Abstract
Economic decision-making depends on our social environment. Humans tend to respond differently to inequity in close relationships, yet we know little about the potential for such variation in other species. We examine responses to inequity in several groups of chimpanzees (Pan troglodytes) in a paradigm similar to that used previously in capuchin monkeys (Cebus apella). We demonstrate that, like capuchin monkeys, chimpanzees show a response to inequity of rewards that is based upon the partner receiving the reward rather than the presence of the reward alone. However, we also found a great amount of variation between groups tested, indicating that chimpanzees, like people, respond to inequity in a variable manner, which we speculate could be caused by such variables as group size, the social closeness of the group (as reflected in length of time that the group has been together) and group-specific traditions.
Keywords: inequity, value, relationship quality, within-species variation, chimpanzee, Pantrogolodytes
1. Introduction
Inequity aversion (IA) appears to have played a key role in the evolution of cooperation in humans (Fehr & Fischbacher 2003), yet little is known about how other species respond to unequal pay-offs. Not all species are expected to show an adverse response to inequity, but such a response is expected in species with high levels of cooperation (Dugatkin 1997). Furthermore, species that are socially tolerant may have expectations about what they should receive (de Waal 1996), again predisposing them for IA.
As laid out by Fehr & Schmidt (1999), there are two kinds of IA: disadvantageous IA, or disliking if another individual receives more than yourself, and advantageous IA, or disliking if you receive more than another individual (i.e. overcompensation; Walster [Hatfield] et al. 1978). Although in humans disadvantageous IA may manifest itself as the willingness to sacrifice potential gain to block another individual from receiving a superior reward (Walster [Hatfield] et al. 1978; Fehr & Schmidt 1999), such complex behaviour probably evolved over a series of simpler, intermediate steps which, at each point, increased the individual’s relative fitness (Brosnan & de Waal 2004a). A first step is the recognition that other individuals obtain rewards that are different from one’s own, which is also important for other behaviours, such as social learning. At the second level, the maligned individual feels strongly enough to react to the discrepancy, presumably leading to the abandonment of the current inequitable relationship. At the third level, the individual will sacrifice their own gains to take away from those of a lucky individual, restoring equity. Advantageous IA, by contrast, consists of responses when either the self or an observed third party is overcompensated, which presumably developed after the evolution of disadvantageous IA. We focus on the second precursor of disadvantageous IA (i.e. whether chimpanzees react if they receive pay-offs different from those of a conspecific partner) and on one element of advantageous IA (i.e. whether ‘lucky’ individuals show any response to overcompensation).
Chimpanzees (Pan troglodytes) are one species from which we might expect to see such variation. First, chimpanzees are capable of modifying their responses as the social situation changes (e.g. food calls; Brosnan & de Waal 2003a). Furthermore, chimpanzees are the species most closely related to humans and commonly thought to elucidate the behaviour of our early ancestors (Boehm 1999; Tomasello 1999), and thus are ideal subjects for elucidating the evolution of the more complex stages of IA in the primate lineage.
For several reasons, we chose to test subjects from two different social groups of chimpanzees as well as two pair-housed groups. First, we know that chimpanzees’ behaviour varies among different groups based upon such characteristics as their housing situation (Aureli & de Waal 1997; Baker et al. 2000) and their social group (Whiten et al. 1999), but, owing to a scarcity of captive chimpanzee groups, it is rare that scientists are able to use individuals from more than one group. Second, the human inequity averse response is quite variable, and social relationships affect responses to inequity. Individuals in positive relationships are more oriented towards equity and averse to getting more than their partner than those in negative relationships (Loewenstein et al. 1989), and those in close relationships follow communal orientation whereas those in more distant relationships follow contingent rules such as equity or equality (Walster [Hatfield] et al. 1978; Clark & Grote 2003; de Waal & Brosnan 2004). Thus, more varied responses to IA may also be expected in animal societies that have strong social relationships and vary their behaviour between situations.
Chimpanzees were tested for their reactions to inequitable situations in which the reward level varied, as well as the amount of effort required to obtain it. Based on what is known about the inequity response in capuchins (Cebus apella; Brosnan & de Waal 2003a), we predicted that chimpanzees would show a strong response to inequity of both reward and effort. However, based on human data we expected significant variation between groups, presumably based on factors such as length of co-housing and the degree of sociality in the group, although with only two groups and a few pair-housed individuals we cannot tease apart these variables with certainty.
2. Material and methods
2.1 Study subjects
Study subjects were adult chimpanzees from the Yerkes National Primate Research Center, Atlanta GA, USA. Four individuals, from the Yerkes Main Center, lived continuously as pairs, housed in indoor–outdoor runs that had a ‘window’ of mesh between neighbouring runs that allows the chimpanzees visual and vocal contact with other pair-housed groups. These pairs (1 male/male pair, 1 female/female pair) were tested in the outdoor section of their runs. Sixteen individuals came from two different social groups at the Yerkes field station. These individuals lived in large outdoor corrals with interior runs. Each social group consisted of 18–22 chimpanzees (only a subset of adults from each group was tested) with a normal demographic distribution. Individuals entered or left the group only through birth, illness or death. One group (G-2) had been housed together for more than 30 years (i.e. long-term social group, eight females, two males), and all subjects but one were born and reared within the group; the exceptional subject was present at the group formation. The other social group (G-12) had been put together a mere 8 years before the study (Seres et al. 2001) (i.e. short-term social group, four females, two males), thus no subject had been born in the group. For testing, unrelated (e.g. individuals who are not related through their female lineage) same-sex pairs were isolated from the rest of their group in indoor cages of their home enclosure. All subjects were adults, and the age distribution between subjects in different groups was similar.
All chimpanzees received daily rations of Purina Large Primate Chow and various produce, and water was available ad libitum. There was no food or water deprivation. Testing depended entirely upon the motivation of the chimpanzees to participate and the desirability of the food reward.
2.2 Experimental testing procedure
The procedure used in this test was an exchange paradigm that had been used in other testing situations for 6–10 months with the pair-housed chimpanzees and the long-term social group and for three months with the short-term social group before this test. However, although familiar with exchange, no chimpanzee had had any experience with a situation in which they were rewarded differently from a partner before this experiment, and no pre-training on reactions to inequity was done before the results reported here.
For exchange, subjects were given a token (a 20 cm long, 3.7 cm diameter white PVC pipe), which they had to return to the experimenter to receive a food reward. Food rewards were placed in identical buckets on the floor in front of the chimpanzees. The chimpanzees could easily see what was in the buckets, but neither individual was shown what reward they would receive for any given exchange until they had successfully returned the token.
Food rewards were chosen on the basis of independent dichotomous-choice food preference tests (Brosnan & de Waal 2004b) to determine which of a pair of food items the chimpanzees preferred. Ultimately, grapes were used as the high-value food item for all individuals, and for the low-value food we used half of a slice of cucumber for the two groups at the field station (G2 and G12) and 4 cm lengths of celery for the Main Center chimpanzees.
Each test session consisted of a series of 50 trials, with trials alternating between the partner and the subject such that each individual received 25 trials per session and the partner always exchanged immediately before the subject. Trials were separated only by the amount of time it took the exchanger to get ready for the next trial (ca. 10–20 s). Individuals were paired with a partner who remained the same throughout testing. Because some of these individuals were pair-housed, each served as the partner for the other.
2.3 Testing paradigms
Each subject underwent four tests. The equity test (ET) was a baseline test in which both the subject and the partner exchanged for a low-value reward (cucumber or celery). For the inequity test (IT), which determined their response to an unequal reward distribution, the partner initially exchanged for a high-value reward (grape) followed by the subject exchanging for a low-value reward. For the effort control test (EC) the partner was initially handed a high-value reward without having to exchange for it (e.g. it was a gift), after which the subject had to exchange to receive the low-value reward. For the food control test (FC), the higher-value reward was present but not given to a conspecific. Before each exchange, a grape was held before the pair, but placed on the ground and not given to either individual.
For each individual, we measured the frequency of refusals to exchange and the latency to exchange. Refusals to exchange were divided into two categories, not returning the token and refusing the reward. Because subjects were not shown what reward they would receive before exchange, we felt both represented an unusual reaction to the testing situation. Both included passive refusals (refusing to return the token or accept the reward) as well as active ones (throwing the token or reward out of the cage). To be conservative, an exchange was only considered a refusal to accept the reward if it never came into the vicinity of the subject’s mouth. Latency to exchange was the amount of time it took for individuals to return the token to the experimenter’s hand from the time they received it from the experimenter, and was measured for all successfully completed exchanges, including those in which the individual failed to consume the food reward.
All testing was taped using a video camera (digital or Super VHS) that time-stamped all video to the nearest hundredth of a second. A second experimenter was always present to record data, but extra information could be extracted from the videotapes as necessary.
2.4 Statistics
Repeated-measures analyses of variance (ANOVA) were performed to look at the variation in refusals to exchange across different testing situations. Methods followed Quinn & Keough (2002) and tests were performed using SAS v. 8.2. Data were inspected for outliers, normality and homogeneity of variance before performing statistical tests. The effects of housing regime, sex, dominance status and time on individual refusals to exchange were determined using mixed within-subjects repeated-measures analyses of variance with individuals nested within housing regime (PROC MIXED in SAS; Wolfinger & Chang 1995). In these analyses, housing regime, sex and dominance status were treated as between-subjects fixed factors, time as a fixed within-subjects repeated measure and individuals as a random factor. Mixed-model statistical analyses require model fitting before examining the hypothesis tests; Akaike (AIC) and Schwarz (BIC) information criteria were used to do so (Wolfinger & Chang 1995). Out of the four covariance structures examined (compound symmetry, Huynh–Feldt, variance components and unstructured), the Huynh–Feldt covariance structure best fit the exchange data. When applicable, least-squares means estimates with sequential Dunn–Sidak adjustments were used to interpret differences among levels of the main and interaction effects and to prevent compounding of type I error. Non-significant interaction terms were excluded from the original model (housing×sex, sex×dominance, sex×time, dominance×time) and the reduced model was used for hypothesis testing.
Housing condition was divided into three categories: long-term group (G12 group), short-term group (G2 group) and pair-housed (both pairs combined into one category). Rank was based on the rank of the subject and partner relative to each other, in the absence of any other individuals, and was determined from independent observations of those two individuals together. Paired comparisons were done using paired t-tests. All tests were performed based on the means of each individual’s score for each test, to avoid artificially inflating the sample size. Comparisons of the changes in level of exchange across the 25 trials were done using Zar’s method for comparing the slopes of the linear regression of each dataset (Zar 1996). This allowed us to see if there was significant variation between the different conditions in the frequency of exchanges over time. All p-values reported are two-tailed.
3. Results
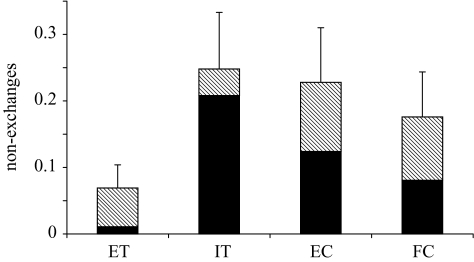
A common reaction to inequity was refusal to exchange, with subjects showing far fewer refusals in the ET than in other tests in which superior rewards were present (figure 1; F24,55=3.87, p < 0.0001). The chimpanzees showed no variation in their willingness to exchange based on gender (F1,13=0.11, p=0.7438). Furthermore, their dominance rank compared with their partner had no effect on their level of response (F1,13=1.04, p=0.3275). Dyadic dominance ranks were determined based on independent observations of the group.
Figure 1.
Mean±s.e.m. of failures to exchange for the chimpanzees across the four test types. The black bars represent the proportion of non-exchanges as a result of refusal to accept the reward, and the hatched bars represent the proportion of non-exchanges as a result of refusal to return the token. ET, equity test; IT, inequity test; EC, effort control; FC, food control.
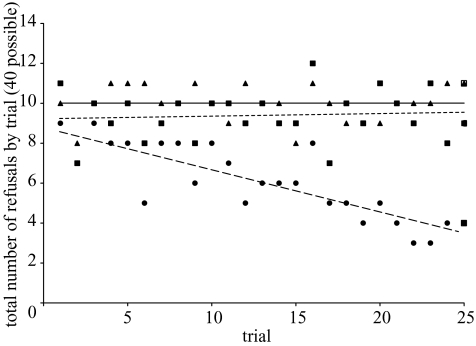
Subjects did not show any significant variation between the IT and the EC tests, indicating that they did not pay attention to effort. However, whereas chimpanzees always show some response, apparently based on the presence of the higher-value food reward, they distinguish situations in which a conspecific receives the better reward from those in which the better reward is merely visible. Not only did subjects show higher levels of refusal to return the token or accept the reward if the food reward was given to a conspecific (IT) than if the food reward was merely visible (comparing IT and FC: reward refusals: t=2.42, d.f.=19, p=0.026; token refusals: t=2.21, d.f.=19, p=0.040), but they also decreased their refusals to exchange as the session progressed in the FC, but not in other tests, as shown by a comparison of the slopes of the linear regression lines (Zar 1996) (figure 2; overall: F2,69=16.83, p < 0.005; paired comparisons: IT versus FC, t=5.03, d.f.=46, p < 0.005; EC versus FC, t=4.95, d.f.=46, p < 0.005, IT versus EC, t=0.32, d.f.=46, NS).
Figure 2.
Sum of the failures to exchange for each subject across sessions within a test type. Lines represent the linear regression of the data. Only those tests in which a higher-value reward was used are included (IT, EC, FC). IT, filled squares; EC, filled triangles; FC, filled circles. (Abbreviatious as in figure 1.)
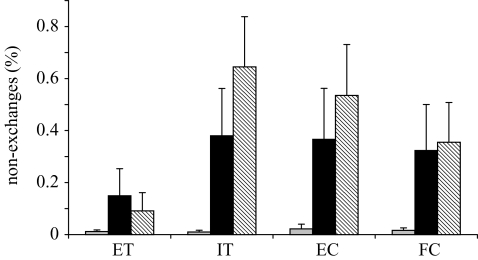
As predicted, the chimpanzees’ willingness to complete the exchange was affected by social and housing conditions (F2,13=4.84, p=0.0269). Individuals who were pair-housed or members of the short-term social group frequently refused to exchange when the partner received a superior reward. However, members of the long-term social group reacted dramatically differently, virtually never refusing to exchange regardless of the situation (figure 3).
Figure 3.
Mean±s.e.m. of failures to exchange for each of the three housing conditions across the four test types. Grey bars represent subjects from the long-term social group, which had been co-housed for more than 30 years, black bars represent subjects from the short-term social group, which had been co-housed for ca. 8 years, and hatched bars represent subjects who were pair housed. Abbreviations as in figure 1.
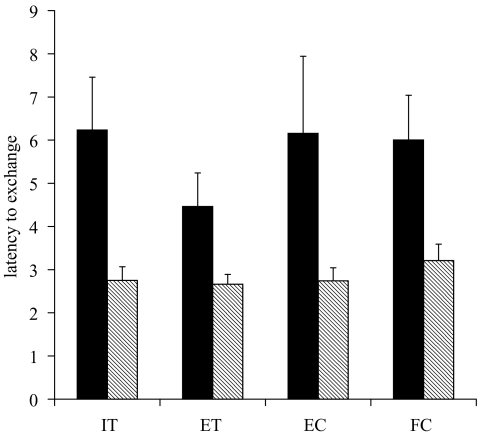
Based on the fact that we had two categories of response, we designated six individuals (all from the short-term or pair-housed group) who refused to exchange at least 10% of the time as ‘high-rate refusers’ (mean proportion of refusals: 0.561±0.072), and 14 individuals who virtually never refused to exchange as ‘low-rate refusers’ (mean proportion of refusals: 0.017±0.004). Intriguingly, the only difference we found between these two groups, other than their variation in refusal rate, was that high-rate refusers took almost twice as long to exchange (if they exchanged) as their low-rate refusing counterparts. This was true across all four tests, including the ET (figure 4: t=7.361, d.f.=4, p=0.005).
Figure 4.
Latency to exchange for high- versus low-rate refusers across the four test types. Black bars represent high-rate refusers (refuse to exchange at least 10% of the time) and hatched bars represent low-rate refusers (refuse to exchange less than 10% of the time). Abbreviations as in figure 1.
Chimpanzees showed no indication of advantageous IA, in which the overcompensated individual reacts to the discrepancy (Fehr & Schmidt 1999). In the IT and EC tests, in which the partner received the superior reward but the subject received the lower-value reward, none of the 20 partners ever refused to exchange the token. Furthermore, partners refused their higher-value rewards in only two instances in the EC and three instances in the IT (out of 2000 trials). Finally, there were no instances of active sharing between the partner and the subject and only four possible instances of passive sharing (the partner dropped his or her higher-value reward and did not protest when the subject collected it). This represents, at best, an active sharing rate of 0.002%, which is much lower than spontaneous sharing within a group of chimpanzees (de Waal 1989). Finally, the partner’s latency to exchange was no different if the subject received a lesser-value reward than if the subject received the same reward (comparing partner latency with exchange in the ET versus IT: t=−1.44, d.f.=19, p=0.168).
4. Discussion
Chimpanzees do show variation in their exchange behaviour consistent with IA. They decline to complete the exchange interaction when their partner receives a superior reward for the same amount of effort. Furthermore, there is no effect of the subject’s gender or their rank relative to their partner.
Although chimpanzees respond negatively to being short-changed relative to their partner, their willingness to participate is gradually restored if the better reward is visible but not given to a conspecific partner. In this case, although their initial response is as strong as in those situations in which the partner gets the higher reward, the reaction consistently declines over the course of the session. This is in sharp contrast to the situations in which the partner receives the superior reward, in which the rate of refusals stays constant. Thus, the chimpanzee’s response is apparently based on the partner receiving the superior reward rather than its mere presence.
Whereas the chimpanzees respond to reward discrepancies, they do not appear to respond to discrepancies in the level of effort. This is surprising, given that we previously found evidence that capuchin monkeys do show such a discrimination (Brosnan & de Waal 2003a). Although it may be that chimpanzees do not distinguish between or react to different levels of effort, it is also possible that their lack of reaction reflects their perception of exchange as too trivial of a physical effort to merit a reaction based upon the effort expended. The chimpanzees, though they on average acquired exchange more readily than capuchins, showed no difference in their exchange ability at the time of testing. However, the capuchins required a full-body motion to return their token (a small granite rock appropriate for their body size), whereas the chimpanzees required only a movement of the arm to return the tokens.
Chimpanzees also do not demonstrate advantageous IA, that is, they do not react when they receive a reward superior to that of their partner. Perhaps chimpanzees do not notice the discrepancy in being overcompensated, yet it is difficult to believe that they notice if they receive the lower-value reward but not the higher-value one. We suspect that they do notice, but it does not cause any modification of behaviour, indicating that, like the capuchin monkeys, they operate at the level of disadvantageous IA, but not advantageous IA (Fehr & Schmidt 1999). Although this may represent a difference from human responses, there is also evidence against human aversion to advantageous IA. In fact, whereas people may prefer equity to any sort of inequity, advantageous inequity is typically preferred to disadvantageous inequity (Loewenstein et al. 1989), most people tend to respond by psychological rather than material compensation—that is, justifying why they deserved a superior reward (Walster [Hatfield] et al. 1978)—d most people will choose to ignore information that could lead to a more fair outcome at a cost to the self (Dana et al. 2003).
As predicted, the chimpanzees’ willingness to complete the exchange was strongly affected by social and housing conditions, apparently including both group size and the strength of the relationship, using the length of time individuals had lived together as a proxy measure. This significant variability in reaction is the first demonstration that reactions to inequity in non-human animals may parallel the strong variation in response in humans based on the quality of the relationship (Loewenstein et al. 1989; Clark & Grote 2003).
There is some precedent for variable reactions between chimpanzee groups. Individual chimpanzees do alter their behaviour dependent upon the current social situation (Brosnan & de Waal 2003b), their housing situation (Aureli & de Waal 1997; Baker et al. 2000) or their social group (Whiten et al. 1999). However, owing to the scarcity of chimpanzees, most behavioural testing uses individuals from only a single social group (or chimpanzees from pair- or single-housed situations). Bearing these results in mind, future behavioural studies need to take the chimpanzees’ background and familiarity into account and test multiple groups whenever possible to avoid bias in the dataset.
Although we are unable to determine the precise reasons for the variation in this study, some possibilities emerged. We consider it likely that, as with humans, this variation is based on the close social relationships within well-established groups. Aside from their response to inequity, the long-term social group in our study shows high levels of reciprocity in food sharing and grooming (de Waal 1997), extensive reconciliation after fights (Preuschoft et al. 2002) and a tendency to avoid confrontation (Hare et al. 2000). There are two non-exclusive explanations for the differences in behaviour. First, in the long-term social group, all but one of the pairs tested had been born and raised together in this group. In the exceptional pair, the younger individual was born and raised in the group when the older partner, a founding member of the group, was an adult. Thus, these individuals, who played together as juveniles before reaching adulthood, may have formed extremely close, almost kin-like, relationships mostly absent in the subjects recruited from other conditions. In humans, current theory proposes that individuals in close relationships (marital, family or friendship) follow communal rules, which do not pay overt attention to fairness and switch to contingent rule-based behaviour such as equity or inequality only when there is stress in the relationship (Clark & Grote 2003). If our long-term group of chimpanzees has similarly close relationships, the inequity presented to them may be largely irrelevant within the context of their relationships. Second, if there is an element of contingent behaviour in this group, there also exists a delicate balance between competition and cooperation, so that any negative reaction may be interpreted as a slight by the partner, and hence have far-reaching consequences. The absence of reactions to the partner’s receipt of a superior reward may therefore reflect a sophisticated conflict-avoidance strategy.
The short-term social group, by contrast, was still working out social issues 4 years after its formation (Seres et al. 2001); thus it is unlikely that they share the close relationships possible in the long-term social group. Further, they may not have reached full stability, which allows for sophisticated conflict avoidance. In fact, whereas in the long-term social group we found all pairs equally acceptable for testing, in the short-term group pairs had to be carefully selected, as several individuals refused to participate with certain partners. Among the pair-housed individuals, while one pair had a long history, the lack of any far-reaching social consequences (e.g. coalitions against third parties are impossible in a pair) may have led to stronger reactions in our experiments. Thus, for these pair-housed individuals the long-term costs of a reaction are minimal, and there is nothing to lose by reacting to inequity. This hypothesis deserves further testing.
Another intriguing bit of evidence is the variation in the latency to exchange between those individuals that frequently refused to exchange and those that virtually always completed the interaction. Because the delay is present in the ET, this does not seem to be a mechanism for responding to inequity, but instead a general aspect of these individuals’ exchange behaviour. Although causation is clearly unknown, it is intriguing that those individuals that take more time, and thus may have time to evaluate the situation, are more likely to react to inequity. This behaviour may be more common in less stable groups.
We found IA to be present and robust in chimpanzees, but only in subjects that lived in pairs or in a relatively newly established social group. In a far older group, with its tightly knit social structure characterized by intense integration and social reciprocity, inequity caused barely a ripple. This finding may parallel human responses in close relationships (Clark & Grote 2003), and inequity may be tolerated more as apes develop the mutual dependencies and bonds that otherwise serve a wide range of benefits derived from sociality. If so, tolerance of inequity may increase with social closeness between partners, such as friends and family, in a wide variety of species, a hypothesis that deserves further testing in humans and non-human primates.
Acknowledgments
Research was supported by a grant from the National Institutes of Health (RR-00165) to the Yerkes National Primate Research Center, and a National Science Foundation Graduate Research Fellowship to the first author. We are grateful to Ryan Earley for statistical assistance, Simon Gächter and several anonymous reviewers for comments on an earlier draft of the manuscript, and Rebecca Singer, Kelly Bouxsein and Jennifer Rybak for assistance with data collection. The Yerkes Primate Center is fully accredited by the American Association for Accreditation of Laboratory Animal Care.
References
- Aureli F., de Waal Frans B.M. Inhibition of social behavior in chimpanzees under high-density conditions. Am. J. Primatol. 1997;41:213–228. doi: 10.1002/(SICI)1098-2345(1997)41:3<213::AID-AJP4>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Baker K., Seres M., Aureli F., de Waal F.B.M. Injury risks among chimpanzees in three housing conditions. Am. J. Primatol. 2000;51:161–175. doi: 10.1002/1098-2345(200007)51:3<161::AID-AJP1>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Boehm C. Hierarchy in the forest: the evolution of egalitarian behavior. Harvard University Press; Cambridge, MA: 1999. [Google Scholar]
- Brosnan S.F., de Waal F.B.M. Monkeys reject unequal pay. Nature. 2003a;425:297–299. doi: 10.1038/nature01963. [DOI] [PubMed] [Google Scholar]
- Brosnan S.F., de Waal F.B.M. Regulation of vocal output by chimpanzees finding food in the presence or absence of an audience. Evol. Commun. 2003b;4:211–224. [Google Scholar]
- Brosnan S.F., de Waal F.B.M. Reply to Henrich and Wynne. Nature. 2004a;428:140. [Google Scholar]
- Brosnan S.F., de Waal F.B.M. A concept of value during experimental exchange in brown capuchin monkeys. Folia Primatologica. 2004b;75:317–330. doi: 10.1159/000080209. [DOI] [PubMed] [Google Scholar]
- Clark M.S., Grote N.K. Close relationships. In: Millon T., Lerner M.J., editors. Handbook of psychology: personality and social psychology. Wiley; New York: 2003. pp. 447–461. [Google Scholar]
- Dana J.D., Weber R.A., Kuang J. Exploiting moral wriggle room: behavior inconsistent with a preference for fair outcomes. 2003 Carnegie Mellon Behavioral Decision Research working paper no. 349. See http://papers.ssrn.com/sol3/papers.cfm?abstract_id=400900. [Google Scholar]
- de Waal F.B.M. Food sharing and reciprocal obligations among chimpanzees. J. Hum. Evol. 1989;18:433–459. [Google Scholar]
- de Waal F.B.M. Good natured: the origins of right and wrong in humans and other animals. Harvard University Press; Cambridge, MA: 1996. [Google Scholar]
- de Waal F.B.M. The chimpanzee’s service economy: food for grooming. Evol. Hum. Behav. 1997;18:375–386. [Google Scholar]
- de Waal F.B.M., Brosnan S.F. Simple and complex reciprocity in primates. In: Kapeller P., van Schaik C.P., editors. Cooperation in primates and humans: evolution and mechanisms. Springer; Berlin: 2004. [Google Scholar]
- Dugatkin L.A. Cooperation among animals: an evolutionary perspective. Oxford University Press; New York: 1997. [Google Scholar]
- Fehr E., Fischbacher U. The nature of human altruism. Nature. 2003;425:785–791. doi: 10.1038/nature02043. [DOI] [PubMed] [Google Scholar]
- Fehr E., Schmidt K.M. A theory of fairness, competition, and cooperation. Q. J. Econ. 1999;114:817–868. [Google Scholar]
- Hare B., Call J., Agnetta B., Tomasello M. Chimpanzees know what conspecifics do and do not see. Anim. Behav. 2000;59:771–785. doi: 10.1006/anbe.1999.1377. [DOI] [PubMed] [Google Scholar]
- Loewenstein G.F., Thompson L., Bazerman M.H. Social utiity and decision making in interpersonal contexts. J. Person. Social Psychol. 1989;57:426–441. [Google Scholar]
- Preuschoft S., Wang X., Aureli F., de Waal F.B.M. Reconciliation in captive chimpanzees: a reevaluation with controlled methods. Int. J. Primatol. 2002;23:29–50. [Google Scholar]
- Quinn G.P., Keough M.J. Experimental design and data analysis for biologists. Cambridge University Press; 2002. [Google Scholar]
- Seres M., Aureli F., de Waal F.B.M. Successful formation of a large chimpanzee group out of two preexisting subgroups. Zoo Biol. 2001;20:501–515. [Google Scholar]
- Tomasello M. The cultural origins of human cognition. Harvard University Press; Cambridge, MA: 1999. [Google Scholar]
- Walster [Hatfield] E., Walster G.W., Berscheid E. Equity: theory and research. Allyn and Bacon; Boston, MA: 1978. [Google Scholar]
- Whiten A., Goodall J., McGrew W.C., Nishida T., Reynolds V., Sugiyama Y., Tutin C.E.G., Wrangham R.W., Boesch C. Cultures in chimpanzees. Nature. 1999;399:682–685. doi: 10.1038/21415. [DOI] [PubMed] [Google Scholar]
- Wolfinger R., Chang M. Comparing the SAS.® GLM and MIXED procedures for repeated measures. Proc 20th Ann. SAS Users Group Conf. 1995:1–11. [Google Scholar]
- Zar J.H. Biostatistical analysis. 3rd edn. Prentice-Hall; Saddle River, NJ: 1996. [Google Scholar]