Abstract
We present evidence that in the absence of the transfer of male gland compounds in the ejaculate as well as of behavioural male traits, such as mate guarding or harming of females, sperm itself affects female life-history traits such as hibernation success, female longevity and female fitness. Using the bumble-bee Bombus terrestris, we artificially inseminated queens (females) with sperm from one or several males and show that sire groups (groups of brother males) vary in their effects on queen hibernation survival, longevity and fitness. In addition, multiply inseminated queens always had a lower performance as compared to singly inseminated queens. Apart from these main effects, sire groups (in situations of multiple insemination) affected queen longevity and fitness not independently of each other, i.e. certain sire group combinations were more harmful to queens than others. So far, the cause(s) of these effects remain(s) elusive. Harmful male traits as detected here are not necessarily expected to evolve in social insects because males depend on females for a successful completion of a colony cycle and thus have strong convergent interests with their mates.
Keywords: life history, cost of mating, artificial insemination, male social insects
1. Introduction
In many species of animals males increase their fitness by manipulating females, sometimes even at the cost of reducing female survival and fitness (Eberhard 1996; Johnstone & Keller 2000; Simmons 2001). Apart from mechanical manipulations such as mate guarding (Duvoisin et al. 1999), physical female damaging (Crudgington & Siva-Jothy 2000) and mating plugs (Simmons 2001), biochemical compounds of male glands influence female fitness (Gillott 1996; Kubli 2003), for example by being toxic for the female (Chapman et al. 1995), acting as anti-aphrodisiac (Chen et al. 1988) or inducing oogenesis and increasing oviposition rates (Simmons 2001).
Far less work has been done to look at possible direct influences of sperm, that is, without any accessory gland products within the ejaculate. Their effects are therefore underestimated (Eberhard 1996). This is especially true for social hymenopteran insects (the ants, wasps and bees), where male reproductive traits have received broader scientific interest only recently (Baer 2003). The sperm of hymenopteran insects have several idiosyncrasies that are rarely found in other organisms (Baer & Boomsma 2004). For example, sperm of an individual male is clonal because of male haploidy and thus, intramale sperm competition is absent (Baer 2003). Because monandry is widespread among social insects (Strassmann 2001), competition between sperm of different males (i.e. sperm competition) is generally also rare in social insects. In addition, the life history of hymenopteran social insects is characterized by a single female mating episode early in adult life but a subsequent long female lifespan, in some cases up to several decades. Similarly, a large number of offspring is usually produced, of which only a few become offspring surviving into the next generation. Consequently, selective forces acting on sperm function and morphology should have been rather different compared with other organisms (Baer et al. 2003). For example, colonies of Pogonomyrmex owyheei survive for up to 30 years (Hölldobler & Wilson 1990) or 27 years in Formica execta (Pamilo 1991) and social insect colonies can maintain worker forces of up to 20 million individuals in army ants (Raignier & Van Boven 1955). In the absence of the queen remating later in life, social insect sperm not only survives within the female for such prolonged periods of time but also maintains high fertility to secure high queen fecundity. Unfortunately, we have only marginal information of these aspects of fertility of social insects (Baer 2003).
Here, we used the annual eusocial bumble-bee B. terrestris, where reproductive male traits have been studied intensively over the last years (Baer 2003). Males manipulate females and paternity by mate guarding and with a mating plug (Duvoisin et al. 1999). The mating plug contains linoleic acid that acts as an anti-aphrodisiac and reduces the queen’s willingness to remate (Baer et al. 2001). Although B. terrestris is monandrous, polyandry would be beneficial for queens because of a reduction in parasitism in worker offspring (Baer & Schmid-Hempel 1999). However, forced polyandry of B. terrestris queens also revealed the presence of a yet unidentified cost of polyandry (Baer & Schmid-Hempel 2001).
Here, we used 27 colonies as a source for groups of brother males (referred to as male sire groups) and tested whether sperm influences the life history of queens, which originated from eight different maternal colonies (queen sire groups). Apart from testing for effects of sire groups on female life history, in particular hibernation success and variation in fitness of the colony founded by the surviving queens, we were interested whether effects of sperm on female life history are additive when sperm of more than one male is present in a female, and in the absence of accessory male gland products. The present experimental design represents two major advances compared with an earlier study by Korner & Schmid-Hempel (2003): in addition to testing for additive effects of sperm on queen life history we also tested for such effects under field conditions.
2. Methods
The present article presents a re-analysis of unpublished data from the experiment of Baer & Schmid-Hempel (2001), where more details on the experimental protocol can be found. In addition to this earlier study, and in line with a different question asked, we included all queens in the present analysis (n=202) and not only those producing colonies with more than 15 workers (n=53). In the experiment, queens of B. terrestris were raised within their maternal colonies, and artificially inseminated according to the standard protocol (Baer & Schmid-Hempel 2000). It is important to mention that this method allows collecting and transferring sperm without any products of the accessory glands, therefore testing for effects of sperm solely on female life history. Males were killed to collect sperm by dissecting the two accessory testes. For the transfer of sperm, queens were briefly anaesthetized with carbon dioxide and sperm was transferred into the female’s bursa copulatrix. To test for effects of sperm, we assigned queens randomly to one of four treatments: (i) inseminated once with sperm of a single male; (ii) inseminated once with a mixture of sperm from two unrelated males; (iii) inseminated in four subsequent rounds with a mixture of sperm from four brothers; and (iv) inseminated in four subsequent rounds with sperm from four unrelated males. For treatment (ii), we only collected sperm from a single accessory testis from each of the two males, thus, queens of treatment (i) and (ii) received approximately the same total amount of sperm. Queens were afterwards artificially hibernated at 4°C for two weeks before they were allowed to start a colony in climate chambers at 28°C and food ad libitum (pollen and sugar water). As soon as 15 workers were present in a colony, they were transferred to a field site close to Zurich (Switzerland) and remained there until the end of the colony cycle. Throughout the experiment we monitored life-history data such as a queen’s hibernation success, a queen’s total lifespan (longevity, measured as survival in days between a queen’s first insemination until her death, which reflects queen survival after sperm acquisition) as well as colony fitness by collecting all emerging sexuals (gynes and drones). Fitness was calculated as the sum of males plus two times the number of virgin queens, a fitness measurement which has been repeatedly used as fitness variable in bumble-bees (Baer & Schmid-Hempel 1999, 2001; Korner & Schmid-Hempel 2003).
In total we used sperm of 584 males from 27 colonies and artificially inseminated 202 queens from eight different colonies. None of the males used for artificial inseminations was related to the queen. Data were analysed with SPSS, v. 11 for Macintosh X and all tests are reported with two-tailed probabilities. Fitness measurements and longevity of queens were transformed using the natural logarithm to ln(value +1) to normalize data and tested using ANOVA; hibernation data were analysed using χ2 statistics and logistic regression models.
3. Results
3.1 Hibernation success
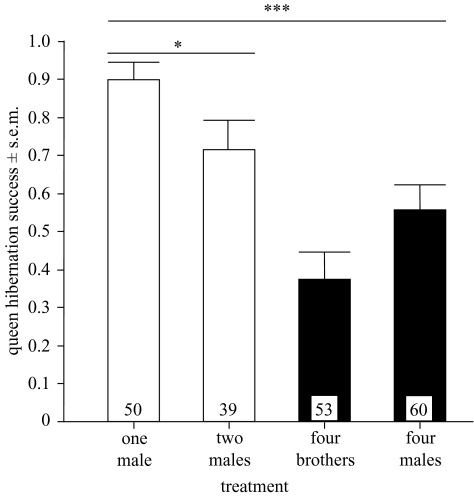
We found that whether or not a queen (female) survived hibernation varied with treatment and that the presence of sperm from more than one male within a female reduced the hibernation success (figure 1; n=202, d.f.=3, Pearson χ2=32.428, p < 0.001). Hibernation success decreased significantly when queens receiving only a single insemination with sperm from a single male were compared with those receiving sperm from two males (figure 1; queens with one versus two males: n=89, d.f.=1, Pearson χ2=4.925, p=0.026). These queens received the same total amount of sperm.
Figure 1.
Hibernation success of bumble-bee queens (probability of survival) according to treatment. Hibernation survival decreased with increasing numbers of males being used for artificial inseminations. The small numbers within the bars refer to the sample sizes used (i.e. number of queens). Significant differences are indicated (*p < 0.05; ***p < 0.001). Treatments were achieved with either one round of insemination (open bars) or four subsequent rounds of insemination (filled bars).
In addition, female hibernation success (survived or died) also varied significantly with male sire group (logistic regression: Wald χ2=64.003, d.f.=26, p < 0.001). The model explained a significant part of the overall variation (Cox & Snell r2=0.483, χ2=385.39, d.f.=26, p < 0.001) but no single sire group had a significant effect of its own.
The model could be improved when, in addition to sire group, insemination treatment (mating frequency) was included as a variable in the logistic regression. In this case, r2=0.499 (χ2=403.51, d.f.=29, p < 001; 83% of cases are correctly classified). In particular, hibernation success again varied with insemination treatment (Wald χ2=13.99, d.f.=3, p=0.003). The biggest effect originated from comparing insemination with sperm from a single versus two unrelated males (odds=2.04, Wald χ2=11.88, d.f.=1, p=0.001; see figure 1). Interestingly, insemination treatment had no interaction with any particular sire group. In other words, insemination treatment had an additive effect on hibernation success regardless of sire group, most significantly by lowering survival when going from the insemination with one male to an insemination with sperm from two unrelated males (figure 1).
3.2 Queen longevity and fitness
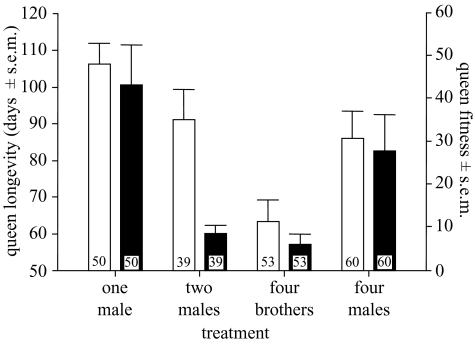
Similar to hibernation success, queen longevity (days from first insemination to death) as well as queen (female) fitness decreased with the presence of sperm from additional males within the queen (figure 2), in agreement with the results of a more restricted sample reported earlier (Baer & Schmid-Hempel 2000). Part of our measurement of queen longevity was already included in the considera tion on hibernation success (yes or no, instead of days of survival). If only post-hibernation survival was considered, queen longevity did significantly differ between treatments (ANOVA: F3,124=3.11, p=0.029) but was not generally lower for queens which received sperm from more than one male compared with singly inseminated queens. Consequently, hibernation survival explained the major part of the variation in queen longevity.
Figure 2.
Queen longevity (open bars) and queen fitness (filled bars; see § 2) in relation to treatment. Queen longevity as well as queen fitness generally decreased with increasing numbers of males used for artificial inseminations. The small numbers within the bars refer to sample sizes (i.e. number of queens used per treatment).
Further new insights emerged, however, because a MANOVA (for hibernation success and queen fitness; table 1a) revealed not only significant differences between the four treatments (p=0.034), but also that male sire group had a significant influence on female fitness (p < 0.001) and a marked albeit marginally non-significant (p=0.051) effect on queen longevity. A highly significant male-sire-group × treatment interaction term indicated that the composition of male sire groups within females was an important factor determining both queen longevity (p=0.002) and colony fitness (p < 0.001).
Table 1.
MANOVA for the effects of male and queen sire group, and treatment on the dependents, colony longevity and colony fitness, for either (a) all queens or (b) queens surviving hibernation only.
| source | dependent variable | d.f. | F | p |
| (a) all queens | ||||
| intercept | fitness | 1 | 68.728 | <0.001 |
| longevity | 1 | 3733.437 | <0.001 | |
| male sire group | fitness | 23 | 4.447 | <0.001 |
| longevity | 23 | 1.547 | 0.051 | |
| queen sire group | fitness | 4 | 8.362 | <0.001 |
| longevity | 4 | 5.439 | <0.001 | |
| treatment | fitness | 3 | 4.778 | 0.003 |
| longevity | 3 | 2.922 | 0.034 | |
| male sire group × queen sire group | fitness | 4 | 0.018 | 0.999 |
| longevity | 4 | 0.012 | 0.999 | |
| male sire group × treatment | fitness | 26 | 3.389 | <0.001 |
| longevity | 26 | 2.045 | 0.002 | |
| queen sire group × treatment | fitness | 2 | 1.268 | 0.282 |
| longevity | 2 | 0.296 | 0.744 | |
| error | fitness | 511 | ||
| longevity | 511 | |||
| (b) queens that survived hibernation | ||||
| intercept | fitness | 1 | 134.402 | <0.001 |
| longevity | 1 | 14552.411 | <0.001 | |
| male sire group | fitness | 17 | 3.982 | <0.001 |
| longevity | 17 | 1.836 | 0.024 | |
| queen sire group | fitness | 2 | 8.362 | <0.001 |
| longevity | 2 | 0.723 | 0.486 | |
| treatment | fitness | 3 | 1.526 | 0.219 |
| longevity | 3 | 2.922 | 0.034 | |
| male sire group × queen sire group | fitness | 1 | 0.040 | 0.842 |
| longevity | 1 | 0.063 | 0.802 | |
| male sire group × treatment | fitness | 19 | 3.847 | <0.001 |
| longevity | 19 | 4.181 | <0.001 | |
| queen sire group × treatment | fitness | 1 | 0.031 | 0.336 |
| longevity | 1 | 0.169 | 0.681 | |
| error | fitness | 268 | ||
| longevity | 268 | |||
When we repeated the analysis for the subset of queens that had survived hibernation (table 1b) we found that treatment still had a significant effect on queen longevity (p=0.034), but no significant effect on fitness (p=0.219) was found any more. The latter result does not contradict our earlier analysis (Baer & Schmid-Hempel 2001) because not every queen which survived hibernation also produced a colony of more than 15 workers, which was our initial criterion to include a colony as having successfully established a colony in the earlier study. Male sire group had a significant effect on queen longevity (p=0.024) and fitness (p < 0.001). Again, the male-sire-group × treatment interaction was highly significant for both queen survival (p < 0.001) and queen fitness (p < 0.001).
4. Discussion
Polyandry and the resulting increase in genetic variation among worker offspring has repeatedly been shown to be beneficial in B. terrestris, because genetically heterogeneous colonies are less parasitized and have higher fitness (Baer & Schmid-Hempel 1999). But multiple mating of B. terrestris queens also induces a substantial cost (Baer & Schmid-Hempel 2001) and as we show here, sperm in combination with sperm of other males induces at least part of this cost (figures 1 and 2). The costs detected here are already expressed very early in the life cycle of a queen with an increase in mortality rate during hibernation and are less pronounced later in queen life. Consequently, polyandry has different fitness consequences during different stages of a queen’s life: it induces serious costs early in life (this study), especially during hibernation, but queens benefit from polyandry during their eusocial phase, when their worker offspring suffer less from parasitism (Baer & Schmid-Hempel 1999). These early costs of polyandry are substantial and might be a reason why queens of B. terrestris are generally monandrous, at least in the population studied here (Schmid-Hempel & Schmid-Hempel 2000).
Similar to Korner & Schmid-Hempel (2003), we used an artificial insemination technique where accessory gland products, which are known to affect at least the behaviour of mated bumble-bee queens (Baer et al. 2001; Sauter et al. 2001), are not transferred and thus cannot be the source of the effects observed. Hence, we must conclude that sperm alone affected hibernation success and fitness of the inseminated females. Even though we have no evidence in B. terrestris, such effects could in principle be a result of sex peptides adhering to the tails of sperm rather than being transferred in the rest of the ejaculate, as is the case in Drosophila melanogaster (Liu & Kubli 2003). Alternatively, if sperm storage is costly (Schoeters & Billen 2000), larger ejaculates or repeated inseminations should result in larger amounts of sperm present in the spermatheca and could consequently increase storage and/or fitness costs to the queen. However, queens inseminated once with a mixture of sperm from two males had a decreased hibernation success compared with queens inseminated with sperm from a single male (figure 1), despite the fact that queens of both treatments received approximately the same total amount of sperm. Hence, storage costs are unlikely to explain our findings. With multiple mating in particular, sperm may compete for storage, within the storage organ or during egg fertilization, which might result in additional energetical demands of sperm (provided by the queen) or the release of chemical compounds during sperm warfare harming queens as well. However, B. terrestris is a monandrous species (Schmid-Hempel & Schmid-Hempel 2000), and inter- and intraejacualtory sperm competition is absent.
A cost to the female could also be caused by sperm incompatibility. Under such a scenario, artificially transferred sperm from different males would interact with each other, inducing potential fitness costs to the queen. The degree to which this cost is established should vary depending on the male genotypes used for artificial insemination, given that certain male pairings induce larger incompatibilities than others. Our significant sire group × treatment interaction terms for queen longevity and fitness is compatible with the effects of sperm incompatibility. However, at the same time, we found that queens inseminated with four brothers performed less well than queens inseminated with four unrelated males (figures 1 and 2), indicating that sperm incompatibility is not fully explaining our data either (and assuming that incompatibility should be worse for unrelated males than for related ones).
Finally our findings could be an experimental artefact because we inseminated some queens only once (treatments (i) and (ii)) but others four times ((iii) and (iv)). However, we already find a decrease in a queen’s hibernation success, longevity and fitness if we compare the queens that received only a single insemination (treatments (i) and (ii); figures 1 and 2). Queens receiving four inseminations cannot really be compared here because they all received sperm from four males. If the number of inseminations per se has a negative effect on queen life-history traits, it remains puzzling why we observe differences within these groups, i.e. between (i) and (ii) as well as (iii) and (iv), where queens received the same number of inseminations. If additional inseminations would have been harmful, queens that received four inseminations should in general have much lower hibernation, longevity and fitness values, but they are in fact very similar between groups of queens with different numbers of total inseminations (figure 2). For example, queen longevity is similar in groups (ii) and (iv), or queen fitness is similar in groups (ii) and (iii). Consequently, the experimental set-up cannot explain our findings either, although we cannot rule out that the different numbers of inseminations between certain queens might have had some (minor) influence.
In conclusion, the results of this study suggest that some as yet unexplained effect of sperm influences female hibernation success, longevity and fitness. This corroborates results of a recent and independent study on the same study system (Korner & Schmid-Hempel 2003), but different to this earlier study we here performed a field study, inseminated queens more than once and were consequently able to test for effects of polyandry on queen life history. As in Korner & Schmid-Hempel (2003), we also find that sire groups have an independent effect on hibernation success, but additionally we find that they interact with each other under conditions of multiple mating when female longevity and fitness are analysed. Further studies will be needed to reveal further insights into the sophisticated male traits of social insects.
Acknowledgments
We thank B. Baer-Imhoof for comments on the manuscript. This work was supported by a Swiss Marie Curie individual fellowship to B.B. (83EU-062441) and a grant by the Swiss NSF (3100-66733.01 to P.S.-H.).
References
- Baer B. Bumble-bees as model organisms to study male sexual selection in social insects. Behav. Ecol. Sociobiol. 2003;54:521–533. [Google Scholar]
- Baer B., Boomsma J.J. Male reproductive investment and queen mating-frequency in fungus-growing ants. Behav. Ecol. 2004;15:426–432. [Google Scholar]
- Baer B., Schmid-Hempel P. Experimental variation in polyandry affects parasite loads and fitness in a bumble-bee. Nature. 1999;397:151–154. [Google Scholar]
- Baer B., Schmid-Hempel P. The artificial insemination of bumble-bee queens. Insectes Soc. 2000;47:183–187. [Google Scholar]
- Baer B., Schmid-Hempel P. Unexpected consequences of polyandry for parasitism and fitness in the bumble-bee, Bombus terrestris. Evolution. 2001;55:1639–1643. doi: 10.1111/j.0014-3820.2001.tb00683.x. [DOI] [PubMed] [Google Scholar]
- Baer B., Morgan E.D., Schmid-Hempel P. A non-specific fatty acid within the bumble-bee mating plug prevents females from remating. Proc. Natl Acad. Sci. USA. 2001;98:3926–3928. doi: 10.1073/pnas.061027998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer B., Schmid-Hempel P., Hoeg J.T., Boomsma J.J. Sperm length, sperm storage and mating system characteristics in bumble-bees. Insect. Soc. 2003;50:101–108. [Google Scholar]
- Chapman T., Liddle L.F., Kalb J.M., Wolfner M.F., Partridge L. Cost of mating in Drosophila melanogaster females is mediated by male accessory gland products. Nature. 1995;373:241–244. doi: 10.1038/373241a0. [DOI] [PubMed] [Google Scholar]
- Chen P.S., Stumm Zollinger E., Aigaki T., Balmer J., Bienz M., Bohlen P. A male accessory gland peptide that regulates reproductive behavior of female Drosophila melanogaster. Cell. 1988;54:291–298. doi: 10.1016/0092-8674(88)90192-4. [DOI] [PubMed] [Google Scholar]
- Crudgington H.S., Siva-Jothy M. Genital damage, kicking and early death. Nature. 2000;407:855–856. doi: 10.1038/35038154. [DOI] [PubMed] [Google Scholar]
- Duvoisin N., Baer B., Schmid-Hempel P. Sperm transfer and male competition in a bumble-bee. Anim. Behav. 1999;58:743–749. doi: 10.1006/anbe.1999.1196. [DOI] [PubMed] [Google Scholar]
- Eberhard W.G. Female control: sexual selection by cryptic female choice. Monographs in behaviour and ecology. Princeton University Press; 1996. [Google Scholar]
- Gillott C. Male insect accessory glands: functions and control of secretory activity. Invertebr. Reprod. Dev. 1996;30:199–205. [Google Scholar]
- Hölldobler B., Wilson E.O. Springer; Berlin: 1990. The ants. [Google Scholar]
- Johnstone R.A., Keller L. How males can gain by harming their mates: sexual conflict, seminal toxins, and the cost of mating. Am. Nat. 2000;156:368–377. doi: 10.1086/303392. [DOI] [PubMed] [Google Scholar]
- Korner P., Schmid-Hempel P. Effects of sperm on female longevity in the bumble-bee Bombus terrestris L. Proc. R. Soc. B. 2003;270(Suppl.):227–229. doi: 10.1098/rsbl.2003.0039. doi:10.1098/rsbl.2003.0039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubli E. Sex-peptides: seminal peptides of the Drosophila male. CMLS Cell. Mol. Life Sci. 2003;60:1689–1704. doi: 10.1007/s00018-003-3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Kubli E. Sex-peptide is the molecular basis of the sperm effect in Drosophila melanogaster. Proc. Natl Acad. Sci. USA. 2003;100:9929–9933. doi: 10.1073/pnas.1631700100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pamilo P. Life span of queens in the ant Formica exsecta. Insectes Soc. 1991;38:111–120. [Google Scholar]
- Raignier A., Van Boven J.K.A. Etude taxonomoque, biologique et biométrique des Dorylus du sous-genre Anomma (Hymenoptera Formicidae) Ann. Mus. R. Congo Belg. 1955;2:1–359. [Google Scholar]
- Sauter A., Brown M.J.F., Baer B., Schmid-Hempel P. Males of social insects can prevent queens from multiple mating. Proc. R. Soc. B. 2001;268:1449–1454. doi: 10.1098/rspb.2001.1680. doi:10.1098/rspb.2001.1680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid-Hempel R., Schmid-Hempel P. Female mating frequencies in Bombus spp. from Central Europe. Insectes Soc. 2000;47:36–41. [Google Scholar]
- Schoeters E., Billen J. The importance of the spermathecal duct in bumble-bees. J. Insect Physiol. 2000;46:1303–1312. doi: 10.1016/S0022-1910(00)00052-4. [DOI] [PubMed] [Google Scholar]
- Simmons L.W. Sperm competition and its evolutionary consequences in the insects. Monographs in behaviour and ecology. Princeton University Press; Oxford: 2001. [Google Scholar]
- Strassmann J. The rarity of multiple mating by females in the social Hymenoptera. Insectes Soc. 2001;48:1–13. [Google Scholar]