Abstract
The hypothesis that social stimulation, derived from the presence and activities of conspecifics, can hasten and synchronize breeding in colonies of birds was tested. A modified playback/recorder system was used to continuously exaggerate the amount of colony sound available to zebra finches throughout their courtship period. Males that heard ‘sound supplements’ generated from their own colony sang more than males in control colonies that did not receive playback; males that heard samples from a different colony, sang at an intermediate level. Females that were exposed to the vocalizations of their mate and playback from a colony other than their own, laid eggs earlier and more synchronously than females in control colonies. Females that heard the vocalizations of their mate along with playback samples generated from their own colony, laid eggs more synchronously but not earlier than control females. Both acoustic treatments caused females to lay larger clutches. Social stimulation influences the breeding schedule and clutch size in zebra finch colonies. If there are advantages associated with these effects, social stimulation may contribute to the maintenance of colonial breeding systems.
Keywords: social stimulation, breeding schedules, clutch size, zebra finch, colony sound, synchrony
1. Introduction
Animals often mate and produce young synchronously, probably because individuals use the same proximate environmental cues (e.g. day length: Farner & Lewis 1971; rainfall: Sossinka 1980; Zann et al. 1995; food availability: Sanders et al. 1981) to predict the onset of favourable breeding conditions. However, breeding pairs that settle near one another appear to become even more synchronized as the breeding season progresses (Gochfeld 1980; Wissel & Brandl 1988; Ims 1990; Waas 1995); this suggests that social factors may also influence an animal’s reproductive schedule.
Several arguments have been proposed to explain why it might be advantageous to breed synchronously with neighbours. For example, the young of synchronous pairs may be more likely to avoid predation (e.g. Kruuk 1964; Patterson 1965; Hunt & Hunt 1976; Westneat 1992) or may be in a better position to glean information about good foraging sites (e.g. Emlen & Demong 1975; Brown 1986); interference by neighbouring birds, in the form of kleptoparasitism (Harris 1970) or attacks on chicks (Fetterolf 1983) may also be reduced (Nelson 1970, 1978; see Smith (2004) for a review of functional explanations). Although synchrony appears to improve the reproductive success of colony occupants (Emlen & Demong 1975; Findlay & Cooke 1982; Westneat 1992; for an example of an exception see Weatherhead & Sommerer (2001)), little effort has been devoted to determining exactly how synchrony comes about.
F. Fraser Darling (1938) suggested that social stimulation, derived from the presence and activities of neighbours, synchronizes and hastens egg laying in colonies by influencing the rate at which females become physiologically ready to be fertilized. If this is true, early and synchronized breeding can be seen as a simple consequence of courting near others.
The process proposed by Darling might also influence how much animals invest in the brood; for example, females that become fertilized earlier in the breeding season may have time to produce larger clutches and fledge more offspring during the breeding episode. In addition, if synchrony reduces the risk of chick loss, synchronized females may be able to successfully fledge larger clutches than those breeding outside the peak period.
For over 60 years ecologists have used Darling’s hypothesis to explain correlations between colony size or density and the timing of egg laying (reviewed in Gochfeld (1980)); however, the idea has never been adequately tested (Wittenberger & Hunt 1985; Waas 1995). Furthermore, Orians (1961) proposed an explanation for synchronized breeding in colonial animals that did not require social stimulation: that is, newly arriving animals may be attracted to individuals already present at breeding sites. The ‘Orians’ Effect’ would lead to the spatial clustering of animals that arrive in colonies at about the same time, causing greater within- than between-colony synchrony.
A critical test of Darling’s hypothesis would require one to manipulate levels of social stimulation in breeding colonies while controlling variation in arrival patterns (i.e. the Orians’ Effect), the distribution of different age classes (Armstrong 1947; Lack 1943) and environmental conditions. We report on such a study to provide the first experimental demonstration of the process that Darling proposed. A unique playback system allowed us to alter the amount and diversity of courtship sounds that the zebra finch (Taeniopygia guttata) could hear in their laboratory colonies throughout their courtship period; we then measured the influence that this extra stimulation had on egg-laying schedules and clutch size.
Zebra finches are monogamous, sexually dimorphic, colonial breeders with strong pair bonds; both birds contribute to nest construction and parental care. Only the male produces song, and song production peaks just before egg production (Slagsvold 1977). Being opportunistic breeders and living in extremely unpredictable environments, the females’ gonads are permanently activated, which allows them to respond rapidly to the sudden appearance of favourable breeding conditions. In contrast to photoperiodically breeding birds, the final stages of ovarian development in the zebra finch are triggered by proximate factors (male courtship, nest site, rainfall, habitat conditions; Sossinka 1980; Zann et al. 1995). These characteristics make zebra finches excellent subjects for the study of how social factors influence reproductive schedules and clutch size.
In the present study, we test four predictions derived from Darling’s hypothesis: (i) perceivers will court more frequently when they are exposed to the courtship activities of others; (ii) females will lay sooner if they are exposed to exaggerated levels of social stimulation; (iii) females will lay more synchronously if they are exposed to exaggerated levels of social stimulation; and (iv) females will lay larger clutches if they are exposed to exaggerated levels of social stimulation. We show that social stimulation influences the breeding schedule and clutch size in zebra finch colonies.
2. Material and methods
Ninety pairs of zebra finches were used during the experiment; 45 pairs were used in each of two replicates. All birds had previous breeding experience and were 1–5 years old. Subjects were bred and raised in captivity, but were not inbred. Birds were maintained in open single-sex flight cages on a 14 L:10 D schedule for at least one month before the experiment began.
Three identical windowless, soundproofed rooms were used to house study colonies; each room was ventilated and maintained at 22°C. One wide-spectrum and two standard fluorescent tubes (each 122 cm and 40 W) provided light for each colony. Fifteen breeding cages (20 cm apart) were held on three shelves installed along one wall of each room. White sheets were hung between cages so that breeding pairs could not see one another.
A Panasonic tape recorder/player (RX-CS750) was used to manipulate the amount of colony sound heard in two of the three rooms; the machine was modified so that it continuously switched between 12 min recording and playback phases. The system ran 12 min endless loop cassettes (TDK EC-12 min); as a result, a unique 12 min sample of sound would be recorded and then played back every 24 min on a continuous basis.
Omni-directional microphones (Realistic Highball-7), hanging above the cages in room 1 (50 cm apart), were used to record colony sounds from room 1 onto both tracks of the stereo tape. One of the two-way speakers supplied with the system provided playback in room 1; the second speaker provided room 2 with playback. The speakers were mounted directly opposite the breeding cages. Room 3 housed the control colony and did not receive playback but was otherwise treated identically. Continuous playback from another species was not selected as a control because the sounds of another species so close to the birds may have been disturbing and/or have masked courtship sounds produced by the birds. Room/treatment combinations were assigned randomly.
When the system was turned on, any sounds produced in room 1 during the 12 min recording phase would be broadcast in both rooms 1 and 2 during the following 12 min playback phase. At the end of the playback phase, a new 12 min segment of material would be recorded and then played back (and so on).
Because no two samples were ever the same, we reduced the possibility that birds would habituate to the playback of sounds during the course of the courtship period.
On the first day of the experiment, a single pair of finches was placed in each room; we matched pair/room combinations for age and breeding experience. The tape unit was turned on and the lights were set to the same schedule experienced by birds in the holding rooms (i.e. 14 L:10 D). In the middle of each light period, birds in each colony were also exposed to one of three different, randomly selected, recordings of rainfall for 30 min (the tape unit was turned off during this period). Rainfall may help trigger the onset of breeding attempts (Immelmann 1965; Sossinka 1980).
The volume control on the tape unit was adjusted until vocaliza tions being played back through the speaker were of the same amplitude as those produced by the live finches; the listener was positioned directly between the speaker and the cages when the volume was being adjusted. The tape unit adjusted recording levels automatically.
A new pair of breeders was placed into each of the rooms every day until colonies contained 15 pairs. This was done to create an initial ‘spread’ from which we could measure changes in synchrony. Colonies and holding rooms received an extra 15 min of light every 3 days until the last pair arrived (increased daylight appears to have a positive influence on follicle size in zebra finches; Sossinka 1980). Subjects were provided with fresh seed and water each morning; their diets were also supplemented with mashed egg or spinach each day.
Wicker nesting baskets were placed in the upper corner of each cage. Burlap threads (8 cm long) were supplied ad libitum as nesting material. Each cage also contained two perches, a cuttlefish bone and a bowl of grit. From the first day onwards, nests were checked every 12 h (morning and evening).
A Tandberg cassette recorder (TCD 310) and a second set of Realistic microphones were used to record 24 min samples of vocal activity from the colonies each morning (when zebra finches vocalize most; Ollason & Slater 1973); this was done to determine whether our manipulations influenced song rates within the colonies. We turned the Tandberg on as soon as the modified tape unit switched to the record mode; as a result, each 24 min sample included vocal activity from one 12 min recording phase and one 12 min playback phase. The Tandberg and modified unit were housed in a separate room so that recordings could be made without disturbing subjects.
The experiment ended after 60 days; this provided birds with sufficient time to raise at least one clutch to the point of fledging. As soon as each clutch was initiated, we removed any unused nesting material to stop pairs from burying their eggs. The second replicate, using 45 new pairs, began one month after the first ended. The three rooms were reassigned with respect to treatment to avoid the possibility of a room effect. The Queen’s University Animal Ethics Committee approved the experimental protocol.
To determine if the treatments influenced how often males sang (females do not sing), we counted the number of songs produced during each 24 min morning sample of vocal activity. A χ2-test was used to determine if males that received playback surpassed the singing rates of control males more often than expected by chance during the 45 days that followed the entrance of the last pair in each colony. A two-sample Kolmogorov–Smirnov test was used to initially determine whether the distribution of laying dates differed among the treatments or replicates. Two-way analyses of variance (ANOVAs) were then used to determine if clutch size or the amount of time taken to initiate a clutch differed among treatments or replicates. Data obtained from each pair were considered independent for our analysis of egg-laying patterns as pairs were visually isolated from others and each female experienced a unique acoustic condition, with the vocalizations of her male set against the background of colony sound. Females can recognize the vocalizations of their mates (Miller 1979; Silcox & Evans 1982; Clayton 1988) and would be able to distinguish playback of her partner’s songs and also calls from those of other males.
Levene’s test was used to examine breeding synchrony (Levene 1960; Van Valen 1978); for each colony, we calculated the difference between the mean laying date for that colony and the time that each individual clutch in that colony was initiated. A two-way ANOVA was used to compare these values between replicates and treatments.
Two or, more commonly, three of the 15 pairs in each colony failed to produce eggs so we used data from the first 12 pairs to lay in each room for analysis.
As suggested by Van Valen (1978), we removed the values from the first and last clutch from the dataset before we analysed data on breeding synchrony or the time between arrival and egg laying (leaving a sample size of 10 per treatment per replicate). Tukey’s multiple range tests were used to distinguish among treatments where the ANOVAs showed no differences between replicates.
3. Results
3.1 Song rates
Males occupying colonies receiving sound supplements from their own room produced more songs than males in control colonies during 76 out of the 90 morning songs samples (χ2=42.71, p<0.01). Males occupying colonies that received sound supplements from a different colony ‘out-sang’ males in the control colony during 55 out of the 90 samples (χ2=4.44, p<0.05).
As a group, males in colonies that received playback from their own room produced about four songs for every three produced in either control colonies or colonies that heard playback from a foreign colony. If songs added by the playback system are included, birds in colonies that received playback from their own room heard twice as many songs as birds in control colonies during the morning samples; birds in colonies that received playback from another colony heard about one and a half times as many songs as birds in the control colonies.
3.2 Egg-laying distribution
There was significant variation in the distribution of laying dates between the three treatments; there were no significant differences between replicates. The distribution of laying dates in colonies that received playback supplements from a foreign colony was different from those in control colonies (two-sample Kolmogorov–Smirnov test, p=0.025) and colonies that received playback supplements from their own colony (p=0.025); colonies that received sound supplements from their own room did not differ significantly from control colonies.
3.3 Time to first egg
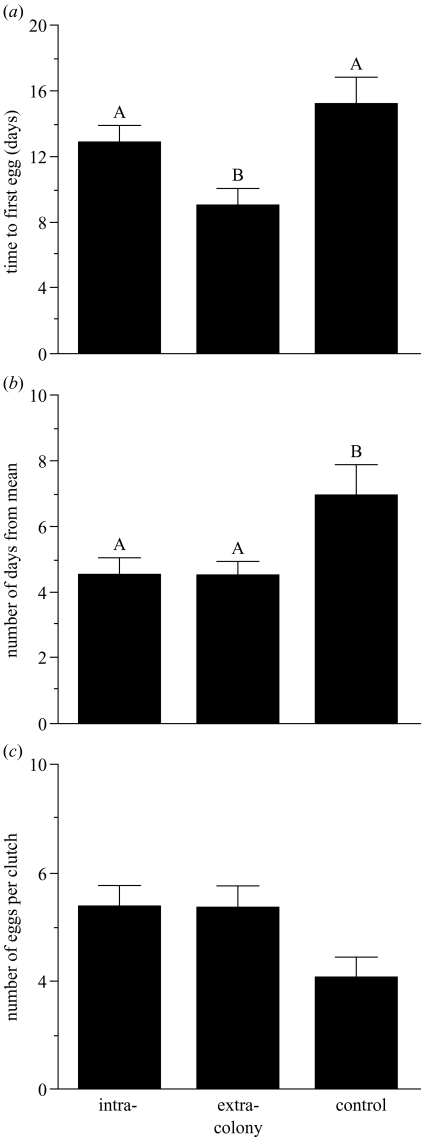
A two-way ANOVA indicated significant variation between treatments in the amount of time required to produce the first egg (F2,54=7.10, p=0.002; figure 1a); there were no significant differences between replicates (F2,54=0.99, n.s.). Colonies that received sound supplements from another colony began clutches ca. 6 days earlier than control colonies and 4 days earlier than colonies that received supplements from their own colony.
Figure 1.
Comparisons of (a) the mean number of days (±s.e.m.) between a pairs’ arrival in the colony and clutch initiation; (b) the mean number of days (±s.e.m.) from the mean laying date of the colony to the date that each pair initiated their clutch; and (c) the mean clutch size (±s.e.m.) for the three treatments (i.e. intra-colony:samples of sound were generated from their own colony; extra-colony:samples of sound were generated from a different colony; control:no sound supplements). Bars not sharing any letter are significantly different (p < 0.05, Tukey’s multiple-range tests).
3.4 Synchrony
A two-way ANOVA also showed significant variation between treatments in how synchronously the birds initiated clutches (F2,54=3.80, p=0.028; figure 1b); once again, there were no statistically significant differences between replicates (F2,54=0.05, n.s.). Birds in colonies that received sound supplements laid ca. 2.5 days closer to the mean laying date of their colony than birds in control colonies.
3.5 Clutch size
Clutch size also differed between treatments (F2,68=3.16, p=0.049; figure 1c) but not replicates (F2,68=0.00, n.s.). Colonies that received sound supplements from their own colony or from a different colony laid larger clutches than birds in control colonies. Note, however, that a Tukey’s multiple-range test did not distinguish between treatments even though the ANOVA indicated that significant variation occurred (figure 1c).
4. Discussion
Our paper provides the first experimental support, to our knowledge, for the predictions of the Darling hypo thesis. Males in colonies receiving playback originating from their own colony sang more frequently than males in control colonies; males in colonies that received playback from another colony sang at an intermediate level. Social stimulation, derived from the social facilitation of song and the playback of courtship sounds, synchronized and hastened egg laying in zebra finch colonies. Playback appeared to influence the rate at which females became physiologically ready for fertilization. We also found that females exposed to elevated song rates and playback tended to lay larger clutches than females in the control colonies.
In contrast to our findings, Bruen & Dunham (1973) found that the sounds of unseen conspecifics suppressed nesting activities (i.e. nest building, manipulating nest material) in male zebra finches. However, in their study, a screen separated the male and female cage-mates. Males normally gather nesting material while females adjust the material inside the nest (Morris 1954). By separating the male and female, Bruen and Dunham did not allow the female to assume a role in nest construction; this might explain why males showed little interest in nest building.
Previous studies of birds (e.g. Brockway 1965; Lehrman & Friedman 1969; Kroodsma 1976; Morton et al. 1985; Tchernichovski et al. 1998) and mammals (McComb 1987) have shown that exposure to courtship sounds can influence how quickly females reach breeding condition (the sight of conspecifics can also have important influences; e.g. Meijer & Langer 1995); other studies show that courtship sounds are socially facilitated in colonies (Fetterolf & Dunham 1985; Waas 1988, 2000). Exposure to male vocalizations may instigate changes in gonadotropic activity (i.e. follicle-stimulating hormone (FSH) and luteinizing hormone (LH)); these changes can lead to an increase in the rate of follicular growth and a decrease in the time to ovulation (e.g. Farner & Lewis 1971; Bluhm 1985).
Zebra finches exposed to sound from their own colony took longer to lay than finches hearing samples from a different colony. This may have been due to habituation; birds exposed to playback of their own vocalizations heard each 12 min segment of sound twice (i.e. once live and once in its recorded form). In addition, hearing their own vocalizations being produced across the room by the playback system may have agitated females and their partners. Alternatively, exposure to the vocalizations of 30 different males may be more stimulating than hearing the same 15 males vocalize more often. This may be particularly important in a laboratory setting where animals may be starved of social diversity. However, the primary source of social stimulation for female zebra finches is most probably the ‘directed songs’ that their own partners performed (cf. ‘undirected songs’ performed by isolated males; Hall 1962; Immelmann 1968; Dunn & Zann 1997); colony sounds may simply alter the number and/or quality of songs produced by each male which in turn influence his female’s reproductive state.
In our study, exposure to courtship sounds also synchronized laying. A simple explanation exists for this effect. The amount of courtship activity perceived by females will increase as more birds arrive in the colony. If a positive relationship exists between exposure to displays and ovarian development, early females (arriving when levels of social stimulation are low) will always take longer to ovulate than females arriving later. The net effect would be to reduce the variance around the mean time of laying. The birds in all colonies, including the control colonies, laid eggs more synchronously than the birds arrived; the playback system simply amplified or exaggerated a process that must occur under natural conditions.
Several field studies also suggest that social stimulation might influence how early and synchronously colony dwellers lay eggs. For example, birds in large or densely packed colonies (where levels of social stimulation are presumably high) tend to lay earlier and more synchronously than birds in smaller and less densely packed colonies (Coulson & White 1960; MacRoberts & MacRoberts 1972; Montevecchi et al. 1979; Birkhead 1980). However, several studies have failed to find support for such relationships (e.g. Orians 1961; Snapp 1976) and other factors (e.g. variation in the distribution of different aged birds) could also explain the effects (Wittenberger & Hunt 1985).
The present study is the first, to our knowledge, to demonstrate a relationship between exposure to colony sound and clutch size. However, Kroodsma (1976) showed that female canaries (Serinus canaria) exposed to playback of a large repertoire of male songs laid eggs at a faster rate than did females exposed to a smaller song repertoire; also, Balzer & Williams (1998) found that female zebra finches produce larger clutches for ‘preferred’ males, which had higher song durations and frequency. Increases in clutch size can also be explained by relationships between playback and circulating FSH because FSH influences the recruitment of vitellogenic follicles. For example, Sinervo & Licht (1991) increased the clutch size of side-blotched lizards (Uta stansburiana) by injecting ovine FSH into vitellogenic females.
Several field studies also link social stimulation with variation in clutch size. For example, McLandress (1983) found a positive relationship between clutch size and nest density in colonies of Ross’ geese (Chen rossii). Finney & Cooke (1978) found a negative relationship between clutch size and clutch initiation dates in the lesser snow goose (Anser caerulescens caerulescens). These trends may be due to temporal and spatial variation in the distribution of different age classes. However, Westneat (1992) controlled for age in a study of red-winged blackbirds (Agelaius phoeniceus) and still found a negative relationship between clutch size and clutch initiation dates. Our results suggest that there is a close link between social stimulation, early breeding and enhanced clutch size.
The link between social stimulation and clutch size in zebra finch colonies may have adaptive value. From a functional perspective, high levels of social stimulation may trigger the recruitment of extra follicles because the level of stimulation is a good predictor of early laying and birds that lay early may be capable of rearing more young. Alternatively, there may be advantages associated with producing more young in seasons when breeder densities (i.e. as revealed by levels of social stimulation) are high.
There are clear reproductive advantages associated with breeding early (e.g. Emlen & Demong 1975; Hunt & Hunt 1976; Mills 1979; Wittenberger & Hunt 1985; Westneat 1992) and synchronously (e.g. Findlay & Cooke 1982; Wittenberger & Hunt 1985; Wissel & Brandl 1988; Waas 1995). Nevertheless, the effects of social stimulation that we demonstrated here should be viewed as a consequence, not a cause, of coloniality (Orians 1961; Hoogland & Sherman 1976; Siegel-Causey & Kharitonov 1990). The possible benefits of social stimulation could only accrue once colonies existed. However, any advantages associated with the effects of social stimulation are likely to contribute to the maintenance of colonial breeding systems, once established.
Acknowledgments
The authors thank Fred Cooke, Ian Jamieson, Bob Lavery, Laurene Ratcliffe, Raleigh Robertson, Kathy Wynne-Edwards and anonymous reviewers for their comments on the manuscript. The playback system was constructed by Roy Young and staff at the Department of Biomedical Engineering (Queen‘s University). Ron Weisman and the Department of Psychology (Queen‘s University) provided laboratory space. Scott MacDougall-Shackleton and Tina Tom maintained the colonies and helped collect the data. The research was funded by a Natural Sciences and Engineering Research Council of Canada (NSERC) post-doctoral fellowship to J.R.W. and operating grants to P.W.C. and P.T.B.Patrick Colgan died on 21 July 2004, at the age of 59, from the effects of amyotrophic lateral sclerosis. Patrick made countless contributions to the study of biology and to the lives of all who knew him. He will be greatly missed.
References
- Armstrong E.A. Bird display and behavior. L. Drummond; London: 1947. [Google Scholar]
- Balzer A.L., Williams T.D. Do female zebra finches vary primary reproductive effort in relation to mate attractiveness. Behaviour. 1998;135:297–309. [Google Scholar]
- Birkhead T.R. Timing of breeding of common guilemonts Uria aalge at Skomer, Wales. Ornis Scand. 1980;11:142–145. [Google Scholar]
- Bluhm C.K. Social factors regulating avian endocrinology and reproduction. In: Follett B.K., Ishii S., Chandola A., editors. The endocrine system and the environment. Springer; Berlin: 1985. pp. 247–264. [Google Scholar]
- Brockway B.F. Stimulation of ovarian development and egg-laying by male courtship vocalizations in budgerigars (Melopsittacus undulatus) Anim. Behav. 1965;13:575–579. doi: 10.1016/0003-3472(65)90123-5. [DOI] [PubMed] [Google Scholar]
- Brown C.R. Cliff swallow colonies as information centers. Science. 1986;234:83–85. doi: 10.1126/science.234.4772.83. [DOI] [PubMed] [Google Scholar]
- Bruen K., Dunham D.W. Effects of social stimuli on nest building in the zebra finch (Poephila guttata) Anim. Behav. 1973;21:183–190. [Google Scholar]
- Clayton N.S. Song discrimination learning in zebra finches. Anim. Behav. 1988;36:1016–1024. [Google Scholar]
- Coulson J., White E. The effect of age and density of breeding birds on the time of breeding in the kittiwake Rissa tridactyla. Ibis. 1960;102:71–86. [Google Scholar]
- Darling F.F. Bird flocks and the breeding cycle. Cambridge University Press; 1938. [Google Scholar]
- Dunn A.M., Zann R.A. Effects of pair bond and presence of conspecifics on singing in captive zebra finches. Behaviour. 1997;134:127–142. [Google Scholar]
- Emlen S.T., Demong N.J. Adaptive significance of synchronized breeding in a colonial bird: a new hypothesis. Science. 1975;188:1029–1031. doi: 10.1126/science.1145188. [DOI] [PubMed] [Google Scholar]
- Farner D.L., Lewis R.A. Photoperiodism and reproductive cycles in birds. In: Giese A.C., editor. Photophysiology: current topics in photochemistry and photobiology. Vol. 6. Academic Press; New York: 1971. pp. 325–370. [Google Scholar]
- Fetterolf P.M. Infanticide and non-fatal attacks on chicks by ring-billed gulls. Anim. Behav. 1983;31:1018–1028. [Google Scholar]
- Fetterolf P.M., Dunham D.W. Stimulation of courtship displays in ring-billed gulls using playback of vocalization. Can. J. Zool. 1985;63:1017–1019. [Google Scholar]
- Findlay C.S., Cooke F. Synchrony in the lesser snow goose (Anser caerulescens caerulescens). II. The adaptive value of reproductive synchrony. Evolution. 1982;36:786–799. doi: 10.1111/j.1558-5646.1982.tb05445.x. [DOI] [PubMed] [Google Scholar]
- Finney G., Cooke F. Reproductive habits in the snow goose: the influence of female age. Condor. 1978;80:147–158. [Google Scholar]
- Gochfeld M. Mechanisms and adaptive value of reproductive synchrony in colonial birds. In: Burger J., Olla B.L., Winn H.E., editors. Behavior of marine animals. Plenum Press; New York: 1980. pp. 207–270. [Google Scholar]
- Hall M.F. Evolutionary aspects of estrildid song. Symp. Zool. Soc. Lond. 1962;8:37–55. [Google Scholar]
- Harris M.P. Breeding ecology of the swallow-tailed gull, Creagus furcatus. Auk. 1970;87:215–243. [Google Scholar]
- Hoogland J.L., Sherman P.W. Advantages and disadvantages of bank swallow (Riparia riparia) coloniality. Ecol. Monogr. 1976;46:33–58. [Google Scholar]
- Hunt F.L., Jr, Hunt M.W. Gull chick survival: the significance of growth rate, timing of breeding and territory size. Ecology. 1976;57:62–75. [Google Scholar]
- Ims R.A. The ecology and evolution of reproductive synchrony. Trends Ecol. Evol. 1990;5:135–140. doi: 10.1016/0169-5347(90)90218-3. [DOI] [PubMed] [Google Scholar]
- Immelmann K. Australian finches in bush and aviary. Angus & Robertson; Sydney: 1965. [Google Scholar]
- Immelmann K. Zur biolischen Bedeutung des Ewtrildidengesanges. J. Ornithol. 1968;109:284–299. [Google Scholar]
- Kroodsma D.E. Reproductive development in a female songbird: differential stimulation by quality of male song. Science. 1976;192:574–575. doi: 10.1126/science.192.4239.574. [DOI] [PubMed] [Google Scholar]
- Kruuk H. Predators and anti-predator behavior of the black-headed gull (Larus ridibundus) Behaviour. 1964;11:1–129. [Google Scholar]
- Lack D. Fisher and Waterston on the fulmar. Ibis. 1943;85:115–116. [Google Scholar]
- Lehrman D.S., Friedman M. Auditory stimulation of ovarian activity in the ring dove (Streptopelia risoria) Anim. Behav. 1969;17:494–497. doi: 10.1016/0003-3472(69)90152-3. [DOI] [PubMed] [Google Scholar]
- Levene H. Robust tests for equality of variances. In: Olkin I., Ghurye S.G., Hoeffding W., Madow W.G., Mann H.B., editors. Contributions to probability and statistics. Stanford University Press; Stanford, CA: 1960. pp. 278–292. [Google Scholar]
- McComb K. Roaring by red deer stags advances the date of oestrus in hinds. Nature. 1987;330:648–649. doi: 10.1038/330648a0. [DOI] [PubMed] [Google Scholar]
- McLandress M.R. Temporal changes in habitat selection and nest spacing in a colony of Ross‘ and lesser snow geese. Auk. 1983;100:335–343. [Google Scholar]
- MacRoberts B.R., MacRoberts M.H. Social stimulation of reproduction in herring and lesser black-backed gulls. Ibis. 1972;114:495–506. [Google Scholar]
- Meijer T., Langer U. Food availability and egg-laying of captive European starlings. Condor. 1995;97:718–728. [Google Scholar]
- Miller D.B. The acoustic basis of mate recognition by female zebra finches (Taeniopygia guttata) Anim. Behav. 1979;27:376–380. [Google Scholar]
- Mills J.A. Factors affecting the egg size of red-billed gulls Larus novaehollandiae scopulinus. Ibis. 1979;121:53–67. [Google Scholar]
- Montevecchi W.A., Impekoven M., Segre-Terdal A., Beer C.G. The seasonal timing and dispersion of egg-laying among laughing gulls Larus atricilla. Ibis. 1979;121:337–344. [Google Scholar]
- Morris D. The reproductive behaviour of the zebra finch (Poephila guttata), with special reference to pseudofemale behavior and displacement activities. Behavior. 1954;6:271–332. [Google Scholar]
- Morton M.L., Pereyra M.E., Baptista L.F. Photoperiodically induced ovarian growth in the white-crowned sparrow (Zonotrichia leucophrys gambelii) and its augmentation by song. Comp. Biochem. Physiol. A. 1985;80:93–97. [Google Scholar]
- Nelson J.B. The relationship between behavior and ecology in the Sulidae with reference to other seabirds. Oceanogr. Mar. Biol. Ann. Rev. 1970;8:501–574. [Google Scholar]
- Nelson J.B. The Sulidae. Oxford University Press; 1978. [Google Scholar]
- Ollason J.C., Slater P.J.B. Changes in the behaviour of the male zebra finch during a 12-hr day. Anim. Behav. 1973;21:191–196. doi: 10.1016/s0003-3472(73)80059-4. [DOI] [PubMed] [Google Scholar]
- Orians G.H. Social stimulation within blackbird colonies. Condor. 1961;63:330–337. [Google Scholar]
- Patterson I.J. Timing and spacing of broods in the black-headed gull. Larus ridibundus. Ibis. 1965;107:433–459. [Google Scholar]
- Sanders E.H., Garner P.D., Berger P.J., Negus N.C. G-methoxybenzoxazolinone: a plant derivative that stimulates reproduction in Microtus montanus. Science. 1981;214:67–69. doi: 10.1126/science.7025209. [DOI] [PubMed] [Google Scholar]
- Siegel-Causey D., Kharitonov S.P. The evolution of coloniality. In: Power D.M., editor. Current ornithology. Vol. 7. Plenum; New York: 1990. pp. 285–330. [Google Scholar]
- Silcox A.P., Evans S.M. Factors affecting the formation and maintenance of pair ponds in the zebra finch, Taeniopygia guttata. Anim. Behav. 1982;30:1237–1243. [Google Scholar]
- Sinervo B., Licht P. Hormonal and physiological control of clutch size, egg size, and egg shape in side-blotched lizards (Uta stansburiana): constraints on the evolution of lizard life histories. J. Exp. Zool. 1991;257:252–264. [Google Scholar]
- Slagsvold T. Bird song activity in relation to the breeding cycle, spring weather, and environmental phenology. Ornis Scand. 1977;8:197–222. [Google Scholar]
- Smith H.G. Selection for synchronous breeding in the European starling. Oikos. 2004;105:301–311. [Google Scholar]
- Snapp B.D. Colonial breeding in the barn swallow (Hirundo rustica) and its adaptive significance. Condor. 1976;78:471–480. [Google Scholar]
- Sossinka R. Ovarian development in an opportunistic breeder, the zebra finch Poephila guttata castanotis. J. Exp. Zool. 1980;211:225–230. [Google Scholar]
- Tchernichovski O., Schwabl H., Nottebohm F. Context determines the sex appeal of male zebra finch song. Anim. Behav. 1998;55:1003–1010. doi: 10.1006/anbe.1997.0673. [DOI] [PubMed] [Google Scholar]
- Van Valen L. The statistics of variation. Evol. Theory. 1978;14:33–44. [Google Scholar]
- Waas J.R. Acoustic displays facilitate courtship in little blue penguins, Eudyptula minor. Anim. Behav. 1988;36:366–371. [Google Scholar]
- Waas J.R. Social stimulation and reproductive schedules: does the acoustic environment influence the egg-laying schedule in penguin colonies? In: Dann P., Norman I., Peilly P., editors. Penguins: ecology and management. Surrey Beatty; Sydney: 1995. pp. 111–137. [Google Scholar]
- Waas J.R. Colony sound facilitates sexual and agonistic activities in royal penguins. Anim. Behav. 2000;60:77–84. doi: 10.1006/anbe.2000.1415. [DOI] [PubMed] [Google Scholar]
- Weatherhead P.J., Sommerer S.J. Breeding synchrony and nest predation in redwinged blackbirds. Ecology. 2001;82:1632–1641. [Google Scholar]
- Westneat D.F. Nesting synchrony by female red-winged blackbirds: effects on predation and breeding success. Ecology. 1992;73:2284–2294. [Google Scholar]
- Wissel C., Brandl R. A model for the adaptive significance of partial reproductive synchrony within social units. Evol. Ecol. 1988;2:102–114. [Google Scholar]
- Wittenberger J.F., Hunt G.L., Jr The adaptive significance of coloniality in birds. Avian Biol. 1985;8:1–78. [Google Scholar]
- Zann R.A., Morton S.R., Jones K.R., Burley N.T. The timing of breeding by zebra finches in relation to rainfall in central Australia. Emu. 1995;95:208–222. [Google Scholar]