Abstract
Analyses at multiple spatial scales may show how important ecosystem services such as biological control are determined by processes acting on the landscape scale. We examined cereal aphid–parasitoid interactions in wheat fields in agricultural landscapes differing in structural complexity (32–100% arable land). Complex landscapes were associated with increased aphid mortality resulting from parasitism, but also with higher aphid colonization, thereby counterbalancing possible biological control by parasitoids and lastly resulting in similar aphid densities across landscapes. Thus, undisturbed perennial habitats appeared to enhance both pests and natural enemies. Analyses at multiple spatial scales (landscape sectors of 0.5–6 km diameter) showed that correlations between parasitism and percentage of arable land were significant at scales of 0.5–2 km, whereas aphid densities responded to percentage of arable land at scales of 1–6 km diameter. Hence, the higher trophic level populations appeared to be determined by smaller landscape sectors owing to dispersal limitation, showing the ‘functional spatial scale’ for species-specific landscape management.
Keywords: biological control, wheat, landscape structure, spatial ecology, temporal changes
1. Introduction
Understanding of population dynamics needs analyses of trophic interactions at multiple spatial and temporal scales (Kareiva 1990; Pimm 1991; Wiens et al. 1993; Kareiva & Wennergren 1995; Pickett & Cadenasso 1995; Rosenzweig 1995; Holt 1996; Roland & Taylor 1997; Wiens et al. 1997; Tischendorf & Fahrig 2000; Cadenasso & Pickett 2000; Ricketts 2001; Menalled et al. 2003; Tscharntke & Brandl 2004). Spatial and temporal dynamics should be particularly important in agricultural landscapes, which are dominated by arable fields. Annual harvesting and soil cultivation almost completely erase herbivores and their natural enemies, so that arable fields have to be recolonized from surrounding habitats yearly. Nevertheless, annual crops can exhibit strong regulation of herbivore populations by natural enemies (Halaj & Wise 2001), this being improved by both changing agricultural practices within crop fields (Wratten & van Emden 1995; Van Driesche & Bellows 1996), and the management of agricultural landscapes (Altieri 1995; Burel & Baudry 1995; Van Driesche & Bellows 1996; Matson et al. 1997; Menalled et al. 1999; Thies & Tscharntke 1999; Tscharntke & Kruess 1999; Tscharntke 2000; Östman et al. 2001; Tscharntke et al. 2002; Van Nouhuys & Hanski 2002a). A basic understanding of the mechanisms of naturally occurring biological control may contribute to the management of environmentally sound crop production systems that use ecosystem services and reduce external effects such as those due to pesticides (Tilman et al. 2002).
In winter wheat fields (Triticum aestivum L.) in northern Germany, the herbivore community is dominated by three species of cereal aphid (Homoptera, Aphididae), Sitobion avenae (Fabricius), Metopolophium dirhodum (Walker) and Rhopalosiphum padi (Linnaeus). Outbreaks of aphid populations causing economic damage have been recorded since the early 1970s. They have been related to increased applications of nitrogen fertilizers in combination with applications of growth regulators and fungicides, which protect the leaf structure (Hanisch 1980; Ankersmit 1988; Honek 1991). The role of natural enemies in preventing cereal aphid outbreaks is emphasized in several studies (e.g. Wratten & Powell 1991; Levie et al. 2000; Kindlmann & Dixon 2001; Sigsgaard 2002; Schmidt et al. 2003), but little is known about whether variability of bio-control can be explained by the surrounding landscape (but see Östman et al. 2001). The landscape context connects to local processes via dispersal and thereby should be expected to filter differences in the species traits such as body size, foraging range, resource specialization, population size variability and trophic position (Holt 1996; Tscharntke & Brandl 2004).
In this three-year study, we analysed changes in the population size of cereal aphids and their mortality owing to parasitism at multiple spatial scales. Our former analyses of experimental exclusion of aphid enemies showed that parasitoids (Hymenoptera: mainly Aphidiidae) that are specialized on one or several aphid host species (Sigsgaard 2002), could best explain differences in aphid densities (Schmidt et al. 2003). However, flying and ground-dwelling predators can also be important (see Holland et al. 1996; Holland & Thomas 1997; Östman et al. 2001; Lang 2003). The fields were located in agricultural landscapes characterized by a gradient from structurally complex landscapes to structurally simple ones (32–100% arable land that was closely correlated with habitat type diversity). The influence of landscape context on aphid–parasitoid interactions was tested from small to large landscape scales, in that we tested seven circular landscape sectors ranging from 0.5 to 6 km diameter. We expected that (i) aphid parasitism would decrease and aphid densities increase with increasing percentage of arable land in a landscape and (ii) that aphids and their parasitoids would respond to landscape context at different spatial scales.
2. Material and methods
The three-year study was conducted in 40 conventionally managed winter wheat fields in the vicinity of the city of Göttingen (51°32′ N, 9°56′ E; see Steffan-Dewenter et al. (2002); Thies et al. (2003)), Lower Saxony (North Germany) in 2001 (n=18 fields), 2002 (n=10 fields) and 2003 (n=12 fields), which differed between years owing to crop rotation. The area is dominated by arable fields with intensive agricultural land use (ca. 75% of the region) and patchily distributed fragments of forest, grassland, fallow, hedge and other semi-natural habitats. The area under cultivation was composed of cereals (71%), sugar beets (12%), oilseed rape (8%) and corn (4%) (I. Roschewitz, unpublished data). The average temperature (°C) and total rainfall (mm) during the study periods from June to July differed considerably (June 2001: 13.9°C, 59.9 mm; June 2002: 16.6°C, 71.4 mm; June 2003: 18.1°C, 47.7 mm; and July 2001: 18.4°C, 68.8 mm; July 2002: 17.3°C, 95.2 mm; July 2003: 18.7°C; 46.9 mm; data from the meteorological station in Göttingen).
The study sites were located in a gradient from structurally simple landscapes (up to 100% arable land) to structurally complex landscapes (less than 35% arable land). The distribution of landscapes of different complexity did not show any north–south or east–west pattern, to avoid spatial autocorrelation in abiotic factors such as soil fertility. For each of the fields the surrounding proportion of habitat types was measured at seven circular sectors of increasing diameter (∅ 0.5 km; ∅ 1 km; ∅ 2 km; ∅ 3 km; ∅ 4 km; ∅ 5 km; ∅ 6 km), representing a nested set of landscape sectors at seven spatial scales. Landscape data were available for 39 (out of the 40) landscape sectors. We quantified the area of arable land, grassland, forest, hedgerow, garden land and settlement using official digital thematic maps (ATKIS–Digitales Landschaftsmodell 25/1; Landesvermessung und Geobasisinformation, Hannover, Germany 1991–1996) and the Geographical Information System ArcView 3.1 (ESRI Geoinformatik GmbH, Hannover, Germany) at these seven spatial scales. The percentage of arable land per landscape was used as an indicator of landscape complexity as annual ploughing and harvesting in crops may greatly affect most organisms, and this simple parameter has been shown to be a good predictor for other landscape metrics such as habitat type diversity and habitat isolation (Thies & Tscharntke 1999; Steffan-Dewenter et al. 2002).
Land-use intensity of the fields, i.e. the amount of nitrogen fertilizers (kg N per hectare) and pesticides (number of applications of herbicides, fungicides, insecticides and growth regulators per year), which was recorded in a questionnaire to farmers in 2001 (n=18 fields), was not related to landscape complexity. For example, percentage of arable land in a landscape sector of 1 km diameter was not related to (i) nitrogen: 208.1±28.1 kg N per hectare, rS=0.282, p=0.244; (ii) herbicides: 1.9±0.5 applications per year, rS=0.218 to p=0.369; (iii) fungicides: 1.9±0.8 applications per year, rS=0.336, p=0.167; (iv) insecticides: 0.7±0.6 applications per year, rS=0.288, p=0.235; or (v) growth regulators: 1.7±0.7 applications per year, rS=0.207, p=0.394 (mean±s.d.; Spearman's rank correlation (rS); I. Roschewitz, unpublished data).
Aphids and parasitized aphids (mummies) were quantified visually on an insecticide-free area of 800 m2 in each of the 40 fields on 100 wheat shoots per field at wheat flowering in June (after the main period of aphid colonization of the fields), and on 100 wheat shoots per field at wheat milk ripening in July (after the main period of aphid reproduction in the fields). Each of the 80 samples consisted of four sub-samples of 25 shoots from different locations, which totalled 100 shoots. In the first year, aphid mummies were additionally sampled (2 h per field) and taken into the laboratory for rearing and identification of the parasitoid genera. The effects of the predictor variables: ‘year’, ‘host plant density’ and ‘percentage of arable land’ on aphid densities, and the effects of the predictor variables: ‘year’, ‘host density’ and ‘percentage of arable land’ on aphid parasitism were analysed individually for each of the seven spatial scales (i.e. landscape sectors) using general linear models (following Wiegand et al. 1999; Steffan-Dewenter et al. 2002; Thies et al. 2003; for theoretical background, see Hanski 1998; Legendre & Legendre 1998; Turner et al. 2001; Holland et al. 2004). Model assumptions were tested by examining the normality of the residuals (Sokal & Rohlf 1995). The skewness and/or kurtosis of the data was compensated by log(X+1)-transformation of aphid densities, and arcsine(√X)-transformation of parasitism rates. In addition, Spearman's rank correlations (rS) were used to analyse the relationship between aphid population growth (the proportion of aphid density at wheat milk ripening (dwmr) and aphid density at wheat flowering (dwfl)=dwmr/dwfl) and percentage of parasitism at the second sample date. Arithmetic means ±s.d. are given.
3. Results
A total of 12234 cereal aphid individuals was counted in 40 winter wheat fields, of which 69.4% were Sitobion avenae, 27.4% Metopolophium dirhodum and 3.2% Rhopalosiphum padi. Aphids were attacked by five genera of parasitoids (mainly Aphidius; Aphidinidae) and the latter by three genera of hyperparasitoids (mainly Dendrocerus; Megaspilidae). The population densities of aphids and their parasitoids are summarized in table 1; they varied considerably between the 40 study sites. Aphid and parasitoid densities were positively correlated at wheat flowering (F1,38=10.0, p=0.003, n=40, R=0.456), but not at wheat milk ripening (F1,38=0.01, p=0.913, n=40, R=0.031).
Table 1.
Densities of cereal aphids and parasitoid mummies (individuals per 100 shoots) in winter wheat fields in three consecutive years.
(Arithmetic mean±s.d., minima and maxima are given for 18 fields in 2001, for 10 fields in 2002 and for 12 fields in 2003.)
| mean±s.d. | minimum | maximum | |
| number of aphids at wheat flowering | |||
| 2001 | 23.7±14.5 | 1 | 53 |
| 2002 | 257.6±161.7 | 30 | 596 |
| 2003 | 121.7±63.9 | 7 | 229 |
| number of aphids at wheat milk ripening | |||
| 2001 | 46.9±32.2 | 13 | 140 |
| 2002 | 101.6±63.9 | 18 | 228 |
| 2003 | 492.6±297.4 | 258 | 1371 |
| number of mummies at wheat flowering | |||
| 2001 | 0.9±1.5 | 0 | 5 |
| 2002 | 5.8±5.4 | 0 | 14 |
| 2003 | 0.3±0.6 | 0 | 2 |
| number of mummies at wheat milk ripening | |||
| 2001 | 8.8±6.4 | 0 | 23 |
| 2002 | 13.2±8.1 | 4 | 26 |
| 2003 | 8.0±6.0 | 0 | 23 |
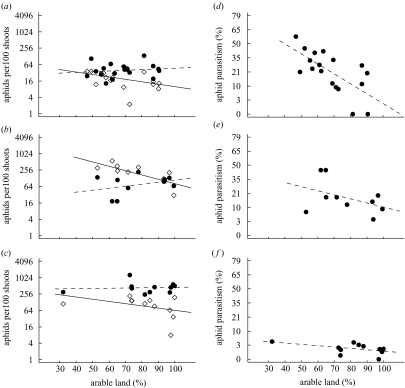
Aphid densities averaged 111.6±127.8 individuals per 100 shoots at wheat flowering and 194.3±258.8 individuals per 100 shoots at milk ripening. They increased from wheat flowering to milk-ripening stage in 2001 and 2003, but not in 2002 (table 1), thereby exceeding the threshold level of economic damage (3–5 per shoot; Pflanzenschutzamt Hannover 2002) in 0% of the fields in 2001, 40% of the fields in 2002, and 100% of the fields in 2003. Aphid densities differed significantly between years on both dates (table 2). At wheat flowering, they correlated negatively with percentage of arable land at scales between ∅ 1 km and ∅ 6 km. At milk ripening, this relationship was not significant at any scale (table 2; figure 1a–c).
Table 2.
F-values and levels of significance from general linear models relating aphid densities and aphid parasitism rates in wheat fields to three predictive factors: (i) the year of the survey (A), (ii) the percentage of arable land per landscape sector at seven spatial scales (B), and (iii) host plant density (C) and host density, respectively.
| diameter of landscape sector (km) |
|||||||
| 0.5 |
1 |
2 |
3 |
4 |
5 |
6 |
|
| aphids per 100 shoots at wheat flowering | |||||||
| A | 28.3*** | 39.1*** | 40.5*** | 37.1*** | 36.6*** | 36.5*** | 34.8*** |
| B (%) | n.s. | 10.2** | 12.3** | 9.9** | 9.4** | 9.5** | 8.9** |
| C | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| A×B | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| A×C | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| B×C | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| model | 28.3*** | 26.1*** | 27.9*** | 25.9*** | 25.5*** | 25.6*** | 25.0*** |
| aphids per 100 shoots at wheat milk ripening | |||||||
| A | 53.1*** | 53.1*** | 53.1*** | 53.1*** | 53.1*** | 53.1*** | 53.1*** |
| B (%) | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| C | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| A×B | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| A×C | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| B×C | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| model | 53.1*** | 53.1*** | 53.1*** | 53.1*** | 53.1*** | 53.1*** | 53.1*** |
| aphid parasitism (%) at wheat milk ripening | |||||||
| A | 7.2** | 3.9* | 3.8* | n.s. | n.s. | n.s. | n.s. |
| B (%) | n.s. | 12.3** | 9.6** | n.s. | n.s. | n.s. | n.s. |
| C | n.s. | 18.8*** | 18.4*** | 57.2*** | 57.2*** | 57.2*** | 57.2*** |
| A×B | 6.9* | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| A×C | n.s. | 5.6* | 5.4* | n.s. | n.s. | n.s. | n.s. |
| B×C | 34.9*** | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| model | 19.8*** | 18.6*** | 15.9*** | 57.2*** | 57.2*** | 57.2*** | 57.2*** |
*** p < 0.001; **p < 0.01; *p < 0.05.
Figure 1.
Dependence of cereal aphid densities (a–c) and percentage of parasitism (d–f) on the percentage of arable land within circular landscape sectors of 1 km diameter in three consecutive years: (a,d) 2001; (b,e) 2002; (c,f) 2003. Open quadrats and solid lines: wheat flowering stage in June (after the main period of aphid colonization of the fields); filled points and dashed lines: wheat milk-ripening stage in July (after the main period of aphid reproduction in the fields). Regression lines are shown for descriptive purposes. See table 2 for statistics. Note the logarithmic scale for aphid densities.
Aphid parasitism was low at wheat flowering with an average of 2.6±5.6% and increased at wheat milk ripening to 15.9±15.8% (for absolute densities, see table 1). At wheat milk ripening, parasitism differed significantly between years, and correlated negatively with percentage of arable land and host density (table 2). In contrast to aphid abundance, the variation in parasitism explained by percentage of arable land was only significant between scales of ∅ 0.5 km and ∅ 2 km diameter of landscape sector (table 2; figure 1d–f). At the smallest spatial scale (∅ 0.5 km), the negative effect of percentage of arable land on parasitism occurred mainly when host density was low (interaction: arable land×host density), and differed between years, being marked only in 2001 and 2002 (interaction: year×arable land). At scales of ∅ 1 km and 2 km, arable land and host density accounted for the main effects, but the effect of local host densities also differed between years, being marked in 2001 and 2002 (interaction: year×host density).
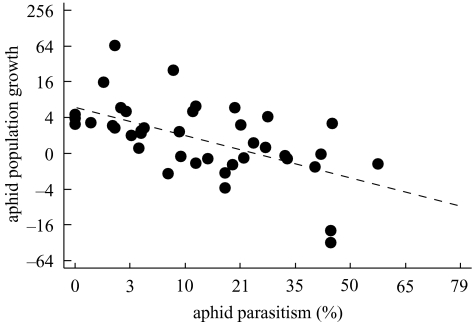
Aphid population growth correlated negatively with parasitism (figure 2). Aphid populations decreased between wheat flowering and milk ripening at parasitism rates higher than 27%, which were found in landscapes with more than 30% non-crop area. The negative correlation between aphid population growth and parasitism rate occurred in each year (2001: p=0.019, n=18, rS=−0.571; 2002: p=0.031, n=10, rS=−0.720; 2003: p=0.060, n=12, rS=−0.566); 34% of the mummies reared in the laboratory were hyperparasitoids.
Figure 2.
Relation of aphid population growth (the proportion of aphid density at wheat milk ripening (dwmr) and aphid density at wheat flowering (dwfl)=dwmr/dwfl) to percentage of parasitism. Spearman's rank correlation: p<0.001, n=40, rS=−0.547.
4. Discussion
The analyses of population density and parasitism of cereal aphids showed that trophic interactions in wheat fields varied greatly between years and were associated with the landscape context at different spatial scales. Non-crop area in the complex landscapes appeared to support larger parasitoid populations, but aphids also profited from complex landscapes, thereby counterbalancing possible biological control of aphids by parasitoids. Aphid densities, after colonization at wheat flowering stage, were higher in structurally complex landscapes than in structurally simple landscapes. However, they only increased substantially between wheat flowering and milk ripening in structurally simple landscapes, thereby resulting in no differences in aphid densities between landscapes after aphid reproduction. As high rates of parasitism could be found only in structurally complex landscapes with a relatively small area of annual crop fields, the parasitoid wasps were most likely to have contributed to the suppression of aphid densities in these landscapes. This implication is supported by the overall negative correlation between aphid population growth and parasitism, and by recent field experiments in our landscapes indicating the great importance of naturally occurring cereal aphid parasitoids (Schmidt et al. 2003). A regulation of cereal aphids by parasitoids released into field cages was also found by Levie et al. (2000), and suggested by other studies reporting similar high rates of cereal aphid parasitism (e.g. Sigsgaard 2002). However, our results showed that parasitism strongly decreased with host density, and parasitoids therefore should be expected to successfully control the aphids only in situations of lower aphid densities. The rates of hyperparasitism that we found reached 34%. Hyperparasitoids mainly act later in the season, namely in the period when aphid population densities break down owing to decreasing resource quality. Nevertheless, they may influence primary parasitoid densities in the following year (Sunderland et al. 1997). Several studies suggest that hyperparasitoids reduce the potential control of herbivore populations by primary parasitoids (reviewed by Rosenheim 1998).
Both aphid populations and their parasitoids appeared to have profited from structurally complex landscapes owing to a high availability of perennial habitats providing shelter from disturbances by agricultural practices, overwintering sites, and alternative host. The cereal aphid S. avenae hibernates on perennial grasses (Leather 1993), which represent a dominant plant family in many perennial habitats. M. dirhodum and R. padi are host alternating on Rosa spp. and Prunus padus, respectively, which are common plant species only in structurally rich landscapes. In addition, other aphid species could be expected to act as a reservoir of cereal aphid parasitoids. For example Acyrthosiphum pisum, which is a key aphid on legumes and known to be highly parasitized by Aphidius sp. (Stary 1976, 1978). Therefore, undisturbed perennial areas should support both high aphid densities and large parasitoid populations, whereas high proportions of arable land should disadvantage these organisms. Overwintering sites for parasitoids adjacent to crop fields are known to increase egg parasitism of grape leafhoppers (Corbett & Rosenheim 1996) and larval parasitism of the rape pollen beetle (Thies & Tscharntke 1999). Moreover, our structurally complex landscapes provide more nectar sources for adult parasitoids owing to a larger cover of flowering plants (Steffan-Dewenter et al. 2001), and there are several examples of enhanced parasitism and extended parasitoid lifetimes by augmented nectar resources (Powell 1986; Wäckers & Swaans 1993; Wratten & van Emden 1995; Wäckers 1994; Wäckers & Steppuhn 2003).
Cereal aphid populations are expected to profit from higher temperatures causing higher immigration and reproduction rates, but to suffer from rainfall events resulting in lower survival rates (Triltsch et al. 1998; Gosselke et al. 2001; for other host–parasitoid systems, see Godfray et al. 1994; Kaitaniemi & Ruohomaki 1999; Van Nouhuys & Lei 2004). In accordance with these findings, our results showed mean aphid densities to be higher in 2002 and 2003, years with higher average temperatures, and population development to be lower in 2002, possibly owing to heavy rainfall in June and July 2002 (two events with 38.5 mm and 36.7 mm per day, respectively, in the period of aphid reproduction). The relative role of interannual weather changes (e.g. Palmer & Räisänen 2002; Hansen 2004) for aphid–natural enemy interactions is not yet well known, but changing temperatures and rainfall events might be expected to be important, because they were related to aphid densities (negatively density-dependent parasitism). Weather conditions are also suggested to influence the aphid control by generalist predators early in the season (Chiverton et al. 1986; Lang 2003).
The geographical scale analyses supported the expectation that species experience the spatial scale of landscape complexity differentially. The predictive power of landscape complexity for aphid parasitism was only significant within smaller landscape sectors of 0.5–2 km diameter. This is in support of the hypothesis that parasitoids often show dispersal limitation (Kruess & Tscharntke 1994, 2000; Tscharntke & Kruess 1999; With et al. 1999, but see Van Nouhuys & Hanski 2002b), and could be related to the relatively small body size of the parasitoids, which is a general predictor of how organisms acquire resources in space (Roland & Taylor 1997; Ritchie & Olff 1999). By contrast, the cereal aphids responded to differences in landscape context within landscape sectors up to 6 km. This is in line with expectations from host-alternating species like cereal aphids (but not parasitoids), as such multi-habitat use should be associated with high dispersal abilities. In addition, this supports findings of other studies that ascribed much importance to long-distance dispersal of aphids of tens or even hundreds of kilometres (Taylor 1977; Halber et al. 1990; Riley et al. 1995).
In conclusion, structurally complex landscapes showed the highest rates of aphid parasitism, but they also enhanced aphid populations, thereby counterbalancing possible control of this major pest, and resulting in an overall neutral effect of landscape context. Hence, the often-stressed argument for conservation or recreation of undisturbed perennial habitats in agricultural landscapes may not generally hold for biological control. Further, parasitoid populations responded to changes of landscape context at smaller scales than their aphid hosts, thereby supporting the idea that spatially explicit approaches may provide a perspective to identify ‘functional spatial scales’ at which species experience their environment, and at which species-specific landscape management may be arranged to enhance biological control. The striking differences in aphid abundance and their interactions with parasitoids between years call for more long-term experiments to better understand the relative role of spatial and temporal factors that shape local ecological processes.
Acknowledgments
F. J. J. A. Bianchi, P. Kindlmann, A. Kruess, M. H. Schmidt and T. Rand contributed with discussions, two anonymous reviewers with helpful comments, and C. Bürger, J. Dauber, D. Gabriel, M. Hücker, W. Koehler, J. Morzfeld, T. Purtauf, L. Reimer, U. Visser, K. Wiegand, S. Vidal and V. Wolters with a productive collaboration within the project BIOPLEX (Biodiversity and spatial complexity in agricultural landscapes under global change). Financial support came from the German Ministry for Research and Education (Bundesministerium für Bildung und Forschung) and the German Science Foundation (Deutsche Forschungsgemeinschaft).
References
- Altieri M.A. Agroecology. Westview Press; Boulder, CO: 1995. [Google Scholar]
- Ankersmit G.W. Integrated control of cereal aphids. In: Helle W., editor. World crop pests: aphids. Elsevier; Amsterdam: 1988. pp. 273–278. [Google Scholar]
- Burel F., Baudry J. Farming landscapes and insects. In: Glen D.M., Greaves M.P., Anderson H.P., editors. Ecology and integrated farming systems. Wiley; Chichester, UK: 1995. pp. 203–220. [Google Scholar]
- Cadenasso M.L., Pickett S.T.A. Linking forest edge structure to edge function: mediation to herbivore damage. J. Ecol. 2000;88:31–44. [Google Scholar]
- Chiverton P.A. Predator density manipulation and its effects on the populations of Rhopalosiphum padi (Hom. Aphididae) in spring barley. Ann. Appl. Biol. 1986;109:49–60. [Google Scholar]
- Corbett A., Rosenheim J.A. Impact of natural enemy overwintering refuge and its interaction with the surrounding landscape. Ecol. Entomol. 1996;21:155–164. [Google Scholar]
- Godfray H.C.J., Hassell M.P., Holt R.D. The population-dynamic consequences of phenological asynchrony between parasitoids and their hosts. J. Anim. Ecol. 1994;63:1–10. [Google Scholar]
- Gosselke U., Triltsch H., Rossberg D., Freier B. Getlaus01—the latest version of a model simulating aphid population dynamics in dependence on antagonists in wheat. Ecol. Model. 2001;145:143–157. [Google Scholar]
- Halaj J., Wise D.H. Terrestrial trophic cascades: how much do they trickle? Am. Nat. 2001;157:262–281. doi: 10.1086/319190. [DOI] [PubMed] [Google Scholar]
- Halber S., Connelly J., Sandvol L. Suction trapping of aphids in western North America (emphasis on Idaho) Acta Phytopathol. Hun. 1990;25:411–422. [Google Scholar]
- Hanisch H.C. Einfluss unterschiedlich hoher Stickstoffdüngung zu Weizen auf die Populationsentwicklung von Getreideblattläusen. Z. Pflkrank. PflSchutz. 1980;87:546–556. [Google Scholar]
- Hansen J. Defusing the global warming time bomb. Sci. Am. 2004;290:68–77. doi: 10.1038/scientificamerican0304-68. [DOI] [PubMed] [Google Scholar]
- Hanski I. Metapopulation dynamics. Nature. 1998;396:41–49. [Google Scholar]
- Holland J.M., Thomas S.R. Quantifying the impact of polyphagous invertebrate predators in controlling cereal aphids and in preventing wheat yield and quality reductions. Ann. Appl. Biol. 1997;131:375–397. [Google Scholar]
- Holland J.M., Thomas S.R., Hewitt A. Some effects of polyphagous predators on an outbreak of cereal aphid (Sitobion avenae F.) and orangewheat blossom midge (Sitodoplosis mosellana Gehin) Agric. Ecosyst. Environ. 1996;59:181–190. [Google Scholar]
- Holland J.D., Bert D.G., Fahrig L. Determining the spatial scale of species' response to habitat. BioScience. 2004;54:227–233. [Google Scholar]
- Holt R.D. Food webs in space: an island biogeographic perspective. In: Polis G.A., Winemiller K.O., editors. Food webs—integration of patterns and dynamics. Chapman & Hall; New York: 1996. pp. 313–323. [Google Scholar]
- Honek A. Nitrogen fertilization and abundance of the cereal aphids Metopolophium dirhodum and Sitobion avenae (Homoptera, Aphididae) Z. PflKrank. PflSchutz. 1991;98:655–660. [Google Scholar]
- Kaitaniemi P., Ruohomaki K. Effects of autum temperature and oviposition date on timing of larval development and risk of parasitism in a spring folivore. Oikos. 1999;84:435–442. [Google Scholar]
- Kareiva P. Population dynamics in spatially complex environments: theory and data. Phil. Trans. R. Soc. B. 1990;330:175–190. [Google Scholar]
- Kareiva P., Wennergren U. Connecting landscape pattern to ecosystem and population processes. Nature. 1995;373:299–302. [Google Scholar]
- Kindlmann P., Dixon A.F.G. When and why top-down regulation fails in arthropod predator-prey systems. Basic Appl. Ecol. 2001;2:333–340. [Google Scholar]
- Kruess A., Tscharntke T. Habitat fragmentation, species loss, and biological control. Science. 1994;264:1581–1584. doi: 10.1126/science.264.5165.1581. [DOI] [PubMed] [Google Scholar]
- Kruess A., Tscharntke T. Species richness and parasitism in a fragmented landscape: experiments and field studies with insects on Vicia sepium. Oecologia. 2000;122:129–137. doi: 10.1007/PL00008829. [DOI] [PubMed] [Google Scholar]
- Lang A. Intraguild interferences and biocontrol effects of generalist predators in a winter wheat field. Oecologia. 2003;134:144–153. doi: 10.1007/s00442-002-1091-5. [DOI] [PubMed] [Google Scholar]
- Leather S.R. Overwintering in six arable aphid pests: a review with particular relevance to pest management. J. Appl. Entomol. 1993;116:217–233. [Google Scholar]
- Legendre P., Legendre L. Numerical ecology. Elsevier; Amsterdam: 1998. [Google Scholar]
- Levie A., Dogot P., Hance T. Release of Aphidius rhopalosiphi (Hymenoptera: Aphidiinae) for cereal aphid control: field cage experiments. Eur. J. Entomol. 2000;97:527–531. [Google Scholar]
- Matson P.A., Parton W.J., Power A.G., Swift M.J. Agricultural intensification and ecosystem properties. Science. 1997;277:504–509. doi: 10.1126/science.277.5325.504. [DOI] [PubMed] [Google Scholar]
- Menalled F.D., Marino P.C., Gage S.H., Landis D.A. Does agricultural landscape structure affect parasitism and parasitoid diversity? Ecol. Applic. 1999;9:634–641. [Google Scholar]
- Menalled F.D., Costamagna A.C., Marino P.C., Landis D.A. Temporal variation in the response of parasitoids to agricultural landscape structure. Agric. Ecosyst. Environ. 2003;96:29–35. [Google Scholar]
- Östman Ö., Ekbom B., Bengtsson J. Farming practice and landscape heterogeneity influence biological control. Basic Appl. Ecol. 2001;2:365–371. [Google Scholar]
- Palmer T.N., Räisänen J. Quantifying the risk of extreme seasonal precipitation events in a changing climate. Nature. 2002;415:512–514. doi: 10.1038/415512a. [DOI] [PubMed] [Google Scholar]
- Pickett S.T.A., Cadenasso M.L. Landscape ecology: spatial heterogeneity in ecological systems. Science. 1995;269:331–334. doi: 10.1126/science.269.5222.331. [DOI] [PubMed] [Google Scholar]
- Pimm S.L. The balance of nature. The University of Chicago Press; 1991. [Google Scholar]
- Powell W. Enhancing parasitoid activity in crops. In: Waage J., Greathead W., editors. Insect parasitoids. Academic; London: 1986. pp. 319–340. [Google Scholar]
- Ricketts T.H. The matrix matters: effective isolation in fragmented landscapes. Am. Nat. 2001;158:87–99. doi: 10.1086/320863. [DOI] [PubMed] [Google Scholar]
- Riley J.R., Reynolds D.R., Mukhopadhyay S., Ghosh M.R., Sarkar T.K. Long-distance migration of aphids and other small insects in northeast India. Eur. J. Entomol. 1995;92:639–653. [Google Scholar]
- Ritchie M.E., Olff H. Spatial scaling laws yield a synthetic theory of biodiversity. Nature. 1999;400:557–560. doi: 10.1038/23010. [DOI] [PubMed] [Google Scholar]
- Roland J., Taylor P.D. Insect parasitoid species respond to forest structure at different spatial scales. Nature. 1997;386:710–713. [Google Scholar]
- Rosenheim J.A. Higher-order predators and the regulation of insect herbivore populations. A. Rev. Entomol. 1998;43:421–447. doi: 10.1146/annurev.ento.43.1.421. [DOI] [PubMed] [Google Scholar]
- Rosenzweig M.L. Species diversity in time and space. Cambridge University Press; 1995. [Google Scholar]
- Schmidt M.H., Lauer A., Purtauf T., Thies C., Schaefer M., Tscharntke T. Relative importance of predators and parasitoids for cereal aphid control. Proc. R. Soc. B. 2003;270:1905–1909. doi: 10.1098/rspb.2003.2469. doi:10.1098/rspb.2003.2469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigsgaard L. A survey of aphids and aphid parasitoids in cereal fields in Denmark, and the parasitoids' role in biological control. J. Appl. Entomol. 2002;126:101–107. [Google Scholar]
- Sokal R.R., Rohlf F.J. Biometry. 3rd edn. Freeman; New York: 1995. [Google Scholar]
- Stary P. Parasite spectrum and relative abundance of parasites of cereal aphids in Czechoslovakia (Hymenoptera, Aphidiidae; Homoptera, Aphidoidea) Acta Ent. Bohemoslov. 1976;73:216–223. [Google Scholar]
- Stary P. Seasonal relations between lucerne, red clover, wheat and barley agro-ecosystems through the aphids and parasitoids (Homoptera, Aphididae; Hymenoptera, Aphiddiidae) Acta Ent. Bohemoslov. 1978;75:296–311. [Google Scholar]
- Steffan-Dewenter I., Münzenberg U., Tscharntke T. Pollination, seed set and seed predation on a landscape scale. Proc. R. Soc. B. 2001;268:1685–1690. doi: 10.1098/rspb.2001.1737. doi:10.1098/rspb.2001.1737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffan-Dewenter I., Münzenberg U., Bürger C., Thies C., Tscharntke T. Scale-dependent effects of landscape structure on three pollinator guilds. Ecology. 2002;83:1421–1432. [Google Scholar]
- Sunderland K.D. Pest control by a community of natural enemies. Acta Jutland. 1997;72:271–326. (and 11 others) [Google Scholar]
- Taylor L.R. Migration and spatial dynamics of an aphid, Myzus persicae. J. Anim. Ecol. 1977;46:411–423. [Google Scholar]
- Thies C., Tscharntke T. Landscape structure and biological control in agroecosystems. Science. 1999;285:893–895. doi: 10.1126/science.285.5429.893. [DOI] [PubMed] [Google Scholar]
- Thies C., Steffan-Dewenter I., Tscharntke T. Effects of landscape context on herbivory and parasitism at different spatial scales. Oikos. 2003;101:18–25. [Google Scholar]
- Tilman D., Cassman K.G., Matson P.A., Naylor R., Polaski S. Agricultural sustainability and intensive production practices. Nature. 2002;418:671–677. doi: 10.1038/nature01014. [DOI] [PubMed] [Google Scholar]
- Tischendorf L., Fahrig L. On the usage and measurement of landscape connectivity. Oikos. 2000;90:7–19. [Google Scholar]
- Triltsch H., Gosselke U., Freier B., Rossberg D. Zum Phänomen der schnelleren Entwicklung von Insekten bei Wechseltemperaturen. Mitt. Biol. Bundesanstalt. 1998;357:1–239. [Google Scholar]
- Tscharntke T. Parasitoid populations in the agricultural landscape. In: Hochberg M.E., Ives A.R., editors. Parasitoid population biology. Princeton University Press; Princeton, NJ: 2000. pp. 235–253. [Google Scholar]
- Tscharntke T., Brandl R. Plant-insect interactions in fragmented landscapes. A. Rev. Entomol. 2004;49:405–430. doi: 10.1146/annurev.ento.49.061802.123339. [DOI] [PubMed] [Google Scholar]
- Tscharntke T., Kruess A. Habitat fragmentation and biological control. In: Hawkins B.A., Cornell H.V., editors. Theoretical approaches to biological control. Cambridge University Press; 1999. pp. 190–205. [Google Scholar]
- Tscharntke T., Steffan-Dewenter I., Kruess A., Thies C. Contribution of small habitat fragments to conservation of insect communities of grassland–cropland landscapes. Ecol. Appl. 2002;12:354–363. [Google Scholar]
- Turner M., Gardner R.H., O'Neill R.V. Landscape ecology in theory and practice. Springer; New York: 2001. [Google Scholar]
- Van Driesche R.G., Bellows T.S. Biological control. Chapman & Hall; New York: 1996. [Google Scholar]
- Van Nouhuys S., Hanski I. Multitrophic interactions in space: metacommunity dynamics in fragmented landscapes. In: Tscharntke T., Hawkins B.A., editors. Multitrophic level interactions. Cambridge University Press; 2002a. pp. 127–147. [Google Scholar]
- Van Nouhuys S., Hanski I. Colonization rates and distances of a host butterfly and two specific parasitoids in a fragmented landscape. J. Anim. Ecol. 2002b;71:639–650. [Google Scholar]
- Van Nouhuys S., Lei G.C. Parasitoid-host metapopulation dynamics: the causes and consequences of phenological asynchrony. J. Anim. Ecol. 2004;73:526–535. [Google Scholar]
- Wäckers F.L. The effect of food deprivation on the innate visual and olfactory preferences in the parasitoid Cotesia rubecula. J. Insect Physiol. 1994;40:641–649. [Google Scholar]
- Wäckers F.L., Steppuhn A. Characterizing nutritional state and food sources use of parasitoids collected in fields with high and low nectar availability. IOBC wprs Bull. 2003;26:209–214. [Google Scholar]
- Wäckers F.L., Swaans C.P.M. Finding floral nectar and honeydew in Cotesia rubecula: random or direct? Proc. Exp. Appl. Entomol. 1993;4:67–72. [Google Scholar]
- Wiegand T., Molony K.A., Naves J., Knauer F. Finding the missing link between landscape structure and population dynamics: a spatially explicit perspective. Am. Nat. 1999;154:605–627. doi: 10.1086/303272. [DOI] [PubMed] [Google Scholar]
- Wiens J.A., Stenseth N.C., van Horne B., Ims R.A. Ecological mechanisms and landscape ecology. Oikos. 1993;66:369–380. [Google Scholar]
- Wiens J.A., Schooley R.L., Weeks R.D. Patchy landscapes and animal movements: do beetles percolate? Oikos. 1997;78:257–264. [Google Scholar]
- With K.A., Cadaret S.J, Davis C. Movement responses to patch structure in experimental fractal landscapes. Ecology. 1999;80:1340–1353. [Google Scholar]
- Wratten S.D., Powell W. Cereal aphids and their natural enemies. In: Firbank I.G., Carter N., Darbyshire J.F., Potts G.R., editors. The ecology of temperate cereal fields. Blackwell Scientific; Oxford: 1991. pp. 233–257. [Google Scholar]
- Wratten S.D., van Emden H.F. Habitat management for enhanced activity of natural enemies of insect pests. In: Glen D.M., Greaves M.P., Anderson H.M., editors. Ecology and integrated farming systems. Wiley; Chichester, UK: 1995. pp. 117–145. [Google Scholar]