Abstract
Evolutionary theory proposes that exaggerated male traits have evolved via sexual selection, either through female mate choice or male–male competition. While female preferences for ornamented males have been amply demonstrated in other taxa, among mammals sexual characters are commonly regarded as weapons whose main function is to enhance male competitiveness in agonistic encounters. One particularly controversial hypothesis to explain the function of male sexual characters proposes that they advertise male fertility. We test this hypothesis in red deer (Cervus elaphus), a species where sexual characters (antlers) reach an extreme degree of elaboration. We find that a global measure of relative antler size and complexity is associated with relative testes size and sperm velocity. Our results exclude the possibility that condition dependence, age or time of culling, drive these associations. Red deer antlers could signal male fertility to females, the ability to avoid sperm depletion throughout the reproductive season and/or the competitive ability of ejaculates. By contrast, male antlers could also signal to other males not only their competitive ability at the behavioural level (fighting ability) but also at the physiological level (sperm competition).
Keywords: sexual selection, antlers, sperm velocity, male fertility, sperm competition, Cervus elaphus
1. Introduction
The concept of sexual selection was developed to explain the evolution of conspicuous traits in males, such as ornaments and weapons, which could not be explained by natural selection for increased survival (Darwin 1871; Andersson 1994). Such traits confer advantages in terms of enhanced reproduction, because either males with elaborated ornaments are more attractive to females, or because males with developed weapons are more competitive in male–male agonistic encounters. Thus, sexual selection can operate through two different processes: female mate choice and male–male competition. Female preferences for males with exaggerated traits have been amply demonstrated in insects, birds and fishes (Andersson 1994; Johnstone 1995; Jennions & Petrie 1997), while in mammals it is assumed that male–male competition is more prevalent. According to this view, most studies on fishes and birds have focused on female preferences for particular traits (referred to as ornaments), whereas among mammals most studies have focused on the role played by secondary sexual characters (this time named weapons) on male competitive ability. It has been suggested that differences in the intensity of female mate choice versus male–male competition between taxa are partly determined by different mechanisms of inheritance of sexual chromosomes (Roldan & Gomendio 1999).
Much attention has been devoted to the benefits derived by females from choosing particular males. Females may obtain direct benefits such as access to territories of good quality, nutrients transferred by the male, and paternal care, all of which are well documented (Andersson 1994). Females could also obtain indirect benefits such as an improvement in the genetic quality of the offspring (Madsen et al. 1992; Hasselquist et al. 1996) or the avoidance of genetic incompatibility (Zeh & Zeh 1997). The phenotype-linked fertility hypothesis suggests that females would also benefit from choosing males with elaborated sexual ornaments if they maximize the chances of mating with fertile males (Sheldon 1994). According to this hypothesis, secondary sexual ornaments would honestly advertise male fertility. In other words, males that are more attractive should deliver higher-quality ejaculates. There have been few attempts to test this hypothesis and they have yielded contradictory results. Among birds, several studies have related male behaviour during the breeding season to specific measures of ejaculate quality (Nitchuk & Evans 1978; Mjelstad 1991). However, these studies do not test the phenotype-linked fertility hypothesis directly since they measure behavioural variables rather than secondary sexual characters. Other studies specifically designed to test the phenotype-linked fertility hypothesis have yielded contradictory results. Several studies have found no relationship between male phenotype and ejaculate quality (Birkhead & Fletcher 1995; Birkhead et al. 1997), while a recent study has revealed a correlation between plumage brightness and testis size (Merilä & Sheldon 1999).
The other group in which the phenotype-linked fertility hypothesis has been examined is fishes, but the findings have not generated a clear picture either. Several studies have provided evidence against Sheldon’s hypothesis, since they find negative correlations between secondary sexual characters (or male sexual activity) and ejaculate quality (Liljedal et al. 1999; Pilastro & Bisazza 1999), whereas others find positive associations (Matthews et al. 1997). Some of these contradictory results seem to be because the relationship between male coloration, as well as mating behaviour and sperm number is population dependent (Pitcher & Evans 2001). Colourful male guppies (Poecilia reticulata) have been found to transfer more sperm to the female at copulation than less colourful males (Pilastro et al. 2002), but this positive association occurs only in female-solicited copulations and not in forced copulations, strongly suggesting that females play a role in accepting more sperm from colourful males. Finally, a recent study has shown that, when ejaculates from rival males compete, more colourful male guppies have higher parentage success than their less conspicuous counterparts (Evans et al. 2003). In this study, the experimental design was such that sperm numbers from each male were equal, thus indicating that the greater competitive ability of the ejaculates from more colourful males was a result of sperm traits other than sperm numbers.
An increasing body of evidence suggests that sperm swimming velocity or vigour influences fertilization success and that, under sperm competition, it may become an important determinant of paternity. Among mammals, sperm velocity influences fertilization success (Holt et al. 1997), and in the sea urchin (Lytechinus variegatus) males with faster sperm had higher rates of fertilization than males with slower sperm (Levitan 2000). When ejaculates from rival males compete within the female reproductive tract, i.e. sperm competition, sperm swimming velocity generally determines fertilization success (Gomendio & Roldan 1991; Birkhead et al. 1995, 1999; Froman et al. 1999, 2002; Anderson & Dixson 2002; Moore et al. 2002; Gage et al. 2004).
We test whether male red deer antlers honestly signal male ejaculate quality as measured by sperm production and sperm velocity. We use this species as a model organism because antlers are so elaborated that they are considered one of the most extreme cases of exaggeration of male traits in the animal kingdom. During the rut, male red deer in Mediterranean populations may form harems, as is common in the north of Europe, or they may defend territories when the food is scarce and females tend to concentrate in those areas where resources are more abundant (Carranza et al. 1996). In male–male encounters males display their antlers presumably to assess their opponents’ strength (Geist 1966), and they are used as weapons when males engage in fights. It is known that fighting success influences harem size which, in turn, is related to copulation success (Clutton-Brock et al. 1982). More recently it has been shown that antler size is associated to lifetime reproductive success (Kruuk et al. 2002), but the question as to whether this is the result of male fighting ability alone, or whether female choice also plays a role remains open (Andersson 1994).
To test the phenotype-linked fertility hypothesis (Sheldon 1994), we collected data from natural red deer populations and tested whether an overall measure of relative antler size is related to relative testes size, a standard measure of investment in sperm production, and to a measure of sperm quality, such as sperm velocity.
2. Material and methods
2.1 Data collection
The study sample included 198 Iberian red deer (Cervus elaphus hispanicus) stags culled during the mating season (October–December) in six different wild populations from the south of Spain, within a period of 50 days. In this region, the reproductive season begins at the end of September and lasts for three months, October to December (Caballero 1985; Sanz & Rodriguez 1993; García et al. 2002). There was no time-dependent selection (hunting) of small or large antlered stags, i.e. males with large antlers were not hunted earlier in the season, as most populations were culled only once in each mating season. Culls were undertaken following Spanish laws that, in turn, conform to European Union regulations. Measurements of body size and antler size were taken in the field. Body length was measured with a tape, while the dead stag lay on its side flat on the ground. Distance in centimetres from the tip of the nose to the sacrococcygeal articulation (beginning of the tail) was recorded. Both testes were removed (in the scrotum) and transported in a cool box at 20–21°C to the laboratory. Time lapse between animal death and sperm analyses ranged from 3 to 6 h, an adequate and safe time interval for evaluating sperm parameters, as it has been shown that only 12 h after the death of a male does a decrease in the quality of sperm traits begins to take place (Garde et al. 1998).
Time of culling had no effect upon relative testes size (F1,112=0.76; r2=0.006; p=0.38) or sperm velocity (F1,110=0.44; r2=0.004; p=0.51).
2.2 Antler size and complexity
Antlers are cast between March and May and the new ones grow from April to August or September, so at the time animals were sampled, antlers had reached their maximum size.
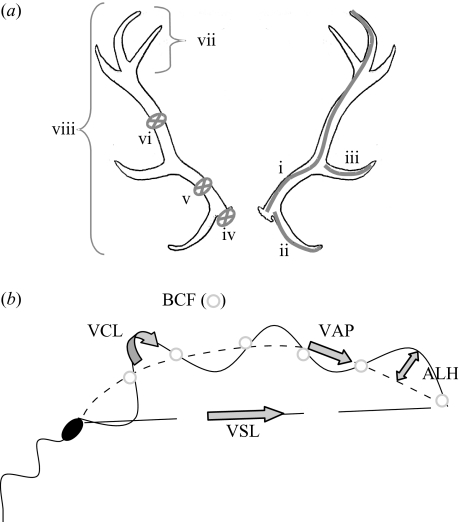
To have a global indicator of relative antler size and complexity we registered eight variables (figure 1a): three lengths, three diameters and two tip counts: (i) length of the main beam from the burr to the furthermost point of the crown, measured along the outer curvature of the antler; (ii) length of the frontal point; and (iii) length of the middle point. The three mean diameters were calculated measuring two transversal diameters at a given level of the antler: (iv) burr diameter; (v) beam diameter was taken halfway above the frontal point and below the middle point (lower diameter); (vi) beam diameter was taken halfway above the middle point and below the crown ramification (upper diameter); (vii) number of crown points; and (viii) total number of points (only those greater than 2 cm were considered). All these variables were corrected for body size before performing multivariate analyses, given that all showed allometry, using each antler size trait as the dependent variable. By correcting for body size we also aim to account for age effects upon antler size, since age strongly determines body size which in turn influences antler size (Kruuk et al. 2002).
Figure 1.
(a) Measures recorded to analyse relative antler size and complexity. (i) Length of the main beam from the burr to the furthermost point of the crown; (ii) length of the frontal point; (iii) length of the middle point. The three diameters were calculated by measuring two transversal diameters at a given level of the antler. (iv) Burr diameter; (v) beam diameter was taken halfway above the frontal tip and below the middle point (lower diameter); (vi) beam diameter was taken halfway above the middle point and below the crown ramification (upper diameter); (vii) number of crown points; (viii) number of total points (only points larger than 2 cm were considered). Given that all variables were correlated with body length, residuals were used. (b) This figure shows five of the six parameters that determine sperm velocity. The one-way grey arrows graphically define the three sperm velocities: curvilinear velocity (VCL), straight-line velocity (VSL) and average path velocity (VAP). The two-way arrow shows amplitude of lateral head displacement (ALH). Circles show beat crosses that define beat cross frequency (BCF). Linearity (LIN), defined as the ratio of distances of straight-line track length and actual track length, is not shown. Actual track, solid line; smoothed track, dotted line; straight-line track, dashed line.
Relative antler size showed a normal distribution (Kolmogorov–Smirnov d=0.068; p>0.20).
2.3 Sperm production
We employed relative testes size as a reliable index of sperm production (Gomendio et al. 1998). Testes and epididymides were removed from the scrotum, and diameters of left and right testes measured with a sliding digital calliper to the nearest 0.01 mm (Mitutoyo, UK). We tested the relationship between testes size and body size to test for allometry. We found a significant relationship (r=0.56, p<0.000 01), and thus calculated relative testes size by adding up major diameters of both testes and dividing by body length. This variable was log transformed and showed a normal distribution (Kolmogorov–Smirnov d=0.044; p>0.2).
There was no effect of age upon relative testes size (F1,126=0.22; p=0.64; r2<0.01).
2.4 Sperm velocity
Spermatozoa were recovered by cutting the caudae epididymides with a surgical blade and collecting the oozing sperm mass and placing it in 1 ml of Dulbecco’s phosphate-buffered saline containing 0.5% bovine serum albumin. Spermatozoa recovered from caudae epididymides are functionally mature and have a fertilizing potential equivalent to that of ejaculated sperm (Cooper 1998; Robaire & Hermo 1988).
Objective measures of sperm motility were recorded in spermatozoa suspended in Dulbecco’s PBS with 0.5% bovine serum albumin and using a computer-aided sperm analyser (CASA; Sperm Class Analyzer, Microptic, Barcelona, Spain). A total of six descriptors of sperm motility were scored by analysing a minimum of 100 tracks per sample: (i) curvilinear velocity (VCL), velocity over total distance moved, including all deviations of sperm head movement; (ii) straight-line velocity (VSL), velocity calculated using the straight-line distance between the beginning and end of the sperm track; (iii) average path velocity (VAP), velocity over a calculated, smoothed path, that is, a shorter distance than that used for calculating VCL; (iv) beat cross frequency (BCF), the frequency with which the actual track crosses the smoothed track; (v) amplitude of lateral head displacement (ALH), the mean value of the extreme side-to-side movement of the sperm head in each beat cycle; (vi) linearity (LIN) is the ratio of the distances (as a percentage) of straight-line track length/actual track length (this value is 100% for a completely linear track) (see figure 1b). None of these six variables showed association with body size (all p>0.1).
2.5 Physical condition
Kidney fat index was used as a reliable indicator of physical condition (Riney 1955; Vanrooyen 1993; Kucera 1997; Yokoyama et al. 2001; Noyes et al. 2002). It was calculated by dividing the weight of kidney fat remaining after trimming by kidney weight.
2.6 Statistical analysis
To ascertain the repeatability of the six antler measurements (the two point counts excluded), we measured the antlers of four randomly selected individuals three times and on both antler sides. Repeatabilities were calculated following Becker (1984). They were in all cases higher than 0.99. All variables were log transformed and checked for normal distribution (all variables p>0.2 after Kolmogorov–Smirnov normality test) before performing the principal component analysis (PCA).
The aim of principal component analysis is to reduce many correlated variables to a small number of new derived variables that adequately summarize the original information (Quinn & Keough 2002). We performed a PCA of the variance–covariance matrix of eight log-transformed relative antler size variables. This analysis extracts the first eigenvector that summarizes multivariate size variation and best extracts ‘size components’ (Jolicoeur 1963). We performed a second PCA including the sperm velocity and track direction set of variables rendered by CASA analysis. Differences in sample size in the two PCAs (see tables 1 and 2) are because sperm velocity was available from fewer individuals than antler size data. To avoid using statistical methods, which artificially increase sample size (pairwise and mean substitution), we chose the casewise deletion technique to perform the factor analysis. This technique excludes from the analysis those individuals with one or more missing values from the respective datasets, implying that the factor score could not be calculated for them. Thus, in subsequent analysis the missing values of the each PCA add together, thus resulting in a smaller sample size than in either PCAs.
Table 1.
Relative antler size and complexity.
(Factor loadings of the eight antler measurements obtained by means of a PCA (n=198 red deer stags). Significant correlations between original variables and the relative antler size component are shown as follows: ***p < 0.0001.)
| antler size variables | factor loadings | p |
| total beam length | 0.85 | *** |
| frontal point length | 0.72 | *** |
| middle point length | 0.67 | *** |
| burr diameter | 0.83 | *** |
| lower diameter | 0.89 | *** |
| upper diameter | 0.91 | *** |
| points in the crown | 0.69 | *** |
| total point number | 0.72 | *** |
| eigenvalue | 4.99 | |
| variance explained | 62% |
Table 2.
Sperm velocity.
(Factor loadings of the six sperm velocity measurements obtained by PCA (n=133 red deer stags). VCL, curvilinear velocity; VSL, straight-line velocity; VAP, average path velocity; LIN, linearity; ALH, amplitude of lateral head displacement; BCF, beat cross frequency. Significant correlations between original variables and the sperm velocity component are shown as follows: **p<0.001; ***p<0.0001.)
| sperm velocity variables | factor loadings | p |
| VCL | 0.47 | *** |
| VSL | 0.97 | *** |
| VAP | 0.90 | *** |
| LIN | 0.80 | *** |
| ALH | −0.33 | ** |
| BCF | −0.55 | *** |
| eigenvalue | 3.03 | |
| variance | 50% |
To test the hypothesis that antler size is a reliable indicator of sperm production and/or sperm velocity, we performed an ANCOVA for relative antler size including four independent variables: relative testes size, sperm velocity and kidney fat index as covariates, and population as a factor. We treated relative testes size, sperm velocity and kidney fat index as independent variables, to be able to control for any confounding effects of nutritional condition (reflected by kidney fat index) upon antler size.
Population was included in the ANCOVA model to account for between-population variation and environmental covariances, as differences in population densities, not controlled for in this study, have been shown to influence antler size in different deer populations (Clutton-Brock & Albon 1989; Schmidt et al. 2001).
In addition, given that condition dependence has been reported in a range of secondary sexual traits (Andersson 1994) including red deer antlers (Kruuk et al. 2002), we included in the ANCOVA model kidney fat index to control for individual physical condition.
All statistical analyses were conducted with Statistica 6.0 (Statsoft 2001).
3. Results
3.1 Multivariate analysis of relative antler size
The PCA performed with eight measures of relative antler size (see figure 1a) rendered a significant factor score (FS antler size) which explained 62% of the variance, with a correspondent eigenvalue of 4.99, that is, the factor generated integrates a total amount of information equivalent to that of almost five variables (see table 1). All the antler variables showed high loadings, with significant positive coefficients that indicate that they are strongly correlated with the component extracted (FS antler size), and thus can be straightforwardly interpreted as a reliable indicator of relative antler size (Jolicoeur 1963).
3.2 Multivariate analysis of sperm velocity
The PCA performed with six measurements of sperm velocity also rendered a significant factor score (FS sperm velocity), which explained 50% of the variance in sperm velocity, with a correspondent eigenvalue of 3. As expected, curvilinear velocity, straight-line velocity, average path velocity and linearity showed a positive significant correlation with the sperm velocity component extracted, since all these variables reflect different aspects of sperm swimming velocity. By contrast, amplitude of lateral head displacement and beat cross frequency showed a significant negative correlation with the sperm velocity component since they reflect features of sperm movement which do not contribute to sperm progressive motility (see table 2). High values for these two variables would imply inefficient or erratic sperm movement.
There was no effect of age upon sperm velocity (FS sperm velocity) (F1,92=0.10; p=0.75; r2=0.001).
3.3 Relationships between relative antler size and reproductive quality
We performed a general linear model (GLM) including relative antler size as the dependent variable and four predictors: three continuous, relative testes size, sperm velocity and kidney fat index; and one categorical: population (see table 3a). In this non-significant full model (F9,49=1.74; p>0.1) only relative testes size and sperm velocity were significant (p=0.023 and p=0.031, respectively) whereas kidney fat index and population were not (p=0.72 and p=0.80, respectively). We then conducted a reduced model, dropping from the analysis the variables that were non-significant, and only including relative testes size and sperm velocity as the independent variables (see table 3b). The analysis rendered a significant model (F2,68=11.51; p<0.0001), which explained 24% of the variance in relative antler size. In this model, relative testes size accounted for 15.37% of the explained variance and sperm velocity for 8.12%.
Table 3.
GLM of relative antler size on relative testes size, FS sperm velocity, kidney fat index and population of red deer stags.
(Full model: r2=0.24; F9,49=1.74; p>0.1. Reduced model: r2=0.25; F2,68=11.5; p<0.0001.)
| dependent variable | independent variable | d.f. | MS | F | p |
| (a) full model | |||||
| relative antler size | relative testes size | 1 | 5.689 | 5.453 | 0.0236 |
| sperm velocity | 1 | 5.085 | 4.875 | 0.0319 | |
| kidney fat index | 1 | 0.133 | 0.128 | 0.7217 | |
| population | 6 | 0.528 | 0.506 | 0.8002 | |
| residual | 49 | 1.043 | |||
| (b) reduced model | |||||
| relative antler size | relative testes size | 1 | 13.139 | 13.600 | 0.0004 |
| sperm velocity | 1 | 6.947 | 7.192 | 0.0091 | |
| residual | 68 | 0.966 |
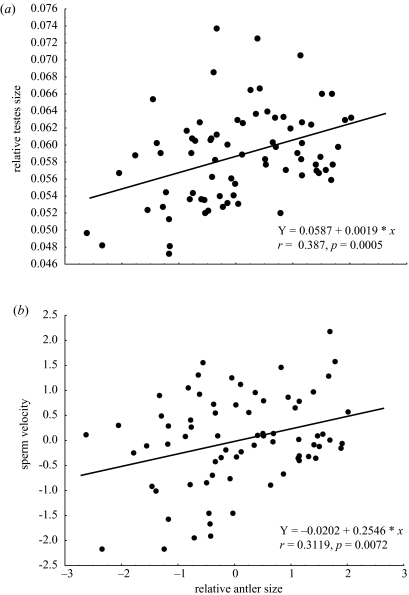
Once we were certain that environmental variation, as measured at the individual level by kidney fat index and at the population level by population, were not generating the association detected between relative antler size and both relative testes size and sperm velocity (Kruuk et al. 2003), and given that the aim of this study was to test whether antlers advertise male reproductive features, we performed two correlations including relative antler size as the predictor variable and relative testes size and sperm velocity as the dependent variables. Both correlations were highly significant for relative testes size (r2=0.15; F1,73=12.86; p=0.0006) and for sperm velocity (r2=0.097; F1,71=7.65; p=0.0072; figure 2). The fact that these two variables are not correlated (r=0.01; p=0.86) precludes the possibility that the effect of one of them is masking the other.
Figure 2.
(a) Relationship between relative antler size and complexity (FS relative antler size) and relative testes size (n=75). (b) Relationship between relative antler size and complexity (FS relative antler size) and sperm velocity (FS sperm velocity) (n=73).
We further explored, whether relative antler size is a better predictor of any of the three univariate measures of sperm velocity, VSL, VAP and LIN, which best correlate with the factor score generated by the PCA. For this purpose, we performed three ANCOVAs with the same predictor variables as in the previous case. Again, population and kidney fat index were not significant in any of the three models (results not shown), so we excluded them from the analysis and performed three regression analyses to test their association with relative antler size. Relative antler size showed significant relationships with VSL (r2= 0.085; F1,71=6.61, p<0.012) and VAP (r2=0.074; F1,71=5.67, p<0.019), but marginally non-significant with LIN (r2=0.052; F1,71=3.92, p<0.051). This result shows that relative antler size better signals the global measure ‘FS sperm velocity’ than any one of the three main sperm velocity variables on their own.
4. Discussion
Red deer antlers have been widely used as an extreme example of exaggerated male secondary sexual characters traits but have always been regarded exclusively as weapons. The fact that males often display their antlers has been interpreted as a display of fighting ability and the possibility that antlers may signal other male attributes has not been considered so far. Similarly, the fact that males with large antlers father more calves (Kruuk et al. 2002) has been interpreted as being the result of these males winning more fights and forming larger harems, excluding the possibility that males may also signal their fertility or their competitive ability after copulation, and that female choice may play a role. Among ungulates, male red deer possess antlers that are not only large in relation to other species, but also very complex, containing many branches and points. We address the question of whether this complex design and extreme size may also convey information about the reproductive potential of the male.
Our results show that relative antler size and complexity are honest indicators of sperm production and of sperm velocity in male red deer (Cervus elaphus hispanicus), thus providing support for the phenotype-linked fertility hypothesis, which states that functional fertility covaries with male phenotype (Sheldon 1994).
The two variables used in our study are known to influence male fertilization success, both in competitive and non-competitive contexts. Relative testes size determines sperm production rate (Setchell 1978; Møller 1989; Gomendio et al. 1998) and thus indicates the number of sperm present in each ejaculate, the number of ejaculates that can be produced in time, and the ability to avoid sperm depletion (Wedell et al. 2002). The number of sperm in each ejaculate determines fertilization success mainly because large numbers of sperm are needed to avoid the dilution effects and the high mortality rates that sperm suffer within the female reproductive tract (Short 1981). The importance of sperm numbers is exacerbated when ejaculates from rival males compete, resulting in a greater investment in testes size when there is sperm competition. The benefits derived by males from producing more sperm when they face competition with other males must be almost universal, since in most taxa, species with high levels of sperm competition have larger testes: butterflies (Gage 1994); fishes (Stockley et al. 1997); birds (Møller 1991; Birkhead 1996); frogs (Jennions & Passmore 1993); primates (Short 1979; Harcourt et al. 1981); mammals (Kenagy & Trombulak 1986); ungulates (Ginsberg & Rubenstein 1990); for a review see Parker et al. (1997). Using an experimental approach, it has been demonstrated that sperm competition does select for an increase in testes size (Hosken & Ward 2001).
More recent evidence has shown that, in addition to numbers, sperm quality also influences male fertilization success. Sperm velocity seems to play an important role, both in competitive and non-competitive contexts (Birkhead et al. 1995, 1999; Holt et al. 1997; Froman et al. 2002; Moore et al. 2002; Gage et al. 2004). Sperm velocity and vigour may determine sperm ability to overcome physical barriers in the female tract, to enter or leave sperm storage tubules, and to penetrate ova vestments (Froman et al. 1999). In addition, when sperm from rival males compete, sperm velocity may determine which sperm arrive first to the vicinity of the ova, and are thus more likely to fertilize.
To obtain an overall measure of sperm velocity, we performed a multivariate analysis including six sperm motility objective parameters. This analysis generated a reliable index of sperm velocity: first, all the variables contribute significantly to the sperm velocity component obtained; second, the variance accounted for by this component was 50%; third, all the variables behaved according to the predictions.
To obtain a global measure that would reflect the size and complexity of red deer antlers we used eight variables, which include length of the main beam, length of the branches, three widths of the main beam at different heights, and two point counts. The results of the multivariate analysis show that all the measures correlate highly significantly with the relative antler size component extracted, and that the variance accounted for by this component is 62%. Since all our antler measurements were corrected for body length, our overall indicator of antler size is not confounded by individual differences in body size.
Our findings show that the global estimate of antler size and complexity is significantly associated with both relative testes size and the overall index of sperm velocity. Since it is known that environmental variables, such as population density, and their effects upon physical condition may influence antler size (Andersson 1994; Kruuk et al. 2002, 2003), we included kidney fat index and population in our analyses to test whether they could be influencing the relationship between antler size/complexity and sperm production/quality. However, our analyses show that both variables had no significant effect in our models, suggesting that the relationship between antlers and sperm production and quality is not confounded by these effects. In addition, male age and time of culling had no effect on relative testes size and sperm velocity, thus eliminating the possibility that they may have explained in part the relationship found.
The finding that antlers are honest indicators of both male sperm production and sperm quality raises the question as to who the signal may be addressed to: other males or females? There are three possible benefits for the female that could explain the function of antlers as honest indicators of male reproductive quality. One of the possible benefits is infertility avoidance. Although it has been argued that this kind of benefit is unlikely because male infertility would be strongly selected against in natural populations (Birkhead 1996), at this stage we cannot rule out the possibility that the differences observed between males in ejaculate quality (e.g. Roldan et al. 1998) may translate into differences between males in fertility rates large enough to influence female choice. The evidence showing that both sperm production and sperm swimming velocity influence fertilization success (Holt et al. 1997; Gomendio et al. 1998; Levitan 2000) lends support to the possibility that antlers may be signalling precisely those reproductive traits that determine male fertility. Second, antlers could signal to females a male’s ability to avoid sperm depletion, since there is evidence that males can become sperm limited as a consequence of sperm production costs and sperm expenditure during reproduction (Wedell et al. 2002). Red deer are seasonal breeders and the rut begins at the end of the summer and lasts for two to three months. During the rut, males may defend harems or territories and copulate frequently with several females (Clutton-Brock et al. 1982; Carranza et al. 1990, 1996). Thus, it is possible that male deer will become sperm-limited as the rut progresses, as has been previously shown in another seasonal ungulate (Preston et al. 2001). However, sperm depletion is mainly the result of a reduction in the number of sperm available. The fact that antler size and complexity are associated with sperm velocity suggests that signalling sperm depletion may not be the main function of antlers.
A third possibility is that male antlers advertise ejaculate competitiveness, which determines fertilization success under sperm competition, although the prevalence of sperm competition in red deer populations is currently believed to be limited. In red deer populations from northern Europe, males tend to defend harems but females may copulate with several males during a given sexual cycle since they change harems. This possibility is supported by the fact that in our model species (Cervus elaphus) males have large testes in relation to their body size when compared with other species from the Cervidae family (Clutton-Brock et al. 1982). Moreover, in populations from southern Europe such as those included in this study, the mating season coincides with a period of food scarcity, since it begins after the hot and dry summer and takes place largely before the rains start. In these populations, males exhibit a flexible mating system so that some males may defend harems while other males defend territories where food resources are concentrated and which are used by females as feeding sites (Carranza et al. 1995, 1996). Under these conditions females move freely in their search for food, moving between territories and between harems rather frequently whether they are in oestrus or not (Carranza et al. 1996). Thus, in this context, sperm competition may be more intense, and females more able to choose which males they copulate with.
These three alternatives suggest that by choosing to copulate with males with large and complex antlers females could increase the chances of mating with fertile males; with males which are unlikely to become sperm depleted soon after the reproductive season begins, and/or with males which are likely to win in a sperm competition context. There are some suggestions in literature supporting the existence of female choice in red deer (Lincoln & Guinness 1973; Bartos 1982; Bubenik 1983), and it has been shown that female red deer choose males because of their roaring performance (McComb 1991). Thus, female mate choice in red deer has been documented, although it has not received enough attention to allow an assessment of its relevance. Both antler size (Williams et al. 1994; Kruuk et al. 2002) and semen quality are, to a certain extent, heritable (Beatty 1971; Ansah et al. 1985; Humblot & Ducrocq 1996; Froman et al. 2002; Simmons & Kotiaho 2002), suggesting that females may also benefit from the inheritance of both traits by their sons. These benefits would be particularly pronounced in species, such as red deer, in which female reproductive success is strongly influenced by their son’s lifetime reproductive success (Clutton-Brock et al. 1982).
Another possibility, which should not be ruled out, is that male antlers could also be signalling to other males the ability to avoid sperm depletion and the competitiveness of the ejaculate. In this way, other males could assess not only a male’s fighting ability, but also the chances that if they copulate with the same females their sperm will be defeated. Males could then use this information to decide whether competing with such a male both at the behavioural (fighting) and physiological (sperm competition) level is a good strategy.
These findings reveal a new function for male red deer antlers and suggest that among mammals the degree of elaboration of male secondary sexual characters may signal important aspects of male reproductive quality to females and males. Previous studies demonstrated that antler size is related to the number of calves fathered by males, and it has been assumed that this is exclusively the result of males with large antlers being able to win more fights with other males (Kruuk et al. 2002). Our findings suggest that males with large antlers could also achieve higher reproductive success through their enhanced ability to win fertilizations both in competitive and non-competitive contexts and the possible preferences shown by females to mate with them.
Acknowledgments
Funding was provided by the Spanish Ministry of Science and Technology (MICYT), FEDER-CICYT and National Institute for Agricultural Research (INIA). The authors are grateful to the landowners and managers of the red deer populations included in this study, for facilitating access to samples. The authors thank Paco García-González and two anonymous reviewers for constructive comments on previous versions of the manuscript. Aurelio Malo enjoyed a studentship from the Spanish Ministry of Science and Technology.
References
- Andersson M. Sexual selection. Princeton University Press; Princeton, NJ: 1994. [Google Scholar]
- Anderson M.J., Dixson A.F. Motility and midpiece in primates. Nature. 2002;416:496. doi: 10.1038/416496a. [DOI] [PubMed] [Google Scholar]
- Ansah G.A., Segura J.C., Buckland R.B. Semen production, sperm quality, and their heritabilities as influenced by selection for fertility frozen-thawed semen in the chicken. Poultry Sci. 1985;64:1801–1803. doi: 10.3382/ps.0641801. [DOI] [PubMed] [Google Scholar]
- Bartos L. A note on the sexual behaviour in red deer hinds. Z. Säugetierk. 1982;47:185–187. [Google Scholar]
- Beatty R.A. The genetics of size and shape of spermatozoan organelles. In: Beatty R.A., Gluecksohn-Waelsch S., editors. The genetics of the spermatozoon. University of Edinburgh; UK: 1971. pp. 97–115. [Google Scholar]
- Becker W.A. Manual of quantitative genetics. Pullman; Washington, DC: 1984. [Google Scholar]
- Birkhead T.R. Sperm competition: evolution and mechanisms. Curr. Top. Dev. Biol. 1996;33:103–158. doi: 10.1016/s0070-2153(08)60338-5. [DOI] [PubMed] [Google Scholar]
- Birkhead T.R., Fletcher F. Male phenotype and ejaculate quality in the zebra finch Taeniopygia guttata. Proc. R. Soc. B. 1995;262:329–334. doi: 10.1098/rspb.1995.0213. [DOI] [PubMed] [Google Scholar]
- Birkhead T.R., Fletcher F., Pellat E.J., Staples A. Ejaculate quality and the success of extra-pair copulations in the zebra finch. Nature. 1995;377:422–423. [Google Scholar]
- Birkhead T.R., Buchanan K.L., Devoogd T.J., Pellat E.J., Szekely T., Catchpole C.K. Song, sperm quality and testes asymmetry in the sedge warbler. Anim. Behav. 1997;53:965–971. [Google Scholar]
- Birkhead T.R., Martnez J.G., Burke T., Froman D.P. Sperm mobility determines the outcome of sperm competition in the domestic fowl. Proc. R. Soc. B. 1999;266:1759–1764. doi: 10.1098/rspb.1999.0843. doi:10.1098/rspb.1999.0843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubenik A.B. The behavioural aspects of antlerogenesis. In: Brown R.D., editor. Antler development in Cervidae. Kingsville, TX: Caesar Kleberg Wildlife Research Institute; 1983. [Google Scholar]
- Caballero R. Hábitat y Alimentación del Ciervo en Ambiente Mediterráneo. Ministerio de Agricultura, Pesca y Alimentación. ICONA; Madrid: 1985. [Google Scholar]
- Carranza J., Alvarez F., Redondo T. Territoriality as a mating strategy in red deer. Anim. Behav. 1990;40:79–88. [Google Scholar]
- Carranza J., Garciamunoz A.J., Vargas J.D. Experimental shifting from harem defense to territoriality in rutting red deer. Anim. Behav. 1995;49:551–554. [Google Scholar]
- Carranza J., FernandezLlario P., Gomendio M. Correlates of territoriality in rutting red deer. Ethology. 1996;102:793–805. [Google Scholar]
- Clutton-Brock T., Albon S.D. Red deer in the Highlands. BSP Professional Books; Oxford: 1989. [Google Scholar]
- Clutton-Brock T., Guinness F., Albon S.D. Red deer. Behavior and ecology of the two sexes. Wildlife behaviour and ecology. Edinburgh University Press; Edinburgh: 1982. [Google Scholar]
- Cooper T.G. Epididymis. In: Knobil E., Neill J.D., editors. Encyclopedia of reproduction. vol. II. Academic Press; San Diego, CA: 1998. pp. 1–17. [Google Scholar]
- Darwin C. The descent of man. London: Murray; 1871. [Google Scholar]
- Evans J.P., Zane L., Francescato S., Pilastro A. Directional postcopulatory sexual selection revealed by artificial insemination. Nature. 2003;421:360–363. doi: 10.1038/nature01367. [DOI] [PubMed] [Google Scholar]
- Froman D.P., Feltmann A.J., Rhoads M.L., Kirby J.D. Sperm mobility: a primary determinant of fertility in the domestic fowl (Gallus domesticus) Biol. Reprod. 1999;61:400–405. doi: 10.1095/biolreprod61.2.400. [DOI] [PubMed] [Google Scholar]
- Froman D.P., Pizzari T., Feltmann A.J., Castillo-Juarez H., Birkhead T.R. Sperm mobility: mechanisms of fertilizing efficiency, genetic variation and phenotypic relationship with male status in the domestic fowl, Gallus gallus domesticus. Proc. R. Soc. B. 2002;269:607–612. doi: 10.1098/rspb.2001.1925. doi:10.1098/rspb.2001.1925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage M.J.G. Associations between body-size, mating pattern, testis size and sperm lengths across butterflies. Proc. R. Soc. B. 1994;258:247–254. [Google Scholar]
- Gage M.J.G., Macfarlane C.P., Yeates S., Ward R.G., Searle J.B., Parker G.A. Spermatozoal traits and sperm competition in Atlantic salmon: relative sperm velocity is the primary determinant of fertilization success. Curr. Biol. 2004;14:44–47. [PubMed] [Google Scholar]
- García A.J., Landete-Castillejos T., Garde J.J., Gallego L. Reproductive seasonality in female Iberian red deer (Cervus elaphus hispanicus) Theriogenology. 2002;58:1553–1562. doi: 10.1016/s0093-691x(02)01048-8. [DOI] [PubMed] [Google Scholar]
- Garde J.J., Ortiz N., Garcia A.J., Gallego L., Landete-Castillejos T., Lopez A. Postmortem assessment of sperm characteristics of the red deer during the breeding season. Arch. Androl. 1998;41:195–202. doi: 10.3109/01485019808994891. [DOI] [PubMed] [Google Scholar]
- Geist V. The evolution of horn-like organs. Behavior. 1966;27:175–214. [Google Scholar]
- Ginsberg J.R., Rubenstein D.I. Sperm competition and variation in zebra mating-behavior. Behav. Ecol. Sociobiol. 1990;26:427–434. [Google Scholar]
- Gomendio M., Roldan E.R. Sperm competition influences sperm size in mammals. Proc. R. Soc. B. 1991;243:181–185. doi: 10.1098/rspb.1991.0029. [DOI] [PubMed] [Google Scholar]
- Gomendio M., Harcourt A.H., Roldan E.R.S. Sperm competition in mammals. In: Birkhead T.R., Møller A.P., editors. Sperm competition and sexual selection. Academic Press; London: 1998. pp. 667–751. [Google Scholar]
- Harcourt A.H., Harvey P.H., Larson S.G., Short R.V. Testis weight, body weight and breeding system in primates. Nature. 1981;293:55–57. doi: 10.1038/293055a0. [DOI] [PubMed] [Google Scholar]
- Hasselquist D., Bensch S., Von Schantz T. Correlation between male song repertoire, extra-pair paternity and offspring survival in the great reed warbler. Nature. 1996;381:229–232. [Google Scholar]
- Holt C., Holt W.V., Moore H.D., Reed H.C., Curnock R.M. Objectively measured boar sperm motility parameters correlate with the outcomes of on-farm inseminations: results of two fertility trials. J. Androl. 1997;18:312–323. [PubMed] [Google Scholar]
- Hosken D.J., Ward P.I. Experimental evidence for testis size evolution via sperm competition. Ecol. Lett. 2001;4:10–13. [Google Scholar]
- Humblot P., Ducrocq V. Genetic determinism of sperm production in bulls. Contracept. Fert. Sex. 1996;24:617–623. [PubMed] [Google Scholar]
- Jennions M.D., Passmore N.I. Sperm competition in frogs—testis size and a sterile male experiment on Chiromantis xerampelina (Rhacophoridae) Biol. J. Linn. Soc. 1993;50:211–220. [Google Scholar]
- Jennions M.D., Petrie M. Variation in mate choice and mating preferences: a review of causes and consequences. Biol. Rev. 1997;72:283–327. doi: 10.1017/s0006323196005014. [DOI] [PubMed] [Google Scholar]
- Johnstone R.A. Sexual selection, honest advertisement and the handicap principle: reviewing the evidence. Biol. Rev. 1995;70:1–65. doi: 10.1111/j.1469-185x.1995.tb01439.x. [DOI] [PubMed] [Google Scholar]
- Jolicoeur P. The multivariate generalization of the allometry equation. Biometrics. 1963;19:497–499. [Google Scholar]
- Kenagy G.J., Trombulak S.C. Size and function of mammalian testes in relation to body size. J. Mammal. 1986;67:1–22. [Google Scholar]
- Kruuk E.B., Slate J., Pemberton J.M., Brotherstone S., Guinness F., Clutton-Brock T. Antler size in red deer: heritability and selection but no evolution. Evolution. 2002;56:1683–1695. doi: 10.1111/j.0014-3820.2002.tb01480.x. [DOI] [PubMed] [Google Scholar]
- Kruuk L.E.B., Merila J., Sheldon B.C. When environmental variation short-circuits natural selection. Trends Ecol. Evol. 2003;18:207–209. [Google Scholar]
- Kucera T.E. Fecal indicators, diet, and population parameters in mule deer. J. Wildl. Mngmt. 1997;61:550–560. [Google Scholar]
- Levitan D.R. Sperm velocity and longevity trade off each other and influence fertilization in the sea urchin Lytechinus variegatus. Proc. R. Soc. B. 2000;267:531–534. doi: 10.1098/rspb.2000.1032. doi:10.1098/rspb.2000.1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljedal S., Folstad I., Skarstein F. Secondary sex traits, parasites, immunity and ejaculate quality in the Arctic charr. Proc. R. Soc. B. 1999;266:1893–1898. doi:10.1098/rspb.1999.0863 [Google Scholar]
- Lincoln G.A., Guinness F. The sexual significance of the rut in the red deer. J. Reprod. Fert. 1973;19:475–489. [PubMed] [Google Scholar]
- McComb K.E. Female choice for high roaring rates in red deer, Cervus elaphus. Anim. Behav. 1991;41:79–88. [Google Scholar]
- Madsen T., Shine R., Loman J., Hakansson T. Why do female adders copulate so frequently? Nature. 1992;355:440–441. [Google Scholar]
- Matthews I.M., Evans J.P., Magurran A.E. Male display rate reveals ejaculate characteristics in the Trinidadian guppy, Poecilia reticulata. Proc. R. Soc. B. 1997;264:695–700. doi:10.1098/rspb.1997.0099 [Google Scholar]
- Merilä J., Sheldon B.C. Testis size variation in the greenfinch Carduelis chloris: relevance for some recent models of sexual selection. Behav. Ecol. Sociobiol. 1999;45:115–123. [Google Scholar]
- Mjelstad H. Displaying intensity and sperm quality in the capercaillie Tetrao urogallus. Fauna Norv. C. 1991;14:93–94. [Google Scholar]
- Møller A.P. Ejaculate quality, testes size and sperm production in mammals. Funct. Ecol. 1989;3:91–96. [Google Scholar]
- Møller A.P. Sperm competition, sperm depletion, paternal care, and relative testis size in birds. Am. Nat. 1991;137:882–906. [Google Scholar]
- Moore H., Dvorakova K., Jenkins N., Breed W. Exceptional sperm cooperation in the wood mouse. Nature. 2002;418:174–177. doi: 10.1038/nature00832. [DOI] [PubMed] [Google Scholar]
- Nitchuk W.M., Evans R.M. A volumetric analysis of Sharp-tailed grouse sperm in relation to dancing ground size and organization. Wilson Bull. 1978;90:460–462. [Google Scholar]
- Noyes J.H., Johnson B.K., Dick B.L., Kie J.G. Effects of male age and female nutritional condition on elk reproduction. J. Wildl. Mngmt. 2002;66:1301–1307. [Google Scholar]
- Parker G.A., Ball M.A., Stockley P., Gage M.J.G. Sperm competition games: a prospective analysis of risk assessment. Proc. R. Soc. B. 1997;264:1793–1802. doi: 10.1098/rspb.1997.0249. doi:10.1098/rspb.1997.0249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilastro A., Bisazza A. Insemination efficiency of two alternative male tactics in the guppy Poecilia reticulata. Proc. R. Soc. B. 1999;266:1887–1891. doi:10.1098/rspb.1999.0862 [Google Scholar]
- Pilastro A., Evans J.P., Sartorelli S., Bisazza A. Male phenotype predicts insemination success in guppies. Proc. R. Soc. B. 2002;269:1325–1330. doi: 10.1098/rspb.2002.2017. doi:10.1098/rspb.2002.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitcher T.E., Evans J.P. Male phenotype and sperm number in the guppy (Poecilia reticulata) Can. J. Zool. 2001;79:1891–1896. [Google Scholar]
- Preston B.T., Stevenson I.R., Pemberton J.M., Wilson K. Dominant rams lose out by sperm depletion. Nature. 2001;409:681–682. doi: 10.1038/35055617. [DOI] [PubMed] [Google Scholar]
- Quinn G.P., Keough M.J. Experimental design and data analysis for biologists. Cambridge University Press; 2002. [Google Scholar]
- Riney T.N. Evaluating condition of free-ranging red deer (Cervus elaphus) with special reference to New Zealand. NZ J. Sci. Technol. 1955;36:429–463. [Google Scholar]
- Robaire B., Hermo L. Efferent ducts, epididymis, and vas deferens: Structure, functions, and their regulation. In: Knobil E., Neill J.D., editors. The physiology of reproduction. Raven Press; New York: 1988. pp. 999–1080. [Google Scholar]
- Roldan E.R., Gomendio M. The Y chromosome as a battle ground for sexual selection. Trends Ecol. Evol. 1999;14:58–62. doi: 10.1016/s0169-5347(98)01567-5. [DOI] [PubMed] [Google Scholar]
- Roldan E.R.S., Cassinello J., Abaigar T., Gomendio M. Inbreeding, fluctuating asymmetry, and ejaculate quality in an endangered ungulate. Proc. R. Soc. B. 1998;265:243–248. doi: 10.1098/rspb.1998.0288. doi:10.1098/rspb.1998.0288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz V., Rodriguez C. XXXIII Reunión científica de la S.E.E.P. 1993. Fechas de concepción en relación con la edad y la condición corporal de la población de ciervos de Quintos de Mora (Montes de Toledo, Toledo) pp. 555–586. Ciudad Real. [Google Scholar]
- Schmidt K.T., Stien A., Albon S.D., Guinness F.E. Antler length of yearling red deer is determined by population density, weather and early life-history. Oecologia. 2001;127:191–197. doi: 10.1007/s004420000583. [DOI] [PubMed] [Google Scholar]
- Setchell B.P. The mammalian testes. Cornell University Press; Ithaca, NY: 1978. [Google Scholar]
- Sheldon B.C. Male phenotype, fertility, and the pursuit of extra-pair copulations by female birds. Proc. R. Soc. B. 1994;257:25–30. [Google Scholar]
- Short R.V. Sexual selection and its component parts, somatic and genital selection, as illustrated by man and the great apes. Adv. Stud. Behav. 1979;9:131–158. [Google Scholar]
- Short R.V. Sexual selection in man and the great apes. In: Graham C.E., editor. Reproductive biology of the great apes. Academic Press; New York: 1981. pp. 319–341. [Google Scholar]
- Simmons L.W., Kotiaho J.S. Evolution of ejaculates: patterns of phenotypic and genotypic variation and condition dependence in sperm competition traits. Evolution. 2002;56:1622–1631. doi: 10.1111/j.0014-3820.2002.tb01474.x. [DOI] [PubMed] [Google Scholar]
- Statsoft Inc. 2001 Statistica for Windows, v.6.0. Tulsa, OK: Statsoft Inc.
- Stockley P., Gage M.J.G., Parker G.A., Møller A.P. Sperm competition in fishes: the evolution of testis size and ejaculate characteristics. Am. Nat. 1997;149:933–954. doi: 10.1086/286031. [DOI] [PubMed] [Google Scholar]
- Vanrooyen A.F. Variation in body condition of impala and nyala in relation to social-status and reproduction. S. Afr. J. Wildl. Res. 1993;23:36–38. [Google Scholar]
- Wedell N., Gage M.J., Parker G.A. Sperm competition, male prudence and sperm-limited females. Trends Ecol. Evol. 2002;17:313–320. [Google Scholar]
- Williams J.D., Krueger W.F., Harmel D.H. Heritabilities for antler characteristics and body weight in yearling white-tailed deer. Heredity. 1994;73:78–83. doi: 10.1038/hdy.1994.101. [DOI] [PubMed] [Google Scholar]
- Yokoyama M., Onuma M., Suzuki M., Kaji K. Seasonal fluctuations of body condition in northern sika deer on Hokkaido Island, Japan. Acta Theriol. 2001;46:419–428. [Google Scholar]
- Zeh J.A., Zeh D.W. The evolution of polyandry II. post-copulatory defenses against genetic incompatibility. Proc. R. Soc. B. 1997;264:69–75. doi:10.1098/rspb.1997.0010 [Google Scholar]