Abstract
Female mate choice has often been proposed to play an important role in cases of rapid speciation, in particular in the explosively evolved haplochromine cichlid species flocks of the Great Lakes of East Africa. Little, if anything, is known in cichlid radiations about the heritability of female mating preferences. Entirely sympatric distribution, large ecological overlap and conspicuous differences in male nuptial coloration, and female preferences for these, make the sister species Pundamilia pundamilia and P. nyererei from Lake Victoria an ideally suited species pair to test assumptions on the genetics of mating preferences made in models of sympatric speciation. Female mate choice is necessary and sufficient to maintain reproductive isolation between these species, and it is perhaps not unlikely therefore, that female mate choice has been important during speciation. A prerequisite for this, which had remained untested in African cichlid fish, is that variation in female mating preferences is heritable. We investigated mating preferences of females of these sister species and their hybrids to test this assumption of most sympatric speciation models, and to further test the assumption of some models of sympatric speciation by sexual selection that female preference is a single-gene trait. We find that the differences in female mating preferences between the sister species are heritable, possibly with quite high heritabilities, and that few but probably more than one genetic loci contribute to this behavioural speciation trait with no apparent dominance. We discuss these results in the light of speciation models and the debate about the explosive radiation of cichlid fishes in Lake Victoria.
Keywords: cichlid fish, genetics of reproductive isolation, female mating preference, Lake Victoria, sexual selection, sympatric speciation
1. Introduction
Female mate choice can be a powerful force of selection on male secondary sexual characteristics in animals (Andersson 1994), and a potential agent of rapid population differentiation and speciation (Fisher 1930; West Eberhard 1979; Lande 1981b, 1982). Several theoretical models suggest that, if disruptive, selection exerted by female choice could cause speciation in the absence of geographical isolation (Lande 1982; Wu 1985; Turner & Burrows 1995; Payne & Krakauer 1997; van Doorn et al. 1998, 2004; Higashi et al. 1999; Kawata & Yoshimura 2000; Takimoto et al. 2000; Takimoto 2002; van Doorn et al. 2004). The conditions for this to happen in complete sympatry are stringent, although speciation by sexual selection along a cline is less controversial (Endler 1977; Lande 1982; Kirkpatrick & Ravigné 2002).
A textbook case of multiple and rapid speciation in which female mate choice has been invoked, is the explosive adaptive radiation of haplochromine cichlids in Lake Victoria. More than 500 species appear to have evolved from very few ancestral species within perhaps as little as 15 000 years (Johnson et al. 1996, 2000; but see 2004). Sexual selection has been proposed as a major player in this rapid diversification, as well as in the radiation of haplochromine cichlids in Lake Malawi (Dominey 1984; McKaye 1991; Seehausen et al. 1997; Galis & Metz 1998; Turner 1998; Kornfield & Smith 2000; Kocher 2004). Closely related sympatric species in these species flocks are often ecologically very similar (Bouton et al. 1997, 1998; Seehausen & Bouton 1997; Genner et al. 1999) but differ strongly in male nuptial coloration (Albertson et al. 1999; Seehausen & van Alphen 1999; Smith & Kornfield 2002; Allender et al. 2003). A previously studied example is the sympatric sibling species pair Pundamilia nyererei and P. pundamilia from Lake Victoria. These completely sympatric species are at most localities reproductively isolated by female mate choice (Seehausen 1997; Seehausen & van Alphen 1998). Given that the geographical range of P. nyererei is completely nested within the larger range of P. pundamilia (Seehausen & van Alphen 1999), and that female mate choice is sufficient and necessary for behavioural reproductive isolation between them (Seehausen & van Alphen 1998), this pair would seem one of the best candidate cases for sympatric speciation through divergent mate choice.
Several modelling approaches suggest that the likelihood of sympatric speciation through divergent mate choice critically depends on the genetic architecture of female mating preferences. In models that start from a mono morphic condition to which a new preference mutant and a matching male trait mutant are introduced at low frequencies, the likelihood of sympatric speciation by divergent mating preferences decreases as the number of loci controlling female mating preference increases above one (Lande 1982; Arnegard & Kondrashov 2004). In fact, models of speciation where female choice is the only source of disruptive selection in complete sympatry, assume an oligogenic nature of female mating preferences: one (Turner & Burrows 1995; Takimoto 2002) or two loci (Payne & Krakauer 1997).
Sister species pairs of Lake Victoria and Lake Malawi haplochromines often consist of a species with yellow-red and one with blue male nuptial dress (Seehausen 1996; Seehausen et al. 1997; Smith & Kornfield 2002; Allender et al. 2003). We investigated the genetic basis of female mating preferences for red and blue male nuptial coloration in the sibling species pair P. nyererei (red) and P. pundamilia (blue). We studied mate preferences of F1 and F2 hybrid females as well as parental type females to test the hypotheses that female mate preference is (i) heritable and (ii) determined by a single genetic locus. Although reproductive isolation in the wild is not complete in the population that we studied (Seehausen 1997), behavioural mate choice of females of both species is species assortative when they can see the male nuptial coloration but not when the differences among the latter are masked by monochromatic light (Seehausen & van Alphen 1998). Similarly, random mating in turbid water, but assortative mating in clear water, had been inferred from phenotype frequency distributions in nature (Seehausen et al. 1997). If female mating preferences are heritable, we expect the first hybrid generation females to express relatively uniform mating preferences, whereas preferences should segregate again among second hybrid generation females. This is the first attempt, to our knowledge, to characterize the genetic basis of divergent female mating preferences in the African radiations of cichlid fish. Given the nested geographical distribution, non-zero gene flow, the divergent female preferences and their effect on male colour variation in clear waters, the species pair that we use is ideally suited for testing genetic assumptions of models of sympatric speciation through divergent mating preferences.
2. Material and methods
2.1 The species and their behaviour
Males and females of P. pundamilia and P. nyererei are morphologically highly similar but P. nyererei males have a red dorsum and yellow flanks, whereas those of P. pundamilia are grey/blue. The females can only be distinguished with considerable experience. Courtship behaviour resembles that of other Lake Victoria haplochromines. For a description of the courtship behaviour, see (Seehausen & van Alphen (1998), with photographs and drawings in (Seehausen (1996). We recorded the following elements of male courtship behaviour: ‘lateral display ’ (LD), ‘quiver’ (QU) and ‘lead swim’ (LE). Each courtship bout began either with an ‘approach’ (AP)–LD sequence or directly with LD. Recorded elements of female courtship, i.e. positive response to male courtship, were AP (upon male courtship) and ‘follow’ (FO, upon male lead swimming).
All males and non-hybrid females were the same laboratory stock used to demonstrate species-assortative female mating preferences earlier (Seehausen& van Alphen 1998), derived from the Python Islands in the Mwanza Gulf of Lake Victoria, except four stimulus red males that were caught from Kissenda Island, also in the Mwanza Gulf. The populations from Python and Kissenda Islands are phenotypically indistinguishable. Hybrids can be easily bred in aquaria by keeping females of one species with males of the other one. They are fully viable and fertile through several generations (Seehausen et al. 1997). F1 hybrids were bred from P. nyererei males (red) and P. pundamilia females (blue) and vice versa. F2 hybrids were bred from randomly chosen F1 hybrids.
2.2 Housing conditions and experimental set-up
Experiments were conducted in two series, one in 2000 (series 1) and the other in 2001 –2002 (series 2). Before the experiments, males and females were kept separately in a large recirculation aquarium system, maintained at 24–26°C, on a 12L:12D cycle. The tanks were illuminated with 40 W daylight fluorescent tubes. Experimental tanks measured 200 cm×50 cm×40 cm, filled up with sand to a depth of 3 cm. At each end of the tank, a cave was built from three to five rocks. One red male was placed at one end of the tank and one blue male at the other end. The caves were dark inside but open to the observer, who could see into them. In the middle of the tank was an additional smaller cave for the female to allow her to settle in quickly when introduced into the experimental tank.
The F2 females had no previous mating experience. They were raised in mono-sex groups from the moment that the sexes became distinguishable. F1 females had prior mating experience exclusively with F1 males. All experiments were free-contact experiments (Turner et al. 2001). Males were introduced several days, and females 5 min before, a trial was started. Males were separated from each other and from a large central section of the tank by plastic grids. This was so that males could not interact with each other. In the first experiment series (2000), the grids were removed for the duration of a trial. In the second series (2001 and 2002), we left them in place during the trials and adjusted the mesh size of the grids such that the females could easily move through but the males were retained in their sections. Because the males had much deeper bodies, this was easily achieved. We tried to match red and blue males by size (standard length) and mass. Only females with ripe ovaries were chosen for trials. Gravidity was determined by swelling of abdomen and urogenital opening and was scored on a 5-point scale (Seehausen & van Alphen 1998). Only females with a minimum score of 3–4 were used.
2.3 Determination of female mating preferences
After a pair of males had established itself and begun to show nuptial coloration and display territorial behaviour, every female within one of the two experiment series was tested with that pair. Each trial lasted 15 min. Such a trial series was completed when every female had been successfully tested with the same male pair. Then the males were exchanged against a new pair. Trials with the new male pair were started as soon as the males showed nuptial coloration and territorial behaviour around their caves. Fifty females were tested with six, 15 with five, and one with four different male pairs. A trial was successful and used for statistical analysis if the following minimum requirements were fulfilled: five or more encounters of the female with each male, plus two or more lateral displays of each male to the female, plus one or more response by the female to at least one male within 15 min. If these minimum requirements were not met, the trial was discarded and repeated at a later stage. If a female hid continuously for 5 min or longer, the trial was extended for 5 min to keep the length of the observed female–male interaction period at ca. 15 min. A total of 379 successful trials were conducted. The frequency of female–male encounters with male courtship display (LD) was quantified as a proportion of all female–male encounters. Female mating preference was measured as the proportion of courtship events of the red male that elicited a positive female response minus the proportion of courtship events of the blue male that elicited a positive female response. We refer to this as a preference index.
2.4 Testing whether female preferences are heritable and biometric estimation of number of genes
If female mating preferences are heritable, and assuming the parental lines were highly homozygous for alternative alleles at their preference loci, the F1 hybrids should be genetically uniform and preference variation observed among them would be environmentally induced or measurement error. As preference genes would segregate in the F2 hybrids, genetic variance adds to the environmental or measurement variance. None of these predictions would be satisfied if preference were not heritable. We used the Castle–Wright estimator (Castle 1921; Wright 1968; Lande 1981a) to biometrically estimate the minimum number of genes contributing to the difference in female mating preference between the species. After subtraction of the variance observed among F1 hybrids and parental lines, the remaining variance in the F2 is a conservative estimate of the heritable component of variance. From the amplitude between the means of the parental lines and the genetic variance (segregating variance, σS) in the F2 hybrids, the number of unlinked genes (= minimum number of genes) controlling the measured trait can be inferred (Lande 1981a).
| 1 |
| 2 |
where nE is the estimate for the minimum number of genes, Δ is the difference between the means of the parental lines and and are the variances of the second and first hybrid generations, respectively, and and are the variances of the two parental lines.
2.5 Data analysis
If males courted conspecific females preferentially, this could influence female mate choice. For males of each species separately, we therefore compared the mean proportions of encounters with conspecific and heterospecific females in which the male courted, using a Wilcoxon matched-pairs test, and we compared the mean between the two species by a Mann–Whitney U-test. We further asked whether individual females received more courtship displays from conspecific than from heterospecific males, by comparing for each female the proportion of encounters with red and blue males in which she was courted, with a Wilcoxon matched-pairs test (pairs are males in the same trial).
We measured a female’s preference as the difference in her responsiveness to courtship from the red male and the blue male. We used the Wilcoxon matched-pairs test to determine for each female whether the raw data suggested a significant preference. To control for effects of variation in male courtship activity, we calculated residual preferences, obtained by regressing female preference against the difference in courtship activity between red and blue males. We then used a one-sample t-test on the residual preference to determine for each female whether a preference remained significant after variation in male courtship activity was controlled for. These residual preferences were used for all further analysis. To compare the variances in the two hybrid generations, we used an F-test. To test for differences between groups of females (e.g. P. nyererei versus P. pundamilia), we used a Mann–Whitney U-test on mean preferences of females calculated over all trials with a female. To combine the 2000 and 2001–2002 datasets Fisher’s combined p and Bonferroni corrections were used to correct for multiple testing.
We calculated repeatability of interspecific female mating preferences using the parental type females, as R=σW2/(σW2 + σE2), where σW2 is the variance between individuals and σE2 is the variance within an individual (Becker 1992). All tests were done using SPSS 10.0 and Minitab release 13.
3. Results
3.1 Males court females of both species equally
Each of 22 males was tested with three or four females of each species. Males of both species exhibited no bias in courtship effort directed to females of either species (P. nyererei males: Z=−1.067, n=11, p=0.286; P. pundamilia males: Z=−0.178, n=11, p=0.859), and there was no difference between males of the two species in their direction of courtship effort towards females of the two species (n1=11, n2=11, U=59, p=0.949). One of 16 females (P. nyererei female R10) received significantly more courtship from conspecific males (Wilcoxon matched-pairs test: n=6, Z=−2.201, p=0.028; all others p≥0.13), none received significantly more courtship from heterospecific males.
3.2 Female mating preferences in Pundamilia nyererei and P. pundamilia
3.2.1 Repeatability of preference measurements
Repeatabilities of individual female mating preferences in the parental lines were R=0.78 in experiment series 1 (n=8), and R=0.50 in series 2 (n=8), suggesting a maximum for possible heritabilities between 50% and 78%. Pooled over both series we obtained a repeatability of R=0.59 (n=16).
3.2.2 Female mating preferences differ between the sibling species
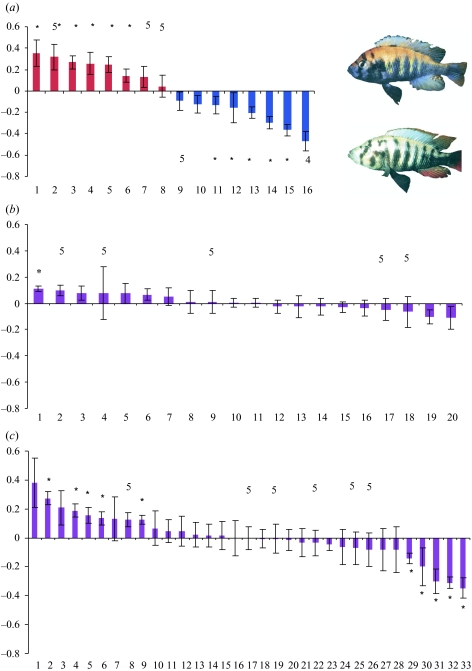
Females of P. nyererei and P. pundamilia exhibited highly significantly different mating preferences (n1=8, n2=8, U=0, p=0.001; figure 1a), consistent with results of earlier investigations that used the same laboratory stock derived from the same populations (Seehausen 1997; Seehausen et al. 1997; Seehausen & van Alphen 1998).
Figure 1.
Mean preference indices and standard errors of all females tested. *Significant preferences (p < 0.05). Each female was tested with six different male pairs unless when indicated otherwise above the bars. (a) Parental-type female preferences, and one example of each of the two male colour phenotypes; (b) F1 hybrid female preferences; (c) F2 hybrid female preferences.
3.3 Hybrid female mating preferences
Only 1 out of 20 F1 hybrid females (as expected by chance at α=0.05) exhibited a significant preference (one-sample t-test, p=0.002; figure 1b). None of the other 19 exhibited mating preferences (p≥0.1). The mean preference of the F1 hybrids was intermediate to those of the two parental species, and the variance among F1 females was small and also intermediate to those among the two parental species (table 1). Nine out of 33 F2 hybrid females (seven more than expected by chance at α=0.05) exhibited significant preferences (figure 1c; one-sample t-test, p≤0.05). The mean preference of F2 females was similar to that of F1 females, but the variance was significantly higher in F2 females (F-test: experiment series 1: n1=15, n2=11, F=0.068, p < 0.001; experiment series 2: n1=5, n2=22, F=0.625, p=0.701; combined dataset: n1=20, n2=33, F=0.167, p < 0.001; table 2).
Table 1.
Means and variance of residual mating preference for the four female genotype classes.
| experiment 2000 |
experiment 2001–2002 |
2000 and 2001–2002 pooled |
|||||||
| female genotype classes | n | mean | variance | n | mean | variance | n | mean | variance |
| red | 4 | 0.2985 | 0.0021 | 4 | 0.1413 | 0.0071 | 8 | 0.2199 | 0.0109 |
| blue | 4 | −0.1689 | 0.0169 | 4 | −0.1398 | 0.0021 | 8 | −0.2321 | 0.0178 |
| F1 hybrids | 15 | 0.0031 | 0.0041 | 5 | 0.0140 | 0.0052 | 20 | 0.0058 | 0.0041 |
| F2 hybrids | 11 | <0.0001 | 0.0627 | 22 | 0.0003 | 0.0076 | 33 | 0.0030 | 0.0250 |
Table 2.
Results of Mann–Whitney U-tests comparing the different female genotype classes.
| experiment 2000 | n1, n2 | Z | p | experiment 2001–2002 | n1, n2 | Z | p |
| red versus blue | 4, 4 | −2.309 | 0.029 | red versus blue | 4, 4 | −2.309 | 0.029 |
| F1 versus red | 15, 4 | −3 | 0.003 | F1 versus red | 5, 4 | −1.960 | 0.050 |
| F1 versus blue | 15, 4 | −3 | 0.003 | F1 versus blue | 5, 4 | −2.449 | 0.014 |
| F2 versus red | 11, 4 | −3 | 0.003 | F2 versus red | 22, 4 | −2.416 | 0.016 |
| F2 versus blue | 11, 4 | −3 | 0.003 | F2 versus blue | 22, 4 | −2.914 | 0.004 |
| F2 versus F1 |
11, 15 |
0 |
1 |
F2 versus F1 |
22, 5 |
−0.375 |
0.708 |
| experiment 2000 and 2001–2002 pooled |
n1, n2 |
Fisher’s combined p |
Bonferroni corrected p |
||||
| red versus blue | 8, 8 | 0.00512 | 0.01024 | ||||
| F1 versus red | 8, 20 | 0.00055 | 0.00218 | ||||
| F1 versus blue | 8, 20 | 0.00017 | 0.00102 | ||||
| F2 versus red | 8, 33 | 0.00156 | 0.00468 | ||||
| F2 versus blue | 8, 33 | 0.00045 | 0.00223 | ||||
| F2 versus F1 | 20, 33 | 0.83491 | 0.83491 | ||||
3.3.1 Relationship between strength and consistency of female preference
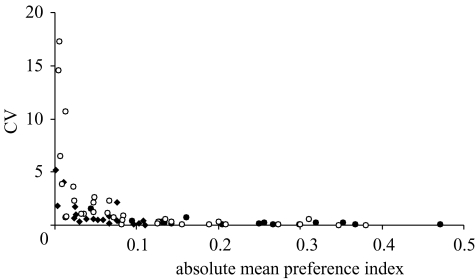
We calculated the coefficient of variation (CV) by dividing the variance by the mean, to compare the variances in the different groups of females (i.e. parental-type, F1 and F2 hybrids). A strong correlation emerged between the sign-removed mean and its CV (figure 3). The weaker the mean preference of a female for either red or blue males, the larger is the variation in her response measured over different male pairs. The CV for F2 hybrid females is greater than that for F1 hybrid females and both parental types, confirming greater variance in the F2 hybrid females (mean CVF2=2.33; mean CVF1=1.07; mean CVnyererei=0.38; mean CVpundamilia=0.25).
Figure 3.
Relationship between strength and consistency of female mating preference: absolute mean preference index and its coefficient of variation (CV) for all females (16 parental type, filled circles; 20 F1 hybrids, filled diamonds; and 33 F2 hybrids, open circles). A high CV reflects lack of consistency in relative response to red and blue males; a high absolute mean preference index reflects a strong mean mating preference.
3.3.2 Inheritance of female mating preference
The mating preferences of females in both hybrid generations are intermediate to those in females of the parental populations, and the variance in the second hybrid generation is greater than in parental females and in the first hybrid generation (figures 1 and 2). This, and the relationship between strength and consistency of preferences, are strong evidence for heritability of the interspecific difference in female mating preferences.
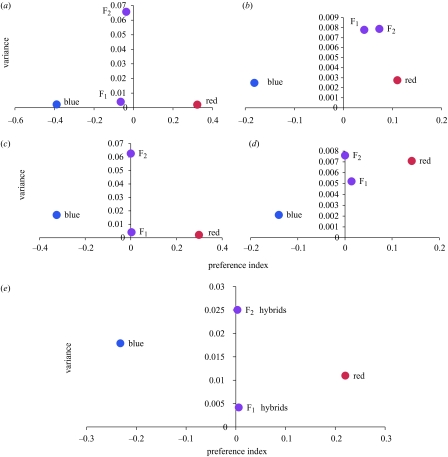
Figure 2.
Mean preference index (x-axis) and variance (y-axis) for parental type, F1 hybrid and F2 hybrid females. (a) Experiment series 1: raw preferences; (b) experiment series 2: raw preferences; (c) series 1: residual preferences; (d) series 2: residual preferences; (e) both experiment series combined: residual preferences.
3.3.3 Estimation of minimum number of genes
The minimum number of genes contributing to the difference in female preference between P. nyererei and P. pundamilia for red versus blue males was calculated from all females pooled, as well as for the two experiment series separately. Using the residual preferences, the estimates are one gene (series 1) and four genes (series 2). With raw data, not corrected for effects of variation in male courtship, we obtained one and five genes, respectively. To pool the data from the two experiment series that differed in the amplitude of observed between-female variation in preference, we calculated, using the raw preferences, for each series the ratio (Q) as
| 3 |
where DR−B is the difference between the mean preferences of the two parental species, and DF2,max−min is the difference between the highest and the lowest preferences of F2 hybrid females. Series 1 yielded DR–B=0.80, DF2,max−min=1.08 and a ratio Q=0.735; series 2 yielded DR−B=0.29, DF2,max−min=0.40 and Q=0.747. Given that the ratios were essentially identical, we calculated an adjustment factor (F) as the ratio of DR−B of the two series, i.e. F=0.29/0.80=0.36. We then calculated adjusted preferences for the females of series 1 by multiplying their mean preference indices with F. The pooled data using all 16 parental, 20 F1 and 33 F2 hybrid females then yielded an estimate of 1.6 for the minimum number of genes.
4. Discussion
Our data strongly suggest that the difference in female mating preferences for different male nuptial dress between the sympatric closely related, and occasionally hybridizing, cichlid species Pundamilia pundamilia and P. nyererei is heritable. We hybridized laboratory lines of these species that exhibited significantly different preferences. The mean preference of females in the first and second hybrid generations was intermediate to those of females of the two parental species. The means of F1 and F2 hybrid generations were not significantly different from each other, but the variances differed, with the F2 generation significantly more variable than the F1 generation. Hence, female preference behaved as expected from a heritable trait. High repeatabilities of parental type preferences are consistent with potentially high heritabilities for the interspecific difference in preferences (Falconer & Mackay 1996).
Males did not direct courtship effort preferentially to either conspecific or heterospecific females. Therefore, we conclude that (i) males did not differentiate between females of the two species and (ii) preferences of P. pundamilia and P. nyererei females cannot be explained by differential male courtship effort, consistent with an earlier study (Seehausen & van Alphen 1998). In experiments of series 1, hybrid females of both generations tended to prefer blue males, whereas hybrid females in the experiments of series 2 tended to prefer red males (figure 2a,b). However, this was largely explained by differences between red and blue males in their mean courtship effort. When differences in male courtship effort were controlled, the means of the female preference indices for both hybrid generations and both experiment series converged on zero (= no preference), whereas those of parental type females were little affected (figure 2c,d).
To obtain accurate estimates of minimum numbers of genes determining a trait difference, Lande (Seehausen (1981a) estimated that a minimum of 20 F1 and 100 F2 hybrid phenotypes are required. Our sample sizes are small with 20 F1 and only 33 F2 phenotypes. Estimates from small sample sizes are likely to vary greatly around the true minimum number of genes. Nevertheless, we feel that it is justified to conclude from our data that the minimum number of genes contributing to the difference in female mating preferences for red versus blue males in this sympatric species pair is small. Our estimates suggest between one and five unlinked loci are involved. The biometrical method tends to underestimate the true number of loci if the assumptions of unlinked loci and equal allelic effects at all loci are violated (Zeng et al. 1990). Hence, it seems likely that the true number of genes in our case is larger than 1.
Our data suggest that preference alleles are largely additive, although the possibility of dominance on some preference loci that could cancel each other out cannot be discarded. Our data further highlight the danger of arriving at the wrong conclusions if male behaviour is not controlled for when female preferences are measured. This is especially obvious in heterozygous preference genotypes that have no strong preference for specific male traits of either species and are hence a lot more affected by random variation in male courtship behaviour (figure 2).
Colour vision is important in cichlid communication (Evans & Norris 1996; Seehausen et al. 1997; Carleton et al. 2004 but simultaneously also plays an important role in food selection (Van der Meer& Bowmaker 1995). Polygenic determination may be expected if multiple selection forces operate on a trait. It is perceivable that a switch in visual colour preference can be achieved with moderate modifications to the visual system, for example through up- or down-regulation of opsin gene expression (Carleton & Kocher 2001). However, mate preferences could also be determined at higher levels of information processing, making it difficult to predict whether species differences should be polygenic or oligogenic. Two studies on genetics of female mating preference in Drosophila found a small number of loci determining mating preference three (loci in Ting et al. (2001), and one locus or one cluster of loci in (Doi et al. (2001)). (Ritchie(2000) found that the genetic control of mate choice in the bushcricket Ephippiger ephippiger could be explained by a simple polygenic additive model. Certainly, the Drosophila results compare favourably with ours.
Given the geographical distribution of the sister species P. nyererei and P. pundamilia, their ecological overlap and only very slight morphological differentiation, but conspicuously different male nuptial colours and female preferences for these, it had been hypothesized that this species pair diverged sympatrically (Seehausen 1997), possibly through disruptive sexual selection (Seehausen & van Alphen 1998). Speciation under disruptive selection on the mating system can potentially be faster than allopatric speciation, where reproductive isolation would have to evolve as a by-product of drift, natural or sexual selection in different habitats (Coyne 1992; Coyne & Orr 1998). Mathematical and simulation models of sympatric speciation, driven by disruptive sexual selection alone, where divergent mating preferences are not reinforced by natural selection against intermediates, fall largely into two categories in terms of the assumptions and conclusions about the genetics of female mating preferences. First, models that begin from monomorphic conditions and introduce both the alternative (novel) mating preference and mating trait as rare alleles, tend to require that mating preferences are determined by just one or two genes (Wu 1985; Turner & Burrows 1995; Payne & Krakauer 1997; Takimoto 2002; Arnegard & Kondrashov 2004). Although our estimate of between one and five genes is a minimum estimate, we cannot rule out that this is the case in Pundamilia, one of the most species-rich genera of Lake Victoria cichlids with one of the highest sympatry indices among East African cichlid species complexes (Genner et al. 2004).
Second, in models of speciation from polymorphic populations that segregate for alternative preference and trait alleles at similar and high frequencies, the number of loci has no inhibiting effect on sympatric speciation by disruptive Fisherian runaway selection (Higashi et al. 1999; Takimoto et al. 2000; Kawata & Yoshimura 2000). Such models have been criticized for their lack of biological plausibility. Although this may often be true, these models may not be completely unrealistic for cases such as the explosive radiation of Lake Victoria cichlids. Conditions similar to those simulated in these models may arise if either preference and trait mutations, before being exposed to disruptive selection, accumulated in an environment in which they were selectively neutral (for example, in turbid water where colour vision is impaired), or if interspecific hybridization injects female preference and male trait genes into a population at high frequencies. Populations of P. nyererei and P. pundamilia hybridize in turbid water (Seehausen 1997; Seehausen et al. 1997) and the single polymorphic populations of Pundamilia found in very turbid water, exhibit broad variation in male nuptial colour (Seehausen et al. 1997) and female mating preferences (O. Seehausen, unpublished data). Similar conditions are likely to have affected other Lake Victoria cichlids in the past. In fact, against the background of far reaching climatic fluctuations within the Holocene and Late Pleistocene (Johnson et al. 1996, 2000), the entire Lake Victoria cichlid species flock may have gone through cycles of conditions that promote hybridization and conditions that promote speciation. Hence, it is not unlikely that the ancestral condition from which sympatric species pairs of Lake Victoria cichlids rapidly originated was one of relatively broad genetic variation in female mating preferences and male traits, as opposed to a monomorphic ancestral condition.
In speciation models that allow mating preferences to diverge or be reinforced by disruptive natural selection, the probability of sympatric speciation is less constrained by the number of genes that determine female mating preferences (Dieckmann & Doebeli 1999; Kondrashov & Kondrashov 1999; Arnegard & Kondrashov 2004). The same may be true without natural selection when male nuptial colour plays a role not just in female choice, but also in male–male competition (Evans & Norris 1996; P. D. Dijkstra, O. Seehausen and T. G. G. Groothuis, unpublished data). The latter may then generate negative frequency-dependent selection on male nuptial colour, assisting the invasion of rare male phenotypes, which in turn could exert selection on initially rare female preference phenotypes (Seehausen& Schluter 2004; van Doorn et al. 2004; P. D. Dijkstra, O. Seehausen and T. G. G. Groothuis, unpublished data).
Although our data do not allow conclusions on the importance of disruptive natural versus disruptive sexual selection during cichlid fish speciation, they suggest that the genetics of female mating preferences in a sister species pair of Lake Victoria cichlids may be relatively simple and permissible of sympatric speciation by divergence in mate choice through a variety of mechanisms. They further suggest that linkage mapping of female preference genes in this system might be feasible. The latter could be an important next step towards identifying the nature of female mating preferences and its role in rapid speciation.
Acknowledgments
We thank Rob Fraser for help with data collection, Nicky Barson, Mairi Knight, George Turner, Dik Heg, Ian Hamilton and Hans Hofman for comments on earlier drafts of the manuscript, and three anonymous reviewers for their criticism. O.S. was initially supported by a European Union Marie Curie Fellowship.
References
- Albertson R.C., Markert J.A., Danley P.D., Kocher T.D. Phylogeny of a rapidly evolving clade: the cichlid fishes of Lake Malawi, East Africa. Proc. Natl Acad. Sci. USA. 1999;96:5107–5110. doi: 10.1073/pnas.96.9.5107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allender C.J., Seehausen O., Knight M.E., Turner G.F., Maclean N. Divergent selection during speciation of Lake Malawi cichlid fishes inferred from parallel radiations in nuptial coloration. Proc. Natl Acad. Sci. USA. 2003;100:14074–14079. doi: 10.1073/pnas.2332665100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson M. Sexual selection. Princeton University Press; 1994. [Google Scholar]
- Arnegard M.E., Kondrashov A.S. Sympatric speciation by sexual selection alone is unlikely. Evolution. 2004;58:222–237. [PubMed] [Google Scholar]
- Becker W.A. Manual of quantitative genetics. Academic Enterprises; Pullman, WA: 1992. [Google Scholar]
- Bouton N., Seehausen O., van Alphen J.J.M. Resource partitioning among rock-dwelling haplochromines (Pisces: Cichlidae) from Lake Victoria. Ecol. Freshwat. Fish. 1997;6:225–240. [Google Scholar]
- Bouton N., van Os N., Witte F. Feeding performance of Lake Victoria rock cichlids: testing predictions from morphology. J. Fish Biol. A. 1998;53:118–127. [Google Scholar]
- Carleton K.L., Kocher T.D. Cone opsin genes of African cichlid fishes: tuning spectral sensitivity by differential gene expression. Mol. Biol. Evol. 2001;18:1540–1550. doi: 10.1093/oxfordjournals.molbev.a003940. [DOI] [PubMed] [Google Scholar]
- Carleton, K. L., Spady, T. & Kocher, T. D. 2004 Visual communication in East African cichlid fishes: diversity in a phylogenetic context. In Fish communication (ed. B. G. Kapor, F. Ladich, S. P. Collin and W. G. Raschi) (In the press.). Enfield, NH: Science Publisher Inc.
- Castle W.E. An improved method of estimating the number of genetic factors concerned in cases of blending inheritance. Science. 1921;54:223. doi: 10.1126/science.54.1393.223. [DOI] [PubMed] [Google Scholar]
- Coyne J.A. Genetics and speciation. Nature. 1992;355:511–515. doi: 10.1038/355511a0. [DOI] [PubMed] [Google Scholar]
- Coyne J.A., Orr H.A. The evolutionary genetics of speciation. Phil. Trans. R. Soc. B. 1998;353:287–305. doi: 10.1098/rstb.1998.0210. doi:10.1098/rstb.1998.0210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieckmann U., Doebeli M. On the origin of species by sympatric speciation. Nature. 1999;400:354–357. doi: 10.1038/22521. [DOI] [PubMed] [Google Scholar]
- Doi M., Matsuda M., Tomaru M., Matsubayashi H., Oguma Y. A locus for female discrimination behaviour causing sexual isolation in Drosophila. Proc. Natl Acad. Sci. USA. 2001;98:6714–6719. doi: 10.1073/pnas.091421598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominey W.J. Effects of sexual selection and life history of speciation: species flocks in African cichlids and Hawaiian Drosophila. In: Echelle A.A., Kornfield I., editors. Evolution of fish species flocks. University of Maine at Orono Press; Orono, ME: 1984. pp. 231–249. [Google Scholar]
- Endler J.A. Geographic variation, speciation, and clines. Princeton University Press; Princeton, NJ: 1977. [PubMed] [Google Scholar]
- Evans M.R., Norris K. The importance of carot enoids in signalling during aggressive interactions between male firemouth cichlids (Cichlasoma meeki) Behav. Ecol. 1996;7:1–6. [Google Scholar]
- Falconer D.S., Mackay T.F.C. Introduction to quantitative genetics. Longman; New York: 1996. [Google Scholar]
- Fisher R.A. The genetical theory of natural selection. Clarendon; Oxford: 1930. [Google Scholar]
- Fryer G. Speciation rates in lakes and the enigma of Lake Victoria. Hydrobiologia. 2004;519:167–183. [Google Scholar]
- Galis F., Metz J.A.J. Why are there so many cichlid species? Trends Ecol. Evol. 1998;13:1–2. doi: 10.1016/s0169-5347(97)01239-1. [DOI] [PubMed] [Google Scholar]
- Genner M.J., Turner G.F., Barker S., Hawkins S.J. Niche segregation among Lake Malawi cichlid fishes? Evidence from stable isotope signatures. Ecol. Lett. 1999;2:185–190. [Google Scholar]
- Genner M.J., Seehausen O., Cleary D.F.R., Knight M.E., Michel E., Turner G.F. How does the taxonomic status of allopatric populations influence species richness within African cichlid fish assemblages? J. Biogeogr. 2004;31:93–102. [Google Scholar]
- Higashi M., Takimoto G., Yamamura N. Sympatric speciation by sexual selection. Nature. 1999;402:523–526. doi: 10.1038/990087. [DOI] [PubMed] [Google Scholar]
- Johnson T.C., Scholz C.A., Talbot M.R., Kelts K., Ricketts R.D., Ngobi G., Beuning K., Ssemmanda I., McGill J.W. Late Pleistocene desiccation of Lake Victoria and rapid evolution of cichlid fishes. Science. 1996;273:1091–1093. doi: 10.1126/science.273.5278.1091. [DOI] [PubMed] [Google Scholar]
- Johnson T.C., Kelts K., Odada E. The Holocene history of Lake Victoria. Ambio. 2000;29:2–11. [Google Scholar]
- Kawata M., Yoshimura J. Speciation by sexual selection in hybridizing populations without viability selection. Evol. Ecol. Res. 2000;2:897–909. [Google Scholar]
- Kirkpatrick M., Ravigné V. Speciation by natural and sexual selection: models and experiments. Am. Nat. 2002;159:S22–S35. doi: 10.1086/338370. [DOI] [PubMed] [Google Scholar]
- Kocher T. Adaptive evolution and explosive speciation: the cichlid fish model. Nature Rev. Genet. 2004;5:288–298. doi: 10.1038/nrg1316. [DOI] [PubMed] [Google Scholar]
- Kondrashov A.S., Kondrashov F.A. Interactions among quantitative traits in the course of sympatric speciation. Nature. 1999;400:351–354. doi: 10.1038/22514. [DOI] [PubMed] [Google Scholar]
- Kornfield I., Smith P.F. African cichlid fishes: model systems for evolutionary biology. A. Rev. Ecol. Syst. 2000;31:163–196. [Google Scholar]
- Lande R. The minimum number of genes contributing to quantitative variation between and within populations. Genetics. 1981a;99:541–553. doi: 10.1093/genetics/99.3-4.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lande R. Models of speciation by sexual selection on polygenic traits. Proc. Natl Acad. Sci. USA. 1981b;78:3721–3725. doi: 10.1073/pnas.78.6.3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lande R. Rapid origin of sexual isolation and character divergence in a cline. Evolution. 1982;36:1–12. doi: 10.1111/j.1558-5646.1982.tb05034.x. [DOI] [PubMed] [Google Scholar]
- McKaye K.R. Sexual selection and the evolution of the cichlid fishes of Lake Malawi, Africa. In: Keenleyside M.H.A., editor. Cichlid fishes. Chapman & Hall; London: 1991. pp. 241–257. [Google Scholar]
- Payne R.J.H., Krakauer D.C. Sexual selection, space, and speciation. Evolution. 1997;51:1–9. doi: 10.1111/j.1558-5646.1997.tb02382.x. [DOI] [PubMed] [Google Scholar]
- Ritchie M.G. The inheritance of female preference functions in a mate recognition system. Proc. R. Soc. B. 2000;267:327–332. doi: 10.1098/rspb.2000.1004. doi:10.1098/rspb.2000.1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie M.G., Phillips S.D. The genetics of sexual isolation. In: Howard D., Berlocher S., editors. Endless forms: species and speciation. Oxford University Press; 1998. pp. 291–307. [Google Scholar]
- Seehausen O. Lake Victoria rock cichlids. Taxonomy, ecology and distribution. Cichlid Press; Zevenhuizen, The Netherlands: 1996. [Google Scholar]
- Seehausen O. Distribution of and reproductive isolation among colour morphs of a rock-dwelling Lake Victoria cichlid (Haplochromis nyererei) Ecol Freshwat Fish. 1997;6:59–66. [Google Scholar]
- Seehausen O., Bouton N. Microdistribution and fluctuations in niche overlap in a rocky shore cichlid community in Lake Victoria. Ecol Freshwat Fish. 1997;6:59–66. [Google Scholar]
- Seehausen O., Schluter D. Male–male competition and nuptial colour displacement as a diversifying force in Lake Victoria cichlid fish. Proc R Soc B. 2004;271:1345–1353. doi: 10.1098/rspb.2004.2737. doi:10.1098/rspb.2004.2737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seehausen O., van Alphen J.J.M. The effect of male colouration on female mate choice in closely related Lake Victoria cichlids (Haplochromis nyererei complex) Behav. Ecol. Sociobiol. 1998;42:1–8. [Google Scholar]
- Seehausen O., van Alphen J.J.M. Can sympatric speciation by disruptive sexual selection explain rapid evolution of cichlid diversity of Lake Victoria? Ecol. Lett. 1999;2:262–271. [Google Scholar]
- Seehausen O., van Alphen J.J.M., Witte F. Cichlid fish diversity threatened by eutrophication that curbs sexual selection. Science. 1997;277:1808–1811. [Google Scholar]
- Smith P.F., Kornfield I. Phylogeography of Lake Malawi cichlids of the genus Pseudotropheus: significance of allopatric colour variation. Proc. R. Soc. B. 2002;269:2495–2502. doi: 10.1098/rspb.2002.2188. doi:10.1098/rspb.2001.2188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takimoto G. Polygenic inheritance is not necessary for sympatric speciation by sexual selection. Popul. Ecol. 2002;44:87–91. [Google Scholar]
- Takimoto G., Higashi M., Yamamura N. A deterministic genetic model for sympatric speciation by sexual selection. Evolution. 2000;54:1870–1881. doi: 10.1111/j.0014-3820.2000.tb01234.x. [DOI] [PubMed] [Google Scholar]
- Ting C-T, Takahashi A., Wu C.-I. Incipient speciation by sexual selection in Drosophila: concurrent evolution at multiple loci. Proc. Natl Acad. Sci. USA. 2001;98:6709–6713. doi: 10.1073/pnas.121418898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner G.F. Explosive speciation in African cichlid fishes. In: Magurran A.E., May R.M., editors. Evolution of biological diversity. Oxford University Press; 1998. pp. 217–229. [Google Scholar]
- Turner G.F., Burrows M.T. A model of sympatric speciation by sexual selection. Proc R Soc B. 1995;260:287–292. [Google Scholar]
- Turner G.F., Seehausen O., Knight M.E., Allender C.J., Robinson R.L. How many cichlid fishes are there in African lakes? Mol. Ecol. 2001;10:793–806. doi: 10.1046/j.1365-294x.2001.01200.x. [DOI] [PubMed] [Google Scholar]
- Van der Meer H.J., Bowmaker J.K. Interspecific variation of photoreceptors in four co-existing haplochromine cichlid fishes. Brain Behav. Evol. 1995;45:232–240. doi: 10.1159/000113552. [DOI] [PubMed] [Google Scholar]
- van Doorn G.S., Noest A.J., Hogeweg P. Sympatric speciation and extinction driven by environment dependent sexual selection. Proc. R. Soc. B. 1998;265:1915–1919. doi:10.1098/rspb.1998.0520 [Google Scholar]
- van Doorn G.S., Dieckmann U., Weissing F.J. Sympatric speciation by sexual selection: a critical re-evaluation. Am. Nat. 2004;163:709–725. doi: 10.1086/383619. [DOI] [PubMed] [Google Scholar]
- West Eberhard M.J. Sexual selection, social competition, and evolution. Proc Am Phil Soc. 1979;51:222–234. [Google Scholar]
- Wright S. Evolution and the genetics of populations. vol. 1. University of Chicago Press; 1968. [Google Scholar]
- Wu C-I. A stochastic simulation study on speciation by sexual selection. Ecology. 1985;39:66–82. doi: 10.1111/j.1558-5646.1985.tb04080.x. [DOI] [PubMed] [Google Scholar]
- Zeng Z-B, Houle D., Cockerham C.C. How informative is Wright’s estimator of the number of genes affecting a quantitative character? Genetics. 1990;126:235–247. doi: 10.1093/genetics/126.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]