Abstract
Reduced levels of antioxidants such as carotenoids and vitamins A and E can increase DNA damage caused by free radicals. Exposure to radiation has been proposed to reduce levels of antioxidants that are used for DNA repair and this reduction may be responsible for increased levels of mutation in radioactively contaminated areas. We test this hypothesis using field measures of antioxidants in blood, liver and eggs of the barn swallow Hirundo rustica while relating these to levels of mutation as reflected by the frequency of abnormal sperm. Antioxidant levels in blood, liver and eggs were reduced in Chernobyl, Ukraine, compared with an uncontaminated control area, and levels of antioxidants correlated negatively with levels of background radiation. The frequency of abnormal sperm was almost an order of magnitude higher in Chernobyl than in the control area and was negatively related to antioxidant levels in blood and liver. This is consistent with the hypothesis of a direct link between radiation and individual levels of antioxidants, suggesting that levels of mutation differ among individuals owing to individual differences in the abundance of antioxidants.
Keywords: barn swallow, carotenoids, Hirundo rustica, sperm abnormality, vitamin A, vitamin E
1. Introduction
A deficiency of antioxidants such as carotenoids and vitamins A and E can increase DNA damage caused by free radicals (Ames 1983; Edge et al. 1997; Bast et al. 1998; Krinsky 1998; Rice-Evans et al. 1997; Møller et al. 2000). Radiation has been proposed to reduce levels of antioxidants that are used for DNA repair because antioxidants are used for removing free radicals that arise owing to radiation (Ben-Amotz et al. 1998; Ivaniota et al. 1998; Neyfakh et al. 1998a, b; Kumerova et al. 2000). It has been suggested that this reduction in antioxidants may be responsible for increased levels of mutation in radioactively contaminated areas, such as those around Chernobyl in Ukraine (Dubrova et al. 1996; Ellegren et al. 1997; Kovalchuk et al. 2000; Møller & Mousseau 2001). This increase in mutations may arise from the direct effects of radiation or indirectly through the effects of a reduction in levels of antioxidants on levels of mutations. We test the hypothesis that elevated levels of radiation are associated with reduced levels of antioxidants thereby causing an increase in mutation rates, using field measures of antioxidants in blood, liver and eggs of the barn swallow Hirundo rustica while relating these to levels of mutation as reflected by the frequency of abnormal sperm.
Antioxidants are important biochemicals that provide organisms with protection against the damaging effects of free radicals on DNA and other molecules (review in Surai 2003). Elevated levels of radiation, as experienced by recovery workers and other people exposed to the Chernobyl accident, are associated with diminished antioxidant defence owing to the use of antioxidants for free-radical scavenging (Ivaniota et al. 1998; Neyfakh et al. 1998a; Kumerova et al. 2000). Such effects of radiation on antioxidant levels are reversible since supplementation with β-carotene and vitamins A and E can reduce the effects of the lipoperoxidative cascade among individuals subject to radiation (Clavere et al. 1996; Ben-Amotz et al. 1998; Neyfakh et al. 1998b). Administration of subjects with antioxidants such as vitamin E reduced the effects of radiation on immunity (Rana& Malhotra 1995; Moriguchi et al. 1996) and the intestine (Empey et al. 1992; Felemovicius et al. 1995). While these studies provide important information about the links between radiation exposure and antioxidant defence, there is no study available that links the effects of radiation to antioxidant defence and level of efficiency measured in terms of mutation rates and DNA repair at the level of individuals. We provide such a preliminary test using a free-living bird in contaminated and control areas in Ukraine. While blood levels reflect circulating levels of antioxidants, the liver comprises the major body storage of antioxidants in birds (Surai 2003). Hence, we quantified both circulating antioxidants in the plasma, and stored antioxidants in the liver, to investigate whether circulating levels or stores of antioxidants are depressed under the influence of radiation.
Barn swallows are migratory birds that winter in sub-Saharan Africa and return to the breeding grounds in Ukraine in May. Egg-laying takes place between two and five weeks after arrival. Natal dispersal in the barn swallow is, on average, 0.7 km in males and 2.5 km in females, while breeding dispersal rarely goes beyond a breeding site (Cramp 1988; Møller 1994). Thus, dispersal between our main study sites around Chernobyl and those in the control area at Kanev ca. 120 km to the southeast is unlikely to affect the results presented here. Previous studies on the barn swallow in the Ukraine have found that birds in areas with elevated radiation around Chernobyl have more pale melanin-based plumage pigmentation than in control areas, in particular long-tailed males (Camplani et al. 1999). In addition, 87 out of the 88 partial albinos caused by mutation had partly albinistic feathers within the red melanin-based plumage, whereas only nine were expected, given the area of the red plumage badge (Møller & Mousseau 2001), suggesting that partial albinism is directly related to this melanin-based coloration. Adult barn swallow males with long tails tend to have a more strongly reflecting red throat plumage colour than do short-tailed males, and such birds also have higher levels of circulating carotenoids in the blood (Saino et al. 1999). Studies of three microsatellites in barn swallows from Chernobyl have revealed that mutation rates are increased by a factor of two to ten when compared with individuals from two uncontaminated control areas (Ellegren et al. 1997).
2. Material and methods
2.1 Study area and population
We studied barn swallows in Ukraine just outside the exclusion zone of the Chernobyl area, by visiting villages and checking collective farms for their presence. Once such a farm had been located, we recorded radiation levels and captured adult barn swallows for measurements and subsequent individual ringing. Our own field measurements of radiation at the ground level using a hand-held Inspector dosimeter (Model: Inspector, SE International, Inc., Summertown, TN, USA) revealed levels of mainly γ radiation of 0.390 mRh−1 (s.e.=0.317) at 14 breeding sites in the Chernobyl region. As a control area, we used Kanev ca. 120 km southwest of Kiev that has a relatively low level of contamination. Mean levels of radiation were 0.025 mRh−1 (s.e.=0.002) at five breeding sites. Our data were validated with correlation against data from the governmental measurements published by Shestopalov (1996), estimated as the midpoint of the ranges from this published source. This analysis revealed a very strong positive relationship (linear regression on log–log transformed data: F=159.46, d.f.=1,18, r2=0.89, p<0.0001, slope (s.e.)=1.28 (0.10)), suggesting that our field estimates of radiation were comparable to other estimates. We studied barn swallows in these two study areas during 6–12 June 2000 and 4–11 June 2002.
2.2 Field procedures
We captured barn swallows with mist nets across open doors and windows in farm buildings. This method is highly efficient during the main breeding season since mark–recapture studies have shown a capture probability of 98% of all individuals with just a few capture sessions (Møller & Szép 2002). During the field season 2000, we collected blood samples of 70μl in capillarity tubes from a sample of 41 individuals of both sexes (21 from Chernobyl and 20 from Kanev), and these samples were later used for quantification of circulating antioxidants in blood (§ 2c). A sample of 58 birds (of which 36 were males) (29 from Chernobyl and 29 from Kanev) was collected with permission from the National Museum of Natural History, Kiev, Ukraine, in 2000 and these individuals were used for quantification of antioxidants in the liver and abnormality of sperm. During the field season 2002, we collected one egg from each of a sample of 45 nests (23 from Chernobyl and 22 from Kanev) for quantifying antioxidants in eggs.
2.3 Laboratory analyses of antioxidants
Antioxidants in plasma, liver and egg yolk were assessed by HPLC-based methods described earlier (Suraiet al. 2001a; Blount et al. 2003). In brief, 15–30μl of plasma was vortexed with an equal volume of 5% sodium chloride, plus two volumes of ethanol, 500μl hexane was then added and vortexed. After centrifugation the hexane phase, containing antioxidants (carotenoids, α-tocopherol and retinol) was collected and extraction was repeated. The resulting hexane extract was combined and evaporated to dryness under nitrogen gas, then redissolved in 300μl of a methanol–dichloromethane mixture (1:1, v/v).
The antioxidants were extracted from the egg yolk and liver as follows. Egg yolk (100–200 mg) or tissue (80–100 mg) were homogenized in 1 ml of 1:1 (v/v) mixture of 5% NaCl solution and ethanol followed by the addition of 2 ml of hexane and further homogenization for 1 min. After centrifugation, the hexane layer was collected and the extraction was repeated twice. Hexane extracts were combined, evaporated to dryness under nitrogen gas, then redissolved in 300μl of a methanol–dichloromethane mixture (1:1, v/v).
Total carotenoids were determined as follows. Samples (10μl) were injected into an HPLC system fitted with a 5μ C18 reverse-phase column (25 cm×4.6 mm) (Spherisorb-type S5NH2; Phase Separations, Clwyd, UK) with a mobile phase of methanol–water (97:3 v/v) at a flow rate of 1.5 ml min−1. Carotenoids were identified as a single peak at 445 nm, and concentrations were determined using lutein as a standard (Sigma-Aldrich, Poole, UK). Previously, using zebra finch plasma (Blount et al. 2003) we showed that carotenoid measurements were highly repeatable (one-way ANOVA, F=3.77, d.f.=22,26, p=0.001; interclass correlation coefficient, r=0.57). Similar results were also obtained when testing α-tocopherol and retinol determinations. The same HPLC system fitted with a Spherisorb type S3ODS2, 3μ C18 reverse phase column, 15 cm×4.6 mm was used (Phase Separation Ltd, UK). Chromatography was performed using the same mobile phase at a flow rate of 1.05 mlmin−1 and retinol and α-tocopherol were determined using fluorescent detection. Tocol was used as an internal standard, and standard solutions of α-tocopherol and retinol (Sigma-Aldrich, Poole, UK) were used for instrument calibration.
All analyses were conducted blind with respect to the origin of birds.
2.4 Assessing sperm abnormality
A sample of sperm was obtained from the cloacal protuberance of all 36 males, using samples that were frozen after collection in the field. The frequency of abnormal sperm was quantified with a microscope at magnification ×400, as the proportion of sperm among 100 spermatozoa that had clearly abnormal morphologies (bent or missing flagella, abnormal head shapes, two heads, two tails, coiled tails, elongated heads, etc.; (Gomes 1977). This measure predicts fertilizing ability in chicken and turkey (Lorenz 1969; Bakst & Cecil 1997). All analyses were done blind with respect to the identity and the origin of birds.
3. Results
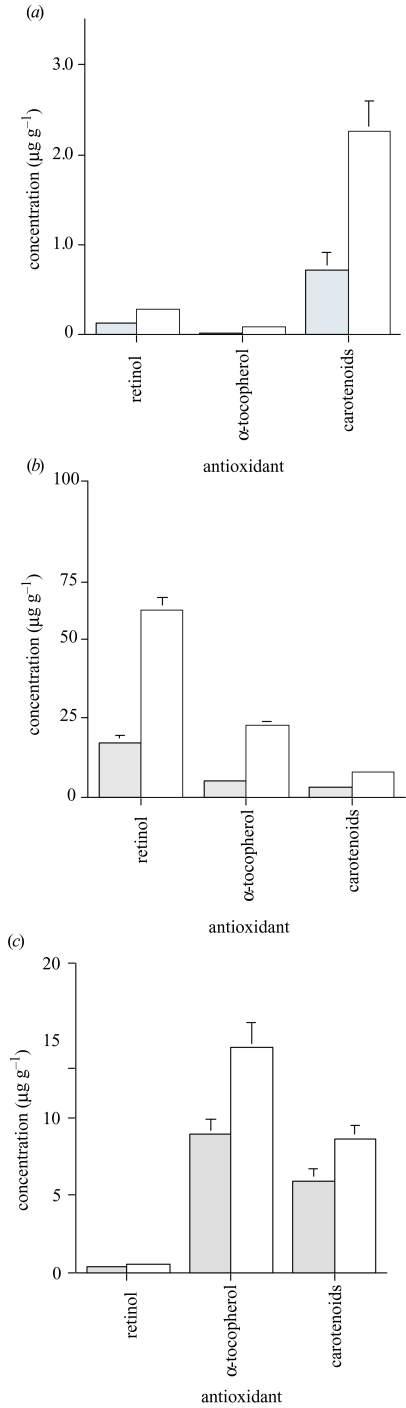
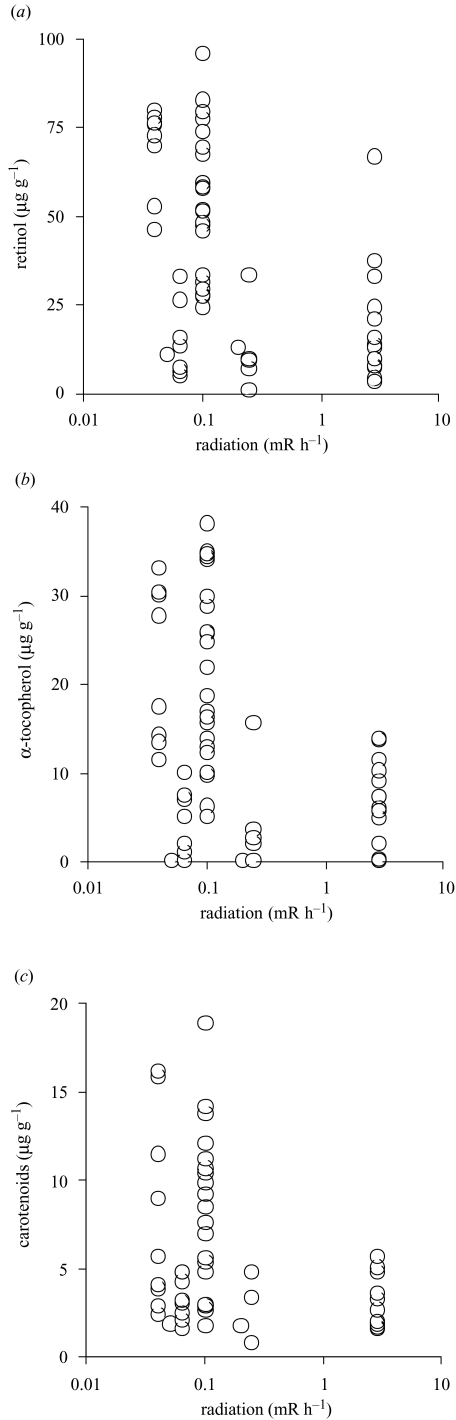
We investigated levels of three common antioxidants in blood and liver of barn swallows from contaminated and control areas in Ukraine. There were weak but significant positive correlations between concentrations in plasma and in liver (log-transformed data: total carotenoids: Pearson r=0.39, t=2.64, d.f.=39, p=0.01; retinol: r=0.38, t=2.59, d.f.=39, p=0.01; α-tocopherol: r=0.36, t=2.41, d.f.=39, p=0.01). Levels of antioxidants in blood and liver differed significantly between Chernobyl and the control area, with concentrations in Chernobyl, on average, being only a half to a quarter of that in the control area (figure 1a, b). There was a significant sex effect on liver concentration of total carotenoids, when entering sex as an additional factor in a two-way ANOVA (analysis based on log-transformed concentration: F=7.56, d.f.=1,48, p=0.008; mean (s.e.), males: 0.70μgg−1 (0.06), n=32; females: 0.56μgg−1 (0.07), n=20). The sex effect was not significant for the other liver antioxidants or for blood antioxidants, and the area by sex interaction was not significant in any of the six analyses. Therefore, we did not enter sex as a factor in the subsequent analyses. Antioxidants were more abundant in areas with lower levels of radiation, but decreased dramatically in the Chernobyl region (figure 2).
Figure 1.
Concentration of antioxidants (μgg−1) in (a) blood and (b) liver of adult barn swallows and in (c) eggs of barn swallows from Chernobyl (grey bars) and Kanev (open bars; uncontaminated control area). Values are means (+1s.e.). One-way ANOVAs revealed significant differences for blood ((a) F=18.58, d.f.=1,39, p < 0.001; F=16.64, d.f.=1,39, p=0.0002; F=16.64, d.f.=1,39, p < 0.001, respectively); for liver retinol, α-tocopherol and carotenoids ((b) F=90.70, d.f.=1,56, p=0.001; F=22.33, d.f.=1,56, p < 0001; F=30.29, d.f.=1,56, p < 0.001, respectively); and for egg α-tocopherol and carotenoids ((c) F=9.23, d.f.=1,43, p=0.004; F=5.31, d.f.=1,43, p=0.03, respectively), while the difference for egg retinol was not significant (F=1.29, d.f.=1,43, p=0.26).
Figure 2.
Antioxidants in liver of adult barn swallows from Ukraine in relation to levels of radiation (mRh−1). The regressions were significant when radiation level was log-transformed ((a) retinol (μgg−1): F=22.01, d.f.=1,56, r2=0.28, p < 0.0001, slope (s.e.)=−21.47 (4.58); (b) α-tocopherol (μgg−1): F=11.84, d.f.=1,56, r2=0.17, p=0.001, slope (s.e.)=−7.00 (2.04); (c) carotenoids (μgg−1): F=9.51, d.f.=1,56, r2=0.15, p=0.003, slope (s.e.)=−2.46 (0.80), respectively).
The concentration of α-tocopherol and total carotenoids in eggs was reduced by 30–60% in eggs from Chernobyl compared with eggs from the control area (figure 1c). This suggests that offspring developed as embryos with initially extremely low levels of antioxidants in the Chernobyl region compared with the control area.
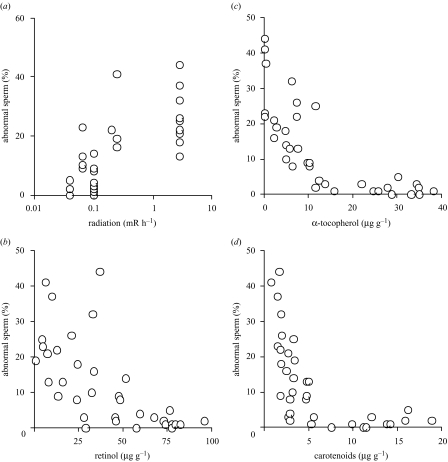
In the barn swallow, the frequency of sperm with abnormal morphology differed significantly between Chernobyl and the control area (one-way ANOVA based on square-root-arcsine-transformed data: F=81.99, d.f.=1,34, p<0.001; mean (s.e.) for Chernobyl: 21.74% (2.41), n=19; control: 2.65% (0.64), n=17). Abnormal sperm were more common among barn swallows from areas with high levels of radiation (figure 3a), and when levels of antioxidants in liver were low (figure 3b–d). Similar results were found for antioxidants in blood (figure 3). This provides evidence of individual levels of mutation, as reflected by the frequency of abnormal sperm, increasing in situations where the background level of radiation is high, and when the concentration of antioxidants is low.
Figure 3.
Frequency of abnormal sperm (%) in barn swallows from Ukraine in relation to radiation and liver antioxidants in adult males. The regressions were significant when radiation level was log-transformed ((a) radiation (mRh−1); F=34.07, d.f.=1,34, r2=0.50, p < 0.0001, slope (s.e.)=0.21 (0.04); (b) retinol (μgg−1): F=35.98, d.f.=1,34, r2=0.51, p < 0.0001, slope (s.e.)=−0.005 (0.001); (c) α-tocopherol (μgg−1): F=71.86, d.f.=1,34, r2=0.68, p < 0.0001, slope (s.e.)=−0.014 (0.002); (d) carotenoids (μgg−1): F=29.43, d.f.=1,34, r2=0.46, p < 0.0001, slope (s.e.)=−0.03 (0.01), respectively). Similar results were found for antioxidants in blood (linear regressions with radiation level log-transformed: retinol (μgg−1): F=12.48, d.f.=1,26, r2=0.32, p=0.002, slope (s.e.)=−0.74 (0.21); (c) α-tocopherol (μgg−1): F=11.33, d.f.=1,26, r2=0.25, p=0.006, slope (s.e.)=−0.07 (0.02); (d) carotenoids (μgg−1): F=8.78, d.f.=1,26, r2=0.30, p=0.002, slope (s.e.)=−1.55 (0.46), respectively).
4. Discussion
Exposure to radiation has been proposed to reduce levels of antioxidant defence owing to their use for free-radical scavenging (Ivaniota et al. 1998; Neyfakh et al. 1998a; Kumerova et al. 2000). However, inflammatory responses could also cause decreased levels of antioxidants in tissues of irradiated birds (Lorimore et al. 2003; Morgan 2003). We have shown, for a free-living migratory passerine bird, that levels of carotenoids and vitamins A and E differ significantly between populations breeding in the contaminated area around Chernobyl and a control area (figure 1). Levels were depressed in the Chernobyl region by more than 50% compared with individuals from the control area. There was no significant sex effect, with the exception of plasma carotenoids, which were less abundant in females than in males. This difference may relate to adult females depositing carotenoids into eggs at this time of the year, although this explanation does not explain the absence of a sex difference for plasma levels of vitamins A and E or liver antioxidants that are also deposited into eggs. These effects of radiation on antioxidant levels were prominent in blood, liver and eggs, suggesting that both circulating levels and storage of antioxidants differed consistently between these regions. These differences between regions may be open to alternative explanations such as differences in availability of antioxidants in the diet. However, we were able to show that levels of antioxidants in individual barn swallows were inversely related to levels of background radiation (figure 2). This suggests that radiation (or a correlated factor) affected antioxidant levels in barn swallows. The exact physiological mechanism responsible for this effect remains to be determined.
Birds’ eggs contain large amounts of antioxidants that are used for free-radical scavenging by the developing embryo (review in Surai2003). We detected dramatically reduced levels of some antioxidants in eggs of barn swallows from the Chernobyl region compared with controls (figure 1c). This suggests that offspring developed with low levels of maternally derived antioxidants in the Chernobyl region. There was a reduction in egg concentration of antioxidants with increasing levels of radiation (figure 2). However, there was considerable variation among sites in the range of antioxidant concentrations recorded (figure 2), and some of this variation may be accounted for by the patchy distribution of high levels of radiation in the Chernobyl region, even on a micro-geographical scale (Shestopalov 1996). Such depression of dietary carotenoids deposited into yolk can increase the susceptibility of embryonic tissues to free-radical attack (Surai& Speake 1998), and reduce hatchling immune function (McWhinney et al. 1989; Haq et al. 1996). Avian embryos and hatchlings are particularly in need of antioxidants because their rapid metabolism incurs high rates of free-radical production, and their tissues are rich in unsaturated lipids that are particularly susceptible to free-radical attack (Suraiet al. 2001b). We suggest that such low levels of antioxidants may have important consequences for offspring when reaching adulthood.
Abnormal sperm with aberrant morphology occur at a low frequency in birds and other vertebrates (5–10% in birds, (Bakst& Cecil (1997); 10–40% in mammals, (Gomes (1977)). High frequencies of abnormal sperm are typical of mutants (Yuet al. 2000; Lamitina & L’Hernault 2002; Mendoza-Lujambo et al. 2002), although other factors may also contribute to the presence of abnormal sperm (Bakst& Cecil 1997). We found low levels of sperm abnormality among barn swallows from the control area, but elevated levels around Chernobyl. Since levels of sperm abnormality were positively related to levels of radiation (figure 3a), but negatively related to levels of antioxidants in blood and liver of adult barn swallows (figure 3), we can conclude that radiation (or a factor associated with radiation) increases levels of sperm abnormality.
Mutations are novel genetic variants, and they are supposed to occur at a constant low rate (Lynchet al. 1999). Individual differences in mutation rates can arise from individual differences in the ability to cope with mutagens and perform DNA repair. This study has shown that levels of antioxidants differ among individuals in relation to levels of radiation owing to the nuclear accident at Chernobyl. These individual differences in levels of antioxidants are related to levels of mutation, as reflected by sperm abnormality, providing a hypothetical mechanism that links individual levels of antioxidants through exposure to radiation to levels of mutations. This hypothesis is testable by investigating the relationship between rates of mutation in molecular markers obtained from sperm and circulating levels of antioxidants in males that produced these sperm.
Acknowledgments
The authors are grateful to G. Milinevski, A. M. Peklo and E. Pysanets for logistic help provided during our visits to Ukraine. The authors received funding from the CNRS (France), the University of South Carolina School of the Environment, the Samuel Freeman Charitable Trust, the National Science Foundation and National Geographic Society to conduct this research.
References
- Ames B.N. Dietary carcinogens and anticarcinogens: oxygen radicals and degenerative diseases. Science. 1983;221:1256–1264. doi: 10.1126/science.6351251. [DOI] [PubMed] [Google Scholar]
- Bakst M.R., Cecil H.C. Techniques for semen evaluation, semen storage, and fertility determination. Poultry Science Association; Savoy, IL: 1997. [Google Scholar]
- Bast A., Haenen G.R., van den Berg R., van den Berg H. Antioxidant effects of carotenoids. Int. J. Vitam. Nutr. Res. 1998;68:399–403. [PubMed] [Google Scholar]
- Ben-Amotz A., Yatziv S., Sela M., Greenberg S., Rachmilevich B., Shwarzman M., Weshler Z. Effect of natural beta-carotene supplementation in children exposed to radiation from the Chernobyl accident. Radiation Environ. Biophysics. 1998;37:187–193. doi: 10.1007/s004110050116. [DOI] [PubMed] [Google Scholar]
- Blount J.D., Metcalfe N.B., Birkhead T.R., Surai P.F. Carotenoid modulation of immune function and sexual attractiveness in zebra finches. Science. 2003;300:125–127. doi: 10.1126/science.1082142. [DOI] [PubMed] [Google Scholar]
- Camplani C., Saino N., Møller A.P. Carotenoids, sexual signals and immune function in barn swallows from Chernobyl. Proc. R. Soc. B. 1999;266:1111–1116. doi: 10.1098/rspb.1999.0751. doi:10.1098/rspb.1999.0751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavere P., Jore D., Gardesalbert M., Lepage S., Bonnefontrousselot D., Delattre J. Prevention of radioinduced LDL peroxidation by vitamin-E derivatives. J. Chim. Phys. Physico-Chimie Biol. 1996;93:53–57. [Google Scholar]
- Cramp S., editor. Handbook of the birds of Europe, the Middle East and North Africa. vol. 5. Oxford University Press; 1988. [Google Scholar]
- Dubrova Y.E., Nesterov V.N., Krouchinsky N.G., Ostapenko V.A., Neumann R., Neil D.L., Jeffreys A.J. Human minisatellite mutation rate after the Chernobyl accident. Nature. 1996;380:683–686. doi: 10.1038/380683a0. [DOI] [PubMed] [Google Scholar]
- Edge R., McGarvey D.J., Truscott T.G. The carotenoids as antioxidants—a review. J. Photochem. Photobiol. 1997;B41:189–200. doi: 10.1016/s1011-1344(97)00092-4. [DOI] [PubMed] [Google Scholar]
- Ellegren H., Lindgren G., Primmer C.R., Møller A.P. Fitness loss and germline mutations in barn swallows breeding in Chernobyl. Nature. 1997;389:593–596. doi: 10.1038/39303. [DOI] [PubMed] [Google Scholar]
- Empey L.R., Papp J.D., Jewell L.D., Fedorak R.N. Mucosal protective effects of vitamin-E and misoprostol during acute radiation-induced enteritis in rats. Digest. Dis. Sci. 1992;37:205–214. doi: 10.1007/BF01308173. [DOI] [PubMed] [Google Scholar]
- Felemovicius I., Bonsack M.E., Baptista M.L., Delaney J.P. Intestinal radioprotection by vitamin-E (alpha-tocopherol) Ann. Surg. 1995;222:504–510. doi: 10.1097/00000658-199522240-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes W.R. Artificial insemination. In: Cole H.H., Cupps T.H., editors. Reproduction in domestic animals. 3rd edn. Academic Press; London: 1977. pp. 257–284. [Google Scholar]
- Haq A.U., Bailey C.A., Chinnah A. Effect of β-carotene, canthaxanthin, lutein, and vitamin E on neonatal immunity of chicks when supplemented in the broiler breeder diets. Poultry Sci. 1996;75:1092–1097. doi: 10.3382/ps.0751092. [DOI] [PubMed] [Google Scholar]
- Ivaniota L., Dubchak A.S., Tyshchenko V.K. Free radical oxidation of lipids and antioxidant system of blood in infertile women in a radioactive environment. Ukrainski Biokhim. Zhur. 1998;70:132–135. [In Ukrainian.] [PubMed] [Google Scholar]
- Kovalchuk O., Dubrova Y.E., Arkhipov A., Hohn B., Kovalchuk I. Wheat mutation rate after Chernobyl. Nature. 2000;407:583–584. doi: 10.1038/35036692. [DOI] [PubMed] [Google Scholar]
- Krinsky N.I. The antioxidant and biological properties of the carotenoids. Ann. NY Acad. Sci. 1998;854:443–447. doi: 10.1111/j.1749-6632.1998.tb09923.x. [DOI] [PubMed] [Google Scholar]
- Kumerova A.O., Lece A.G., Skesters A.P., Orlikov G.A., Seleznev J.V., Rainsford K.D. Antioxidant defense and trace element imbalance in patients with postradiation syndrome: first report on phase I studies. Biol. Trace Element Res. 2000;77:1–12. doi: 10.1385/BTER:77:1:1. [DOI] [PubMed] [Google Scholar]
- Lamitina S.T., L’Hernault S.W. Dominant mutations in the Caerorhabditis elegans Myt1 ortholog wee-1.3 reveal a novel domain that controls in-phase entry during spermatogenesis. Development. 2002;129:5009–5018. doi: 10.1242/dev.129.21.5009. [DOI] [PubMed] [Google Scholar]
- Lorenz F.W. Reproduction in domestic fowl. In: Cole H.H., Cupps T.H., editors. Reproduction in domestic animals. Academic Press; New York: 1969. pp. 569–608. [Google Scholar]
- Lorimore S.A., Coates P.J., Wright E.G. Radiation-induced genomic instability and bystander effects: inter-related nontargeted effects of exposure to ionizing radiation. Oncogene. 2003;22:7058–7069. doi: 10.1038/sj.onc.1207044. [DOI] [PubMed] [Google Scholar]
- Lynch M., Blancard J., Houle D., Kibota T., Schultz S., Vassilieva L., Willis J. Perspective: spontaneous deleterious mutation. Evolution. 1999;53:645–663. doi: 10.1111/j.1558-5646.1999.tb05361.x. [DOI] [PubMed] [Google Scholar]
- Mendoza-Lujambo I., Burfeind P., Dixkens C., Meinhardt A., Hoyer-Fender S., Engel W., Neesen J. The Hook t gene is non-functional in the abnormal spermatozoon head shape (azh) mutant mouse. Hum. Mol. Genet. 2002;11:1647–1658. doi: 10.1093/hmg/11.14.1647. [DOI] [PubMed] [Google Scholar]
- McWhinney S.L.R., Bailey C.A., Panigrahy B. Immunoenhancing effect of β-carotene in chicks. Poultry Sci. 1989;68(Suppl.1) 94 (Abstract) [Google Scholar]
- Møller A.P. Sexual selection and the barn swallow. Oxford University Press; Oxford: 1994. [Google Scholar]
- Møller A.P., Mousseau T.A. Albinism and phenotype of barn swallows Hirundo rustica from Chernobyl. Evolution. 2001;55:2097–2104. doi: 10.1111/j.0014-3820.2001.tb01324.x. [DOI] [PubMed] [Google Scholar]
- Møller A.P., Szép T. Survival rate of adult barn swallows Hirundo rustica in relation to sexual selection and reproduction. Ecology. 2002;83:2220–2228. [Google Scholar]
- Møller A.P., Biard C., Blount J.D., Houston D.C., Ninni P., Saino N., Surai P.F. Carotenoid-dependent signals: indicators of foraging efficiency, immunocompetence or detoxification ability? Poultry Avian Biol. Rev. 2000;11:137–159. [Google Scholar]
- Morgan W.F. Non-targeted and delayed effects of exposure to ionizing radiation. Radiat. Res. 2003;159:567–596. doi: 10.1667/0033-7587(2003)159[0567:nadeoe]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Moriguchi S., Oonishi K., Kishino Y., Umegaki K. Vitamin-E supplementation induces an early recovery of cellular-immunity decreased following X-ray-irradiation. Nutr. Res. 1996;16:645–656. [Google Scholar]
- Neyfakh E.A., Alimbekova A.I., Ivanenko G.F. Vitamin E and A deficiencies in children correlate with Chernobyl radiation loads of their mothers. Biochemistry (Moscow) 1998a;63:1138–1143. [PubMed] [Google Scholar]
- Neyfakh E.A., Alimbekova A.I., Ivanenko G.F. Radiation-induced lipoperoxidative stress in children coupled with deficit of essential antioxidants. Biochemistry (Moscow) 1998b;63:977–987. [PubMed] [Google Scholar]
- Rana K., Malhotra N. Modification of radioresponse of chick spleen with vitamin-E treatment. Natl Acad. Sci. Lett. 1995;18:115. [Google Scholar]
- Rice-Evans C.A., Sampson J., Bramley P.M., Holloway D.E. Commentary: why do we expect carotenoids to be antioxidants in vivo. Free Radical Res. 1997;26:381–398. doi: 10.3109/10715769709097818. [DOI] [PubMed] [Google Scholar]
- Saino N., Stradi R., Ninni P., Møller A.P. Carotenoid plasma concentration, immune profile and plumage ornamentation of male barn swallows (Hirundo rustica) Am. Nat. 1999;154:441–448. doi: 10.1086/303246. [DOI] [PubMed] [Google Scholar]
- Shestopalov V.M. Atlas of Chernobyl exclusion zone. Ukrainian Academy of Science; Kiev: 1996. [Google Scholar]
- Surai P.F. Natural antioxidants in avian nutrition and reproduction. Nottingham University Press; Nottingham: 2003. [Google Scholar]
- Surai P.F., Speake B.K. Distribution of carotenoids from the yolk to the tissues of the chick embryo. J. Nutr. Biochem. 1998;9:645–651. [Google Scholar]
- Surai P.F., Speake B.K., Decrock F., Groscolas R. Transfer of vitamins E and A from yolk to embryo during development of the king penguin. Physiol. Biochem. Zool. 2001a;74:928–936. doi: 10.1086/338062. [DOI] [PubMed] [Google Scholar]
- Surai P.F., Speake B.K., Sparks N.H.C. Carotenoids in avian nutrition and embryonic development. 2. Antioxidant properties and discrimination in embryonic tissues. J. Poultry Sci. 2001b;38:117–145. [Google Scholar]
- Yu Y.E., Zhang Y., Unni E., Shirley C.R., Deng J.M., Russell L.D., Weil M.M., Behringer R.R., Meistrich M.L. Abnormal spermatogenesis and reduced fertility in transition nuclear protein 1-deficient mice. Proc. Natl Acad. Sci. USA. 2000;97:4683–4688. doi: 10.1073/pnas.97.9.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]