Abstract
Müllerian mimicry, where two unpalatable species share a warning pattern, is classically believed to be a form of mutualism, where the species involved share the cost of predator education. The evolutionary dynamics of Müllerian mimicry have recently become a controversial subject, after mathematical models have shown that if minor alterations are made to assumptions about the way in which predators learn and forget about unpalatable prey, this textbook case of mutualism may not be mutualistic at all. An underlying assumption of these models is that Müllerian mimics possess the same defence chemical. However, some Müllerian mimics are known to possess different defence chemicals. Using domestic chicks as predators and coloured crumbs flavoured with either the same or different unpalatable chemicals as prey, we provide evidence that two defence chemicals can interact to enhance predator learning and memory. This indicates that Müllerian mimics that possess different defence chemicals are better protected than those that share a single defence chemical. These data provide insight into how multiple defence chemicals are perceived by birds, and how they influence the way birds learn and remember warningly coloured prey. They highlight the importance of considering how different toxins in mimicry rings can interact in the evolution and maintenance of Müllerian mimicry and could help to explain the remarkable variation in chemical defences found within and between species.
Keywords: taste, perception, novelty, domestic chick, learning, memory
1. Introduction
Aposematic insect species gain protection from avian predators by advertising their unpalatability using bright or conspicuous coloration (Cott 1940;Edmunds 1974). Müllerian mimics are sympatric aposematic species that share the same or similar warning patterns (Wickler 1968). These species are classically thought to benefit by sharing the cost of predator education: if a predator learns to avoid a warningly patterned species by a fixed number of encounters, then Müllerian mimics benefit as fewer individuals of each species will be killed educating naive predators (Müller 1879).
More recent mathematical models have suggested that the exact predictions of classical mimicry theory are sensitive to small changes in the learning and forgetting algorithms used to simulate predator behaviour (Speed 1993, 1999; Speed & Turner 1999). This work has been particularly important in highlighting the effect that different levels of chemical defences between mimics can have on the evolutionary dynamics of unpalatable prey. These models generate the controversial prediction that less unpalatable mimics may raise predation rates on their more unpalatable models Speed 1993, 1999; Speed & Turner 1999). This idea assumes that avian predation rates depend upon the ability of birds to perceive and respond to different levels of unpalatability in mimics, for which there is some evidence from groups of garden birds (Speed et al. 2000), and also from individual laboratory birds (J. Skelhorn & C. Rowe, unpublished data).
These models simulate the way in which predators learn and forget about Müllerian mimics with different levels of the same defence chemical, but they do not consider how the interaction of different defence chemicals may influence predator learning and memory. Unfortunately, there is no systematic study of the toxins possessed by Müllerian mimics but several reviews of insect defence chemistry indicate that some Müllerian mimics do possess different chemicals (Blum 1981; Brower 1984; Nishida 2002). In addition, since many defence chemicals have characteristic flavours (Nishida 2002), it is also possible that avian predators could perceive Müllerian mimics with different chemicals as a single visual signal indicating one of two different toxins.
Given our limited knowledge of insect defence chemicals it is difficult to predict how multiple toxins and tastes will influence predator learning and memory. One possibility is that learned avoidance is dosage dependent, meaning that predators will reach the dose of the toxin equal to the asymptotic level of avoidance quicker when Müllerian mimics share the same toxin than when they possess different toxins (Turner & Speed 1999). Alternatively, the chemicals could have synergistic effects that increase or decrease the potency of the joint toxic loads compared to the effect of each chemical alone. This could produce either beneficial or detrimental effects on predator learning and memory depending on the type of interaction between the chemicals in the mimics. Although these two ideas rely upon toxicity, it could be that taste is also important. Predators may learn to avoid Müllerian mimics that possess different-tasting toxins more quickly than those sharing a single flavour because of the increased novelty that the ‘extra’ taste brings to the mimicry complex, or even because of the reduced predictability of the toxin associated with a particular pattern. There are currently no data to support or refute these ideas.
In the following experiments, we investigated how two bitter-tasting defence chemicals change the efficacy of a colour signal during predator avoidance learning, as well as the subsequent memory for that signal. These are the first experiments, to our knowledge, to investigate the effects of multiple defence chemicals on learning and memory in birds, and also provide insight into how predators perceive toxin differences between prey, and how this could influence the evolution of Müllerian mimicry.
2. Material and methods
2.1 Subjects and housing
Domestic chicks (Gallus gallus domesticus) of mixed sex were hatched in the laboratory, and housed in two cages measuring 100 cm×50 cm×50 cm. One cage housed the experimental chicks, and the other the buddy chicks (see §2c). They were all subject to a 14L: 10D cycle using uncovered fluorescent lights with no UV component, and temperatures were maintained at 24–25°C using room heaters and heat lamps. Water was provided ad libitum, as were brown chick starter crumbs, except during training and experimenting when food deprivation was necessary. All deprivation periods were in accordance with Home Office regulations and guidelines. At the end of the experiment all chicks were donated to free-range smallholdings.
2.2 Artificial prey
We chose quinine and Bitrex (a solution used to prevent nail biting) as our defence chemicals since they are both known to be unpalatable to domestic chicks (e.g. J. Skelhorn & C. Rowe, unpublished data; Marples & Roper 1997), and like many insect defence chemicals they taste bitter. The gustatory effects of quinine on domestic chicks are well documented (Gentle 1971), although it is not clear whether Bitrex has any gustatory effects or is aversive to chicks solely because of its bitter taste (Marples & Roper 1997). In our first experiment, we used crumbs flavoured with either quinine or Bitrex, while in our second experiment we also used crumbs sprayed with a solution of both chemicals, which we refer to as ‘cocktail’ crumbs. To produce crumbs flavoured with quinine, 150 g of brown chick starter crumbs were sprayed with 100 ml of 2% quinine sulphate solution. Crumbs flavoured with Bitrex were produced by spraying 150 g of brown chick starter crumbs with one drop of 2% Bitrex solution made up to 100 ml with water. These concentrations were chosen because they tasted equally bitter to us. The ‘cocktail’ crumbs were made by mixing 50 ml of the Bitrex solution (one drop made up to 100 ml with water) with 50 ml of the 2% quinine sulphate solution. To control for differences in texture and appearance, palatable crumbs were sprayed with 100 ml of tap water.
Crumbs were then allowed to dry for 24 h before being coloured red or green by spraying 150 g of chick starter crumbs with either 2 ml of Supercook red food dye diluted to 90 ml with tap water, or 0.5 ml of Sugarflair spruce-green food dye diluted to 90 ml with tap water. These concentrations were chosen because they produced a similar degree of colour saturation in the crumbs. Crumbs were allowed to dry for 24 h before being sieved to ensure that they were all of similar size.
2.3 Experimental arena
The arena consisted of a cage similar to the housing cages, with a section measuring 25 cm×50 cm×50 cm partitioned off using a wire mesh screen to create a separate ‘buddy arena’. In all training and experimental trials, two chicks were placed in the buddy arena to reduce any potential distress from placing experimental chicks alone in the arena. These buddy chicks were selected from a stock of individuals not used in the experiments, and were changed every three trials. They had free access to food and water throughout the experiment.
Chicks were trained to eat brown crumbs from the green laminated cardboard floor of the experimental arena. The floor was divided into 80 equal-sized rectangles in an 8×10 grid using faint black lines so that crumbs could be identified by their position. The green floor made the green prey appear cryptic and the red prey conspicuous.
2.4 Training
On the first day post-hatch, chicks were placed in the experimental arena for three 10 min sessions in groups of three, followed by one 5 min session in pairs. These trials allowed chicks to habituate to the arena and no food deprivation was necessary. However, on day 2, chicks were food-deprived for ca. 1.5 h before each training session. In the first of these trials chicks were placed in the arena in pairs for 5 min, while in the following three trials chicks were placed in the arena individually for 5 min. All chicks ate readily in the arena at the end of this training.
3. Experiment 1: predator avoidance of Müllerian mimics with different defence chemicals
3.1 Methods
Forty domestic chicks were hatched from a single batch of eggs: 30 were trained to eat brown chick starter crumbs from the green laminated cardboard floor of the experimental arena, while 10 were used as buddy chicks. On day 3, trained chicks were assigned to one of three groups, ensuring that sexes were distributed equally across groups. After ca. 1.5 h of food deprivation, chicks were individually placed in the experimental arena where they encountered 20 green crumbs and 20 red crumbs. Chicks in each of the three groups were given 20 palatable green crumbs, but groups differed in the type of red crumbs they received: the Bitrex group received 20 red Bitrex-flavoured crumbs; the quinine group received 20 red quinine-flavoured crumbs; and the mixed group received 10 red Bitrex-flavoured crumbs and 10 quinine-flavoured crumbs.
Crumbs were placed singly in the rectangles drawn on the floor of the experimental arena. The position of each crumb was determined by randomly generated maps produced prior to the experiment. Chicks were allowed to attack (peck or eat) 16 crumbs before being removed from the arena. All chicks received seven trials in total: two on each of days 3, 4, 5, and one on day 6. However, three female chicks (one from each experimental group) refused to eat coloured crumbs in the first trial, and were excluded from the experiment.
On day 10, 96 h after the final learning trial, chicks received a single extinction trial to test how chicks recalled their learned aversions. They were presented with 20 palatable red crumbs and 20 palatable green crumbs in the arena, and the order and colour of the crumbs that each chick attacked were recorded.
We used a priori contrasts within ANOVA to test for differences in chicks’ responses to red crumbs among our test groups, avoiding the within-subject nature of the data by testing only trial 1 and trial 7, and by adding up the total number of crumbs attacked across all trials for each chick. With the two degrees of freedom among our three groups, we made the following orthogonal contrasts: (i) the responses of chicks in the single-taste groups with those in the mixed group (i.e. quinine ; Bitrex versus mixed); (ii) the two single-taste groups (quinine versus Bitrex). Our prediction is that having two different bitter tastes will enhance the impact, and therefore that contrast (i) will show a significant difference. Since we attempted to make the quinine and Bitrex solutions equally aversive we also predict that there will be no difference between the quinine and Bitrex groups, and therefore that contrast (ii) will reveal no significant difference. Where the dependent variable could not be normalized by transformation, we used a non-parametric ANOVA (i.e. a Kruskal–Wallis test). Preliminary analyses indicated that chick sex had no effect on any of our measurements of learning and memory; sex was also balanced across groups and was therefore discarded as a factor in our final analysis.
3.2 Results
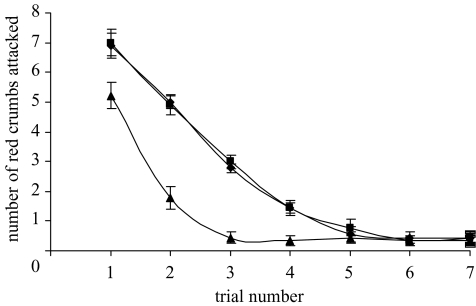
All groups acquired the discrimination and learned to avoid the unpalatable red crumbs by the end of the learning phase (see figure 1). In the first trial, groups differed significantly in the number of red crumbs attacked (F2,24=5.30, p < 0.05; figure 1). As predicted, there was a significant difference between the mixed group versus the single-taste groups combined (contrast F1,25=10.98, p < 0.01). There was no significant difference between the single-taste groups (contrast F1,16=0.033, p > 0.05).
Figure 1.
The mean number (±s.e.) of red crumbs attacked in trials during the learning phase for each experimental group in experiment 1 (n=9 for each group). Bitrex, filled squares; mixed, filled triangles; quinine, filled circles.
To investigate the effect of taste differences on predator learning rates, we compared the total number of red crumbs attacked by each chick from our three groups. There were significant differences among the groups in the total number of unpalatable prey attacked over the seven trials (F2,24=32.70, p < 0.001): chicks in the mixed group attacked significantly fewer red crumbs than chicks in either of the single-taste groups (contrast F1,25=67.85, p < 0.001). Again there was no significant difference between the quinine and Bitrex groups (contrast F1,16=0.064, p > 0.05).
The final asymptotic levels of attack could not be normalized, but non-parametric tests showed there were no significant differences among the groups in the number of unpalatable crumbs attacked in trial 7 (Kruskal–Wallis test, χ2=0.30, p > 0.05, d.f.=2; figure 1). Looking at where the first red crumb fell in the sequence of crumbs attacked in the final learning trial can also be used as a measure of asymptotic performance, since it indicates how reluctant chicks were to attack a red crumb within a single trial. We calculated the number of green crumbs attacked before the first red crumb, assigning a nominal value of 16 to all chicks that only ate green crumbs. Again this measure did not differ among groups (Kruskal–Wallis test, χ2=0.49, p > 0.05, d.f.=2).
Despite learning the discrimination, chicks did not consistently avoid all red crumbs, so that the asymptotic levels of attack were somewhere between zero and one red crumb attacked per trial (this is consistent with data from a similar experiment; J. Skelhorn & C. Rowe, unpublished data). We therefore calculated the number of trials in which a chick attacked either zero or one red crumb and used this as a measure of the speed of achieving asymptotic performance. Groups differed in the mean number of trials in which chicks performed at asymptote, i.e. attacked zero or one unpalatable crumbs (F2,24=28.74, p < 0.001). Chicks in the mixed group spent more trials at asymptote than chicks in the single-taste groups combined (contrast F1,25=59.32, p < 0.001). Once again the quinine group did not differ significantly from the Bitrex group (contrast F1,16=0.138, p > 0.05).
These results indicate that having both chemicals to taste increased the speed with which chicks in the mixed group learned to avoid red crumbs. To ensure that this was an appropriate interpretation, we looked at the numbers of quinine-flavoured and Bitrex-flavoured crumbs eaten by chicks in this group. Consistent with our interpretation, all chicks in the mixed grattacked at least one crumb of each type, and there was no consistent difference between the total numbers of quinine crumbs and Bitrex crumbs attacked across the seven trials by each chick (paired t-test, t<0.001, p=1.000, d.f.=8).
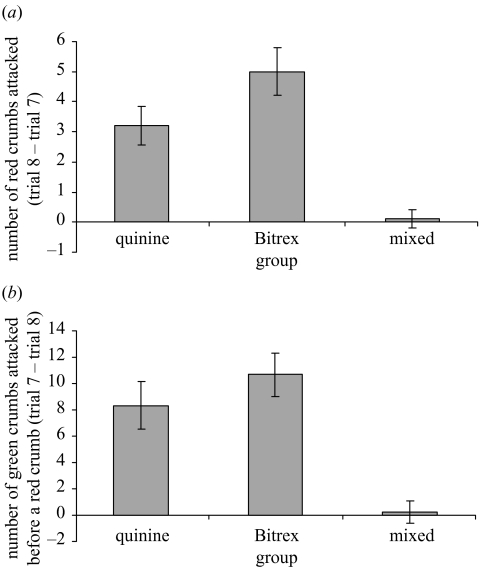
Despite there being no significant differences among the groups in the number of red crumbs attacked in trial 7, the three groups appeared to attack different numbers after a delay of 96h. The difference between the birds’ scores in trial 7 and the extinction trial is a measure of how well they retained their learned aversions (see figure 2a). These scores differed significantly among groups (F2,24=16.44, p < 0.001). The mixed group displayed a smaller difference in the number of red crumbs attacked than the single-taste groups combined (contrast F1,25=41.82, p < 0.001). The difference between the quinine group and the Bitrex group approached significance (contrast F1,16=3.09, p=0.098).
Figure 2.
(a) Mean (±s.e.) number of red crumbs attacked in trial 8−number of red crumbs attacked in trial 7 in experiment 1 (n=9 for each group). (b) Mean (±s.e.) number of green crumbs attacked before a red in trial 7−number of green crumbs attacked before a red in trial 8 in experiment 1 (n=9 for each group).
In the extinction trial, both the red and the green crumbs were palatable, and therefore the number of red crumbs attacked in the extinction trial may have been dependent upon both memory and re-learning about the palatability of the red crumbs. To remove any effect of re-learning, we calculated the number of green crumbs attacked before the first red crumb in both trial 7 and the extinction trial, and found that the difference between these scores differed significantly among groups (F2,24=13.54, p < 0.001; figure 2b). The mixed group displayed a smaller difference in the number of green crumbs attacked before the first red crumb than the single-taste groups combined (contrast F1,25=39.21, p < 0.01). The quinine group did not differ significantly from the Bitrex group (contrast F1,16=0.916, p > 0.05). These results suggest that the use of two unpalatable flavours was important in increasing the memorability of red crumbs.
3.3 Discussion
Chicks presented with a mixture of quinine crumbs and Bitrex crumbs learned to avoid red crumbs significantly faster, and also attacked fewer red crumbs after a delay of 96 h, than those presented with either quinine or Bitrex crumbs. This indicates that quinine and Bitrex interact to enhance both the memorability of red prey, and the speed of avoidance learning in naive avian predators.
Chicks find the quinine crumbs and the Bitrex crumbs equally aversive since they learned and remembered to avoid them at similar rates. However chicks trained on quinine crumbs displayed a smaller difference in the number of red crumbs attacked between trials 7 and 8 than chicks trained on Bitrex crumbs. Although this difference did not quite reach significance, this may indicate that chicks differ in the rate at which they re-learn that red crumbs are palatable. This is explicable if quinine and Bitrex have different gustatory effects.
These results suggest that Müllerian mimics that differ in their bitter defence chemicals may be better protected than those that share the same chemical. The generality of this finding will be determined by the specific mechanism by which it occurs. If the differences in learning and memory are caused by the individual flavours interacting to produce a more unpalatable taste, then it may be that only specific flavour combinations will result in enhanced protection for Müllerian mimics that differ in their bitter defence chemicals. However, if the differences in learning and memory are due to the increased novelty of the aversive taste, then this finding will be true for all Müllerian mimics that differ perceptibly in flavour.
To discriminate between these mechanisms, we conducted a second experiment that explored the potential differences in attack rates when chicks were given a mixture of Bitrex- and quinine-flavoured crumbs compared with when they are given crumbs sprayed with a mixture of both chemicals.
4. Experiment 2: the effects of taste novelty and taste synergy on predator learning and memory
4.1 Methods
Forty domestic chicks of mixed sex were hatched from a single batch of eggs: 28 were used in the experiment while 12 were used as buddy chicks. Chicks were randomly assigned to one of two groups, with equal numbers of males and females in each group. Chicks were then given a series of seven trials where they were offered 20 palatable green crumbs and 20 unpalatable red crumbs in the experimental arena. Although both groups received palatable green crumbs, groups differed in the type of red crumbs received; the mixed group received 10 red crumbs flavoured with Bitrex, and 10 flavoured with quinine, while the cocktail group received 20 red cocktail crumbs all flavoured with both chemicals. Using the same methods as in experiment 1, chicks were given seven learning trials in which they were allowed to attack 16 crumbs before being removed from the arena, and a single extinction trial 96 h after the final learning trial where all crumbs were palatable.
4.2 Results
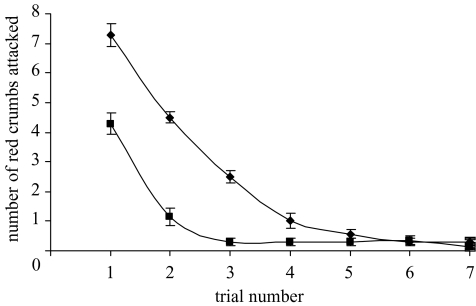
Both groups acquired the discrimination and learned to avoid the unpalatable red crumbs by the end of the learning phase (figure 3). Chicks in the mixed group attacked significantly fewer red crumbs than those in the cocktail group, both in the first trial (F1,26=32.95, p < 0.001), and in total across all trials (F1,26=83.87, p < 0.001). There was no significant difference between the number of quinine crumbs attacked and the number of Bitrex crumbs attacked by the mixed group (paired t-test, t=0.51, p > 0.05, d.f.=13), and all chicks attacked at least one crumb of each flavour.
Figure 3.
The mean number (±s.e.) of red crumbs attacked in trials during the learning phase for each experimental group in experiment 2 (n=14 for each group). Mixed, filled squares; cocktail, filled circles.
In trial 7, there was no significant difference between the groups in either the number of red crumbs attacked (Kruskal–Wallis test, χ2=0.818, p > 0.05, d.f.=1), or the number of green crumbs attacked before the first red crumb (Kruskal–Wallis test, χ2=0.641, p > 0.05, d.f.=1), indicating that the asymptotic level of attack did not differ between groups. Chicks in the mixed group spent significantly more trials at asymptote (as measured by the number of trials in which one or zero red crumbs were attacked), than those in the cocktail group (F1,26=82.9, p < 0.001).
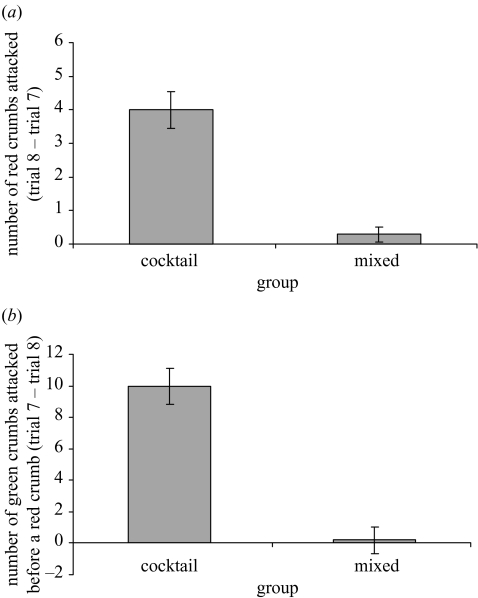
Chicks also appeared to differ in their response to red crumbs after 96h (figure 4a). The difference in the number of red crumbs attacked between trial 7 and the extinction trial was significantly larger in the cocktail group than the mixed group (F1,26=38.71, p < 0.001), as was the difference in the number of green crumbs attacked before the first red crumb (F1,26=47.31, p < 0.001; figure 4b). This indicates that chicks in the mixed group found the red crumbs more memorable than chicks in the cocktail group.
Figure 4.
(a) Mean (±s.e.) number of red crumbs attacked in trial 8−number of red crumbs attacked in trial 7 in experiment 2 (n=14 for each group). (b) Mean (±s.e.) number of green crumbs attacked before a red in trial 7−number of green crumbs attacked before a red in trial 8 in experiment 2 (n=14 for each group).
4.3 Discussion
This experiment clearly shows that the method of presentation of the two defence chemicals is crucial to the learning and retention rates in avian predators. Birds learned to avoid the red prey faster and retain the discrimination better when the same defensive chemical burden was presented separately on different red crumbs than when presented together on single crumbs. This experiment demonstrates that the enhanced learning and memory is not generated by synergistic effects of either toxins or taste, since chicks presented with both quinine crumbs and Bitrex crumbs learned to avoid red crumbs significantly faster than chicks presented with crumbs sprayed with a cocktail of quinine and Bitrex. The important factor that influences learning and memory appears to be the presence of two distinct defence chemicals, indicating that our findings are not restricted to the specific chemicals we used, but may be generally applied to any defence chemicals that avian predators perceive as different.
5. General discussion
Our experiments show that the presence of two defence chemicals in a Müllerian mimicry system enhances predator learning and memory. However, this is only true when the species involved possess different defence chemicals and not when they share the same combinations. Little is known about how defence chemicals are partitioned between Müllerian mimics, but chemical analyses suggest that some defended species that share warning patterns do differ in the chemicals they possess. Tentative evidence suggests that the monarch butterfly (Danaus plexipupus) may possess different defence chemicals than its mimic the viceroy (Limenitis archippus) (see review by Nishida 2002), while the two-spot ladybird (Adalia bipunctata) and the seven-spot ladybird (Coccinella septempunctata) are known to synthesize different alkaloids for use in the reflex bleeding process (see review by Gilsan King & Meinwald 1996), although there is no evidence that birds can discriminate between them on the basis of taste.
Unfortunately, even less is known about how avian predators detect and respond to insect defence chemicals. Although birds are known to reject water containing low levels of unpalatable compounds (Matson et al. 2001, 2004), the ability of birds to taste chemicals contained in food items has been questioned (Kassarov 1999). However, our experiment clearly shows that birds can perceive the difference between two relatively similar bitter tasting food items. One explanation for this is that the relatively few taste receptors that birds possess may be particularly good at detecting flavours that have adaptive significance such as bitter tasting toxins. Although it is not yet known how the flavour of an insect relates to its toxin content, our experiment suggests that taste cues may well influence birds foraging decisions.
The benefits of having different defence chemicals occurs within a few encounters in the first trial, perhaps caused either by differences in learning, or by differences in the attack biases incited against warningly coloured crumbs by the tastes (Rowe & Skelhorn 2005). The fact that the presence of two defence chemicals in a Müllerian mimicry system enhances predator learning and memory overall, can be explained in one of several ways. Given that the gustatory effect of mixed crumbs and the cocktail crumbs were likely to be the same, it seems probable that the differences in learning and memory are due to taste. The relative novelty that the second taste brings to the mimicry system may increase the saliency of the signal by either increasing the attention paid to the visual signal, or by allowing birds to judge more accurately how many unpalatable crumbs they have attacked. The latter may happen if taste novelty serves to clear the predator’s palate between encounters. If this explanation is true, it is unclear whether this effect would hold in nature where predators may encounter aposematic prey at lower rates. One important exception may be where aposematic prey is found in mixed-species aggregations, such as ladybird over-wintering sites (Majerus & Kearns 1989).
Two distinct tastes may also alert the predator to the unpredictability of the effects of the defence chemicals. This may be particularly important in deterring predators that would otherwise attack unpalatable prey until they became saturated with the defence chemical it possessed (Turner & Speed 1999). As predators could no longer predict a ‘safe dosage’ they would be forced to reduce their attack rates, or increase the risk of ingesting a lethal dose of the toxin(s) (Sherratt et al. 2004). Our experiment was not designed to test this specific prediction, so it is difficult to draw conclusions about whether predators continue to attack unpalatable prey when the availability of palatable prey is restricted.
Irrespective of the exact mechanism, the findings are striking, and indicate that defence chemicals possessed by aposematic species could affect the likelihood of a Müllerian resemblance evolving by influencing the benefit of the resemblance. The benefit of Müllerian mimicry would be greater when the species involved possess different defence chemicals.
In addition, differences in protection may also lead to selection for the diversification of defence chemicals after the initial evolution of mimetic visual signals. Our findings could therefore help to explain the remarkable variation in chemical defences found both within and between species (Ruxton et al. 2004). New defence chemicals could evolve by relatively small mutations causing sequestered toxins to be metabolized in slightly different ways (Nishida 2002). For selection to favour polymorphisms in defence chemicals, predators must sample unpalatable prey and release some unharmed on the basis of taste. Although the ability of birds to taste reject butterflies has been questioned because of the position of taste buds on the tongue (Kassarov 1999), it seems likely that insects with hard bodies and defence secretions may well be released unharmed (reviewed by Eisner & Meinwald 1966), but this remains to be tested with avian predators. If birds are prepared to eat unpalatable prey under some conditions, warning coloration may function to advertise unpredictability, resulting in avoidance in favourable foraging conditions and cautious attacks when alternative prey is scarce.
Recent models of Müllerian mimicry have considered variation in palatability along a single chemical dimension (Speed 1993; Speed & Turner 1999). Our experiments suggest that the predictions of such models may be altered significantly if Müllerian mimics differ in the defence chemicals they possess, since the enhanced learning may overcome any potential differences in learning and memory associated with differences in protection. It is, however, still unclear to what scale both toxin differences and differences in the level of unpalatability, affect predator learning and memory.
These data provide insight into how birds perceive variations in chemical defence and how they influence the way in which birds learn and remember warningly coloured prey. They highlight the importance of considering how different toxins in mimicry rings interact if we are to understand the evolution and maintenance of Müllerian mimicry. However, since the chicks in our experiment had experienced only a limited number of different tastes in their short lives our findings should be verified using experienced wild birds, and several different defence chemicals, to ensure that our findings can be generalized to a range of natural situations.
Acknowledgments
The authors thank Lin Hedgecock and Michelle Waddle who looked after their animals, Francis Gilbert for statistical advice and many helpful comments on the manuscript, Mike Speed and two anonymous referees for useful comments. J.S. is supported by a School of Biology studentship, and C.R. holds a Royal Society Dorothy Hodgkin Research Fellowship. This work was supported by The Royal Society, the BBSRC and a Wellcome Trust JIF Award.
References
- Blum M.S. Chemical defences of arthropods. Academic; New York: 1981. [Google Scholar]
- Brower L.P. Chemical defences I. Butterflies. In: Vane-Write R.I., Ackery P.R., editors. The biology of butterflies. Academic; London: 1984. pp. 109–134. [Google Scholar]
- Cott H.B. Adaptive colouration in animals. Methuen; London: 1940. [Google Scholar]
- Edmunds M. Defence in animals. Longman; Harlow: 1974. [Google Scholar]
- Eisner T., Meinwald J. Defensive secretions of arthropods. Science. 1966;153:1341–1350. doi: 10.1126/science.153.3742.1341. [DOI] [PubMed] [Google Scholar]
- Gentle M.J. Taste and its importance to the domestic chicken. Br. Poultry Sci. 1971;12:77–86. doi: 10.1080/00071667108415855. [DOI] [PubMed] [Google Scholar]
- Gilsan King A., Meinwald J. Review of the defence chemistry of Coccinellids. Chem. Rev. 1996;96:1105–1122. doi: 10.1021/cr950242v. [DOI] [PubMed] [Google Scholar]
- Kassarov L. Are birds able to taste and reject butterflies based on ‘beak mark tasting’ ? A different point of view. Behaviour. 1999;136:965–981. [Google Scholar]
- Majerus M.E.N., Kearns P. Ladybirds. Richmond Publishing; Slough, UK: 1989. [Google Scholar]
- Marples N.M., Roper T.J. Response of domestic chicks to methyl anthranilate odour. Anim. Behav. 1997;53:1263–1270. doi: 10.1006/anbe.1996.0433. [DOI] [PubMed] [Google Scholar]
- Matson K.D., Millam J.R., Klasing K.C. Thresholds for sweet, salt, and sour taste stimuli in cockatiels (Nymphicus hollandicus) Zoo Biol. 2001;20:1–13. doi: 10.1002/zoo.1001. [DOI] [PubMed] [Google Scholar]
- Matson K.D., Millam J.R., Klasing K.C. Cockatiels (Nymphicus hollandicus) reject very low levels of plant secondary compounds. Appl. Anim. Behav. Sci. 2004;85:141–156. [Google Scholar]
- Müller F. Ithuna and Thyridia: a remarkable case of mimicry in butterflies. Trans. R. Entomol. Soc. Lond. 20021879:xx–xxix. [Google Scholar]
- Nishida R. Sequestration of defensive substances from plants by Lepidoptera. A. Rev. Entomol. 2002;47:57–92. doi: 10.1146/annurev.ento.47.091201.145121. [DOI] [PubMed] [Google Scholar]
- Rowe, C. & Skelhorn, J. 2005 Colour biases are a question of taste. Anim. Behav.69 (doi:10.1016/j.anbehav.2004.06.010) (In the press.)
- Ruxton, G.D., Sherratt, T.N. & Speed, M.P. 2004 Avoiding attack: the evolutionary ecology of crypsis, mimicry and aposematism Oxford University Press. (In the press.)
- Sherratt T.N., Speed M.P., Ruxton G.D. Natural selection on unpalatable species imposed by state-dependent foraging behaviour. J. Theor. Biol. 2004;228:217–226. doi: 10.1016/j.jtbi.2003.12.009. [DOI] [PubMed] [Google Scholar]
- Speed M.P. Müllerian mimicry and the psychology of predation. Anim. Behav. 1993;45:571–580. [Google Scholar]
- Speed M.P. Robot predators in virtual ecologies: the importance of memory in mimicry studies. Anim. Behav. 1999;57:203–213. doi: 10.1006/anbe.1998.0943. [DOI] [PubMed] [Google Scholar]
- Speed M.P., Alderson N.J., Hardman C., Ruxton G.D. Testing Müllerian mimicry: an experiment with wild birds. Proc. R. Soc. B. 2000;267:725–731. doi: 10.1098/rspb.2000.1063. doi:10.1098/rspb.2000.1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speed M.P., Turner J.R.G. Learning and memory in mimicry: II. Do we understand the mimicry spectrum? Biol. J. Linn. Soc. 1999;67:281–312. [Google Scholar]
- Turner J.R.G., Speed M.P. How weird can mimicry get? Dedicated to Miriam Rothschild. Evol. Ecol. 1999;13:807–827. [Google Scholar]
- Wickler W. Mimicry in plants and animals. Weidenfeld & Nicolson; London: 1968. [Google Scholar]