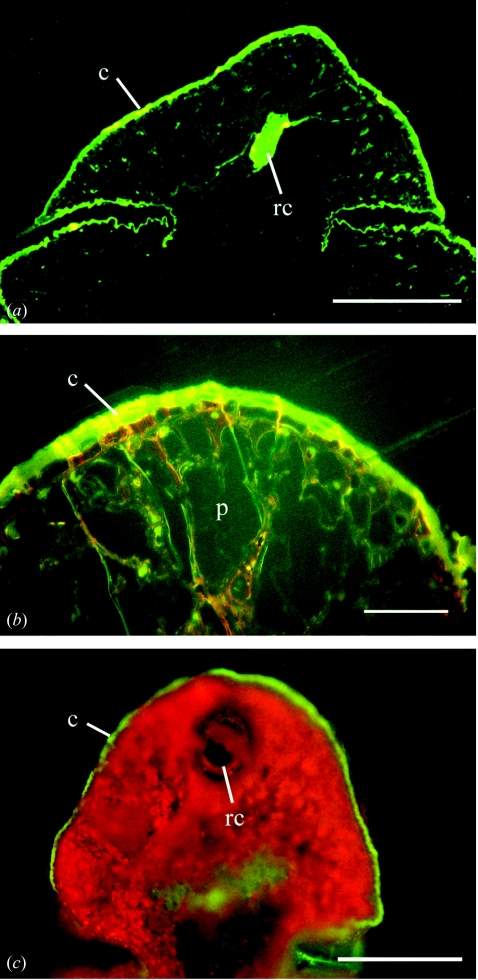
Examination of unstained sections of the amber cypress tissue by fluorescence microscopy (figure 3a,b) revealed a strong green auto-fluorescence that is primarily located in the cuticle and in the central resin canal. Other parts of the tissue showed very low fluorescence. The clear amber matrix and the synthetic resin did not fluoresce. Embedded tissue of recent Glyptostrobus or Juniperus plants did not show any autofluorescence. However, when fresh leaves of Glyptostrobus (figure 3c) were cut with a razor blade and irradiated under the same conditions as the amber tissue, a similar green fluorescence of the cuticle and the resin canals and the strong red fluorescence of the chlorophyll were detected.
Figure 3.
Fluorescence microscope images of (a, b) an unstained thin section of the amber cypress and (c) of a non-imbedded leave from a Glyptostrobus plant. (a) Overview of the cypress leaf with a strong fluorescence of the cuticle (c) and the large resin canal (rc). (b) Enlargement of (a) showing the parenchyma tissue (p) and the cuticle (c). (c) A razor blade cut of a fresh leaf of Glyptostrobus pensilis showing a green fluorescence of the cuticle and a red fluorescence of chlorophyll. The resin canal (rc) shows weak green fluorescence. Size bars: (a, c) 50μm; (b) 300μm.
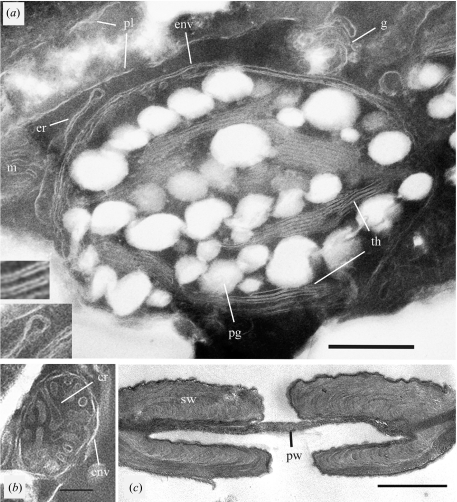
Ultrathin sections of the amber tissue were prepared and examined by transmission electron microscopy (figure 4). Well-preserved cell structures and cellular organelles were observed. A section of a parenchyma cell shows a chloroplast (figure 4a) with the double membrane envelope, thylakoid membranes and large plastoglobuli. Plasmalemma and membranes of the endoplasmic reticulum and golgi system are seen in the cytoplasm. The lumen of the thylakoid membranes and of the double envelope membrane of the chloroplast can be distinguished (inserts in figure 4a). Well-preserved mitochondria (figure 4b) with outer envelope and cristae were observed frequently. A double-bordered pit (figure 4c) with preserved internal structures of the primary and secondary walls from the tracheid-like cells is shown.
Figure 4.
Transmission electron micrographs of ultrathin cross-sections of the amber cypress tissue. (a) Section of a parenchyma cell with a chloroplast, the double membrane envelope (env), thylakoid membranes (th) and large plastoglobuli (pg), membranes of the endoplasmic reticulum (er), the golgi aparatus (g), the plasmalemma (pl) and part of a mitochondrion (m). (b) Cross-section of a mitochondrion with the outer envelope (env) and cristae (cr). (c) Cross-section of a double-bordered pit from a tracheid-like cell with fine structures of the primary and secondary cell walls. Size bars: (a) 500 nm; (b) 200 nm; (c) 1μm.
4. Discussion
With internal embedding, thin and ultrathin sections of the dry and fragile tissue of amber inclusions can be prepared and used for various analytical methods which could not be applied to these fossils previously. Cellular and subcellular structures can now be compared with those of recent forms and could help to resolve taxonomical questions. For example, cross-sections of the amber cypress revealed large resin canals at the centre of the leaves and not visible from the outside, which is similar to the location of resin canals in recent Glyptostrobus. In Thuja and Juniperus, resin canals occur close to the surface of the leaves. This observation supports the assumption that this amber cypress is related to the genus Glyptostrobus.
It should be possible to apply this method to animal inclusions in amber. Although it may be difficult to embed a whole insect in one piece, internal embedding of larger fragments of an organism could give information about the inner structure of organs and tissues. The method may be further developed by using various types of synthetic resins or by employing a slight vacuum during embedding.
Analytical histochemical and cytochemical methods can be applied to the sections. The finding that positively charged cytological stains, which bind to acidic groups of biological macromolecules like lignin or DNA in recent tissues, also react with amber tissue indicates that traces of biomolecules may still be present. Similarly, the autofluorescence of the cuticle and the resin canals of the fossil tissue corresponds to that seen in the cuticle of fresh leaves of recent Glyptostrobus. The fact, that no fluorescence was observed in imbedded recent tissue is a result of the application of organic solvents during dehydration. Although it cannot be assumed that the similarity of these reactions is based on an identical molecular composition, it may indicate that the geomolecules derived from the ancient biomolecules may have retained some of their original molecular structures and chemical characteristics.
Information about the physiological conditions of the tissue at the time of capture by the liquid amber may be deduced from the fine structure of organelles and membranes, such as the variation of the cristae structure or the size and number of plastoglobuli. The strong negative contrast of the fossil membranes is similar to the negative staining of membranes of recent tissue that has been embedded without fixation of lipids by osmium tetroxide. The question of the molecular composition of these structures remains to be solved. Ultrathin sections could be used for molecular biological electron microscopy, like immunolabelling and in situ polymerase chain reaction (PCR), in the search for traces of biomolecules in fossil tissues.
Up to now, to our knowledge, no direct evidence for biological macromolecules in amber tissue has been described. The earlier reports about the detection of DNA in amber inclusions were not reproducible (Austin et al. 1997). Although chitin was identified in a 25 Myr old beetle from the Enspel Lagerstätte in Germany with pyrolysis-gas chromatography-mass-spectrometry, and traces of lignin, cellulose and chitin were found in fossils preserved in copal, a subfossil resin, none of the large biomolecules was detected with this method in inclusions of Dominican amber (Stankiewicz et al. 1997, 1998). Indirect evidence for macromolecules in amber tissue comes from the measurements of the racemization reaction of amino acids. The presence of almost pure l-amino acids in inclusions of Baltic amber led to the assumption that they are preserved in polymeric molecules that would inhibit the racemization reaction (Bada et al. 1994; Wang et al. 1995; Poinar et al. 1996b).
During the formation of amber inclusions, the liquid resin enclosed the plants and animals but usually did not penetrate the tissue. Nevertheless cellular fine structures are preserved, even at the level of cell organelles and membranes. The geochemical basis of the mechanisms of fixation and preservation is not known. It has been suggested that biomolecules in fossil tissue might undergo extensive polymerization, like the non-enzymatic Maillard reactions (Bada et al. 1999; Briggs 1999), which would lead to chemical and mechanical fixation. This hypothesis was underlined by the detection of volatile compounds, characteristic for Maillard reaction products, which were released from fossilized plants found in Egyptian graves (Evershed et al. 1997). High molecular weight Maillard polymeres could be dissolved in an in vitro experiment with the chemical phenacyl-thiazolium bromide (PTB), which breaks the specific crosslinks and allows rescue of the trapped proteins and DNA (Vasan et al. 1996). In accordance with this reaction, incubation with PTB released PCR-amplifiable DNA from insoluble polymers in ancient faeces, which are ca. 10000 years old (Poinar et al. 1998).
These results have led to the hypothesis that carbohydrates, peptides or short chains of DNA or RNA in the tissue of amber fossils may also be trapped in a three-dimensional insoluble network of geopolymers formed by Maillard reactions and therefore cannot be detected as native biological molecules (Bada et al. 1999). The hypothesis is supported by the observation that the colouring of the amber tissue, as seen here in the thin sections, is a darker or lighter shade of brown, which is reminiscent of melanoidins, the insoluble products of the Maillard reaction (browning reaction). The highly preserved fine structures and the demonstration of histochemical reactions described here add to the earlier observation of unracemized amino acids in amber tissue and make it seem more likely that at least remnants of biological macromolecules could be preserved in some modified form in this unique fossil tissue.
Acknowledgments
We sincerely thank Freya Kaulbars for her help during this work, especially for her skills in laboratory and microscopical techniques.
References
- Ascaso C., Wierzchos J., Corral J.C., Lopez R., Alonso J. New applications of light and electron microscopic techniques for the study of microbiological inclusions in amber. J. Palaeontol. 2003;77:1182–1192. [Google Scholar]
- Austin J.J., Ross A.J., Smith A.B., Fortey R.A., Thomas R.H. Problems of reproducibility: does geological ancient DNA survive in amber-preserved insects? Proc. R. Soc. B. 1997;264:467–474. doi: 10.1098/rspb.1997.0067. doi:10.1098/rspb.1997.0067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bada J.L., Wang X.S., Poinar H.N., Paäbo S., Poinar G.O. Amino acid racemization in amber-entombed insects: implications for DNA preservation. Geochimica et Cosmoschimica Acta. 1994;58:3131–3135. doi: 10.1016/0016-7037(94)90185-6. [DOI] [PubMed] [Google Scholar]
- Bada J.L., Wang X.S., Hamilton H. Preservation of key biomolecules in the fossil record: current knowledge and future challenges. Phil. Trans. R. Soc. B. 1999;354:77–87. doi: 10.1098/rstb.1999.0361. doi:10.1098/rstb.1999.0361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs D.E.G. Molecular taphonomy of animal and plant cuticles: selective preservation and diagenesis. Phil. Trans. R. Soc. B. 1999;354:7–17. doi:10.1098/rstb.1999.0356 [Google Scholar]
- Caspary R. Die Flora des Bernsteins und anderer fossiler Harze des ostpreussischen Tertiärs. Abhandlungen der Königlich Preussischen Geologischen Landesanstalt. Neue Folge Heft. 1907;4:1–174. [Google Scholar]
- Conwentz H. Monographie der baltischen Bernsteinbäume. Commisions-Verlag Wilhelm Engelman Leipzig; 1890. [Google Scholar]
- Evershed R.P., Bland H.A., Van Bergen P.F., Carter J.F., Horton M.C., Rowley-Conwy P.A. Volatile compounds in archaeological plant remains and the Maillard reaction during decay of organic matter. Science. 1997;278:432–433. [Google Scholar]
- Göppert H.R. Über die Bernstein Flora. Monatsberichte der Köngl. Akademie der Wissenschaften zu Berlin. 1853;Juli:1–28. [Google Scholar]
- Goeppert, H. R. & Menge, A. 1883 Die Flora des Bernsteins Band 1+2. Naturforschende Gesellschaft in Danzig. Commisions Verlag von Wilhelm Engelmann in Leipzig.
- Grimaldi D.A. The age of Dominican amber. In: Anderson K., Crelling J.C., editors. Amber, resinite and fossil resins. ACS Symposium Series 617; Washington, DC: 1995. pp. 203–217. [Google Scholar]
- Grimaldi D.A., Bonwich E., Delannoy M., Doberstein S. Electron microscopic studies of mummified tissue in amber fossils. Am. Mus. Novitates. 1994;3097:1–31. [Google Scholar]
- Iturralde-Vinent W., MacPhee R.D.E. Age and palaeogeographical origin of Dominican amber. Science. 1996;273:1850–1853. [Google Scholar]
- Kohring R. REM-Untersuchungen an harzkonservierten Arthropoden. Entomol. Gener. 1998;23:95–106. [Google Scholar]
- Poinar G.O., Hess R. Ultrastructure of 40 million year old insect tissue. Science. 1982;215:1241–1242. doi: 10.1126/science.215.4537.1241. [DOI] [PubMed] [Google Scholar]
- Poinar G.O., Poinar H.N. The amber forest. Princeton University Press; 1990. [Google Scholar]
- Poinar H.N., Melzer R.R., Poinar G.O. Ultrastructure of 30–40 million year old leaflet from Dominican amber (Hymenaea protera, Fabaceae: Angiosperm) Experientia. 1996a;52:387–390. [Google Scholar]
- Poinar H.N., Hoss M., Bada J.L., Paäbo S. Amino acid racemization and the preservation of ancient DNA. Science. 1996b;272:864–866. doi: 10.1126/science.272.5263.864. [DOI] [PubMed] [Google Scholar]
- Poinar H.N., Hofreiter M., Spaulding W.G., Martin P.S., Stankiewicz B.A., Bland H., Evershed R.P., Possnert G., Paabo S. Molecular coproscopy: dung and diet of the extinct ground sloth Nothrotheriops shastensis. Science. 1998;281:402–406. doi: 10.1126/science.281.5375.402. [DOI] [PubMed] [Google Scholar]
- Ritzkowski S. K-Ar Altersbestimmungen der bernsteinführenden Sedimente des Samlandes (Paläogen, Bezirk Kaliningrad) In: Ganzelewski M., Rehren T.H., Slotta R., editors. Metalla, Sonderheft 66 zum Symposium Neue Erkenntnisse zum Bernstein. Deutsches Bergbau Museum; Bochum, Germany: 1997. pp. 19–24. [Google Scholar]
- Spurr A.R. A low-viscosity epoxy resin embedding medium for electron microscopy. J. Ultrastructure Res. 1969;210:57–69. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Stankiewicz B.A., Briggs D.E.G., Evershed R.P., Flannery M.B., Wuttke M. Preservation of chitin in 25 million-year-old fossils. Science. 1997;276:1541–1543. [Google Scholar]
- Stankiewicz B.A., Pioneer H.N., Briggs D.E.G., Evershed R.P., Poinar G.O. Chemical preservation of plants and insects in natural resins. Proc. R. Soc. B. 1998;265:641–647. doi:10.1098/rspb.1998.0342 [Google Scholar]
- Vasan S. An agent cleaving glucose-derived protein crosslinked in vitro and in vivo. Nature. 1996;382:275–278. doi: 10.1038/382275a0. (and 12 others) [DOI] [PubMed] [Google Scholar]
- Wang X.S., Poinar H.N., Poinar G.O., Bada J.L. Amino acids in the amber matrix and in entombed insects. In: Anderson K., Crelling J.C., editors. Amber, resinite and fossil resin. ACS Symposium Series 617; Washington, DC: 1995. pp. 255–262. [Google Scholar]
- Weitschat W., Wichard W. Atlas of plants and animals in Baltic amber. Dr Friedrich Pfeil; Verlag, Munich: 2002. [Google Scholar]