Abstract
Mothers would often benefit from producing more offspring of one sex than the other. Although some species show an astonishing ability to skew their sex ratio adaptively, the trends found in many studies on vertebrates have proved inconsistent. Furthermore, evidence for a mechanism by which such a bias is achieved is equivocal at best. Here, we examine sex-ratio variation over 30 years, both at an individual and a population level, in the highly polygynous, size-dimorphic springbok (Antidorcas marsupialis). Many previous studies of similar species have shown that mothers in superior condition preferentially produce sons, whereas those in poorer condition produce more daughters. We found the opposite to be true in springbok, perhaps because daughters provide mothers in superior condition with a more rapid and secure fitness return. This theory was supported by the findings that earlier-conceived offspring tended to be female and that an increased proportion of daughters were produced with increasing rainfall (which was likely to reduce nutritional stress). We also show that selective reabsorption of embryos is unlikely to be the main mechanism by which deviations from an equal sex ratio are achieved. Hence, either differential implantation occurs or females are able to influence the sex of the sperm fertilizing an egg.
Keywords: embryo mass; maternal condition; rainfall, sex ratio; Trivers–Willard hypothesis
1. Introduction
Whenever maternal investment differentially affects the breeding success of the sexes, mothers of different condition should bias their offspring production in relation to future reproductive benefits (Hamilton 1967; Trivers & Willard 1973; Charnov 1982; Hewison & Gaillard 1999). In polygynous mammals, mothers in superior condition are often suggested to prefer sons over daughters, because the extra investment of a high-quality mother translates into an advantage for her son(s) in male–male competition for mating opportunities (Clutton-Brock et al. 1984; Clutton-Brock & Iason 1986; Cassinello & Gomendio 1996; Kruuk et al. 1999; Krackow 2002; West & Sheldon 2002). In many sexually dimorphic species, the costs of rearing sons are significantly greater than those of rearing daughters (Clutton-Brock et al. 1981), and in such species, mothers in superior condition overproduce sons. However, this need not always be the case. For example, when daughters obtain a higher dominance status from high-ranking mothers (Skogland 1986; Lloyd & Rasa 1989; Mendl et al. 1995), or when there is local resource competition and females are the dispersing sex (Hewison & Gaillard 1996), daughters may be more valuable than sons (Leimar 1996).
Recently, it has become apparent that environmental conditions affecting nutritional stress in mothers can also have a profound influence on offspring sex ratios (Kruuk et al. 1999; Post et al. 1999; Mysterud et al. 2000). Failure to consider this additional variable may explain some of the contradictory findings among related species (Kruuk et al. 1999). It is therefore imperative that studies of sex-ratio variation incorporate both phenotypic and environmental factors. In this paper, we used 30 years of data on a semi-wild, Kalahari population of polygynous, size-dimorphic springbok (Antidorcas marsupialis) to investigate the influence of maternal condition and conception date, as well as population density, population age structure and rainfall patterns, on embryo sex-ratio variation at both an individual and a population level.
The springbok is an abundant antelope of the arid savannahs and steppes of southern Africa. Females form herds with their young, while males are territorial during the mating season and defend favourable grazing patches from two years of age (Bigalke 1970; Estes 1991). Females can conceive at the age of six months and give birth to a single lamb after a gestation period of approximately six months. Under good conditions, they can lamb twice in a year (Estes 1991). While there is large variation in the timing of reproduction between years, females of a given population tend to breed highly synchronously (Estes 1991).
Here, we tested a series of specific predictions concerning sex-ratio variation in springbok. First, we predicted that mothers in superior condition would produce sons, whereas those in poorer condition will produce daughters. Despite differences between females in the age at which they begin reproducing, males are likely to exhibit greater variation in lifetime reproductive success than females: as in many other polygynous ungulates (e.g. red deer (Cervus elaphus); Clutton-Brock et al. 1984), male springbok compete for and defend harems, which vary greatly in size (2–35 females; Bigalke 1972). Springbok are therefore expected to fit the classic Trivers–Willard model (Trivers & Willard 1973). Furthermore, because male springbok are significantly larger than females at adulthood, and thus expected to be more costly, mothers in superior condition should produce sons, whereas those in poorer condition should produce daughters. Second, we predicted that females conceiving early in the season (when available levels of nutrition are likely to be at their highest) would produce sons, whereas those conceiving later will favour daughters. Conditions experienced during early development affect survival and reproductive performance in many bird and mammal species (Lindström 1999). The fitness of males in some species has been shown to be more negatively affected by a late birth date than the fitness of females (e.g. Dijkstra et al. 1990; Visser & Verboven 1999). Third, we predicted that high population densities would lead to an increase in competitive interactions between individuals for limited food resources, resulting in nutritional stress among mothers and a reduction in the production of sons (Kruuk et al. 1999; Mysterud et al. 2000). Fourth, we predicted that increased rainfall would increase the amount of fresh grass available in the normally arid Kalahari, thus reducing the nutritional stress of mothers and resulting in an increase in the number of sons produced (Kruuk et al. 1999).
While the Trivers–Willard model refers to individual optimization of offspring sex ratios, and hence does not apply at a population level, the important predictors of individual condition and offspring sex should also be important correlates of sex-ratio variation at the population level. Sutherland (1996) suggests that individual behaviour should be followed up to population-level phenomena, and our third and fourth predictions in particular made it imperative to include a population-level analysis here.
Despite the vast and ever-increasing literature on vertebrate sex-ratio variation, little is known about the underlying physiological mechanisms causing the deviations in question (Krackow 2002; Cameron 2004). Such knowledge is vital if we are to assign biological meaning to any statistical deviation and to differentiate adaptations from confounding effects (James 1993; Krackow 1995). There are three main stages at which sex-ratio adjustment could occur in mammals: (i) sex-chromosome selection at first meiotic division, or gamete selection; (ii) differential implantation of embryos; or (iii) differential mortality of foetuses (Krackow 1995). Rarely has it been possible to distinguish between these, but the examination of culled females in our study allowed an assessment of the likelihood of selective reabsorption as the proximate mechanism of sex-ratio manipulation.
2. Material and Methods
2.1 Data collection
Springbok were studied on the 113 km2 Benfontein farm near Kimberley, South Africa (28°40′ S and 24°10′ E) between 1972 and 2001. The region is part of the southern Kalahari ecosystem, with highly variable annual rainfall (range, 245–770 mm). During the period of study, the population fluctuated between 1383 and c. 6000 animals, as assessed by a combination of aerial and ground head counts completed in February each year to set the culling quota. In some years, post-culling head counts were also conducted and the two population figures (after deducting the number of culled animals from the February count) were always within 10%, and usually much closer. Springbok have been on the farm since at least 1900, and the only restriction is a fence around the property. Animals were effectively culled at random each winter (June–August) as part of a management scheme, and all animals in our study came from this culling regime. Culling was completed by commissioned hunters who were told not to focus on any particular age group or on seemingly ill animals. Despite these instructions, a potential bias could result from the hunters’ greater focus on males (see § 4).
A total of 9699 males and 7591 females was obtained, and 3974 embryos were sexed by two of the authors (R. L. and C. A.). Detailed anatomical examination, involving looking for scrotal development and other sex-specific signs, meant it was often possible to sex embryos of less than 10 grams in weight. Embryo mass was measured to the nearest 0.1 g. Male and female embryos of the same age do not differ in mass (R. L., unpublished data). Because animals were obtained at different times within and between years, embryo mass represents both an estimate of the time conception occurred and relative maternal condition. However, given that we have other measures of maternal condition (see below), we interpret embryo mass essentially as a measure of conception date.
Adult age classes were defined by horn shape and development, and tooth eruption and wear, following the classification of Rautenbach (1971): 1=1–2 months, 2=3–6 months, 3=7–10 months, 4=11–14 months, 5=15–18 months, 6=19–24 months, 7=25–60 months, 8=61–80 months and 9 is more than 80 months of age. Age classes have been found to correspond well with ages of known-age animals (Rautenbach 1971) We determined body condition by measuring body mass (to the nearest 500 g), kidney weight, kidney-fat weight and the kidney-fat index (KFI; see Anderson et al. (1990) for details of calculation). Reproductive status in females was determined by careful examination of the animal, including its uterus, and was categorized as: not pregnant, pregnant, lactating, pregnant and lactating, re-absorption, lambed within last four months (distinctively spotted uterus), lambed twice in last 12 months (currently pregnant and with spotted uterus), recently mated. Early stages of reabsorption were apparent because embryo extremities become rounded off as they begin to break down. In later stages of reabsorption, the embryo is broken down and appears as an amorphous mass.
Although there was variation in culling date between years (mean date varied between 67 and 104, where 1 April=1; overall mean±s.d. date=86±8.9), there was no significant correlation between mean annual culling date and embryo sex ratio (r28=−0.014, n.s.), embryo mass (r27=0.142, n.s.) or female KFI (r28=0.015, n.s.). Hence, these variables did not need to be standardized according to culling date, and they provided an estimate of conception date and maternal condition independent of when culling occurred.
Population density was estimated each year in February from detailed aerial and ground counts of individuals. Rainfall data were available from the meteorological station at Kimberley (South African weather bureau).
2.2 Statistical analysis
Prior to further analysis, we checked whether the sample size of embryos was correlated with the amount of deviation from an equal sex ratio. As the correlation is highly significant (r28=−0.28, p=0.003, r2=0.28), we checked for the robustness of our analysis by excluding years with fewer than 50 sexed embryos from both the individual (n=225 embryos removed) and population-level (n=9 years removed) analyses. The significant correlation between sample size of embryos and the amount of deviation from an equal sex ratio disappeared (r19=−0.42, p=0.059, r2=0.18), but only just. However, none of our results changed qualitatively by excluding years with fewer than 50 sexed embryos and hence we report the results using the whole dataset. The one missing year of population sex ratio (1994) was replaced with the 30-year mean. We tested for deviations from a 1:1 sex ratio using binomial tests. Sex ratio over the 30 years was normally distributed and hence not transformed.
In the generalized linear model, embryo sex (1=male, 0=female) was linked to the following predictors using a logit link function and binomial errors: female age, mass and body condition, female reproductive status, embryo mass, annual population density at time t and t−1, culling date, annual rainfall (Julyt−1−Junet), monthly rainfall levels from January to May and their sum. All possible two-way interactions between variables were also included. The same variables were included with all two-way interactions for the stepwise forward multiple regression model of population sex ratio. Errors in the best model were distributed normally. The 5 years lacking female KFI measures were initially excluded from the analysis. We checked for robustness of the regression model by performing backwards-elimination regression, and found that the final model was the same. Replacing the missing data with means did not change the results qualitatively; the same three predictor variables were chosen. In cases where multiple tests (families of tests) were performed, the sequential Bonferroni correction was applied (Rice 1989).
3. Results
3.1 Sex-ratio variation
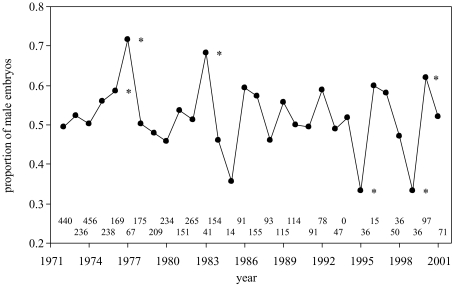
There was considerable between-year variation in the sex ratio of springbok embryos (range: 33.3–71.6% males, n=14–456; figure 1), with a significant excess of males overall (mean: 51.9%, z=2.44, p=0.015). Multivariate models at the individual and population levels revealed similar variables to be predictors of offspring sex and population sex ratio, respectively (table 1). At the individual level (table 1a), the probability of carrying a male embryo decreased with embryo mass; earlier-conceived embryos tended to be female. The interactions between embryo mass and both maternal mass and annual rainfall levels also entered the model, indicating that a higher maternal mass and greater rainfall levels decreased the probability of a female producing a son.
Figure 1.
Sex-ratio variation of springbok embryos between 1972 and 2001. Starred years denote a significant deviation from a 1:1 sex ratio, although only two were significant after sequential Bonferroni correction. Numbers at the bottom refer to the sample size for each year.
Table 1.
Significant variables in (a) a generalized linear model with logit link function predicting the sex of an individual female’s lamb (1=male, 0=female), and (b) a multiple regression model for the proportion of male embryos in the population, between 1972 and 2001.
| (a) | ||||||
| variable |
beta |
s.e.m. |
F1,3905 |
p |
||
| constant | 0.0773 | 0.0320 | — | 0.016 | ||
| embryo mass | −0.000295 | 0.000081 | 13.815 | 0.001 | ||
| embryo mass × maternal mass | −0.0000083 | 0.0000026 | 8.594 | 0.003 | ||
| embryo mass × annual rainfall |
−0.00000043 |
0.00000016 |
7.197 |
0.007 |
||
| (b) | ||||||
| variable |
beta |
s.e.m. |
t |
p |
cumulative r2 |
tolerance |
| constant | 0.765 | 0.049 | 15.578 | 0.001 | — | — |
| embryo mass | −0.0002 | 0.0001 | 3.914 | 0.001 | 0.636 | 0.635 |
| female kidney-fat index | −0.0019 | 0.0006 | 3.238 | 0.004 | 0.724 | 0.580 |
| rainfall January–May | −0.0002 | 0.0001 | 2.174 | 0.042 | 0.766 | 0.873 |
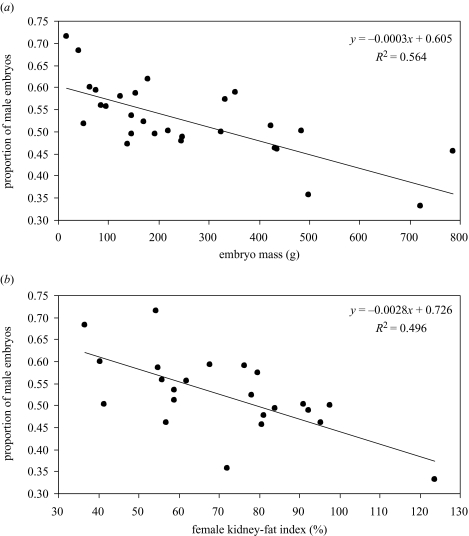
At the population level, embryo sex ratio was predicted by three independent variables, together explaining 76.6% of the variation between years (F3,20=21.78, p<0.001, table 1b). The proportion of male embryos decreased with increasing embryo mass (figure 2a) and with increasing maternal body condition, as assessed by KFI (figure 2b). Female population age structure per se was not correlated with embryo sex ratio (r28=−0.11, n.s.), hence the importance of female KFI was not merely a consequence of a changing age structure and associated changes in body condition over the lifetime in this population. In addition, increased rainfall between January and May (the period before, during and immediately following the main mating season) led to an increase in the proportion of daughters.
Figure 2.
Proportion of male embryos each year in relation to (a) embryo mass and (b) female kidney-fat index. The slopes are highly significant (t28=5.92, p<0.001 and t22=4.66, p<0.001, respectively).
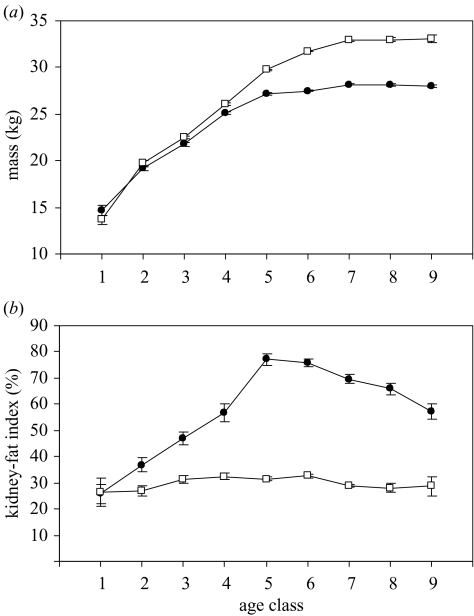
A further factor that is assumed to influence sex-ratio variation is the relative direct metabolic costs incurred by the mother as a result of gestation and lactation. By age class 3 (7–10 months), male springbok were significantly heavier than females (t1147=3.17, p=0.002; figure 3a), with an eventual 17% dimorphism in adulthood. However, springbok males and females did not differ in size during gestation and, if anything, there was a statistical trend for females to be heavier (females=323.1±9.9 g, males=296.4±9.6 g, s.e.m., F1,3726=3.514, p=0.061). Furthermore, there was no significant difference in the mass of the two sexes during lactation (age class 1: t78=1.30, n.s.; age class 2: t575=1.83, n.s.).
Figure 3.
Intersexual differences in springbok (a) mass and (b) kidney-fat index with age. Values are means±s.e.m. Open squares, males; filled circles, females.
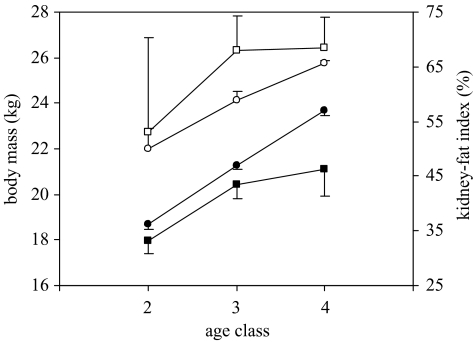
Why might maternal investment be more important to daughters than sons in springbok? To reproduce early in life, females need to accumulate sufficient body reserves, and indeed females possess a much higher KFI than males by the end of lactation (age class 2: t269=2.81, p=0.005; figure 3b). The percentage of pregnant females increased dramatically over the first year of life, from 6.8% at age class 2 (3-6 months) to 18.1% at age class 3 (7-10 months) and 64.9% at age class 4 (11-14 months). Pregnant females were heavier (age class 2: F1,299=19.22, p<0.001; age class 3: F1,426=36.34, p<0.001; age class 4: F1,511=82.66, p<0.001) and in better condition (KFI, age class 2: F1,142=4.10, p=0.045; age class 3: F1,235=10.18, p=0.002; age class 4: F1,137=9.63, p=0.002) than non-pregnant females at the same stage (figure 4). Hence, daughters might potentially profit more than sons from higher maternal investment because they need to put on fat reserves to be in sufficient condition to conceive early.
Figure 4.
Differences between non-pregnant (filled symbols) and pregnant (open symbols) females in terms of body mass (circles) and kidney-fat index (squares) during the first year of life. Values are means±s.e.m.
3.2 Mechanism of manipulation
Only 98 (i.e. 1.7%) out of the 5899 pregnant springbok females that we examined showed evidence of embryo reabsorption, and 75% of reabsorption cases occurred in the years 1972, 1973 and 1975. Even in these three years, there were insufficient cases to change the sex ratio from uniform to a significant bias, or vice versa (see figure 1). Hence, even if mothers were selectively reabsorbing only one sex of embryo, this effect would have been much too small to account for the population-level effects we have documented here.
It is possible that females classified as ‘not pregnant’ had reabsorbed an embryo at an earlier stage, and this went undetected. Out of all females examined over the 30 years, 1354 (17.2%) were classified as not pregnant, and this percentage varied between 1.2 and 29.9% annually. However, 50% of the non-pregnant females were in the age classes 1–3 and hence were highly unlikely to be pregnant anyway. In addition, there was a significant negative correlation between the percentage of non-pregnant females and the mean age over the 30 years (r28=−0.42, p=0.021), indicating that in years where a higher percentage of non-pregnant females was observed, these females were mostly younger ones. Rather than pointing towards adaptive early reabsorption behaviour, this suggests variation in the age structure of the culled females.
4. Discussion
At both an individual and a population level, springbok mothers in superior condition were more likely to produce a daughter than a son. Offspring conceived earlier in the season were more likely to be female. Increased rainfall levels resulted in an increase in the proportion of daughters produced, but there was no significant effect of population density. It might be argued that sire quality influences the sex ratio. Although there are no such data available for springbok, it is reasonable to assume that territorial, high-quality males will father most of the early-conceived offspring because of the polygynous mating system. The ‘successful-sons’ hypothesis (Hewison & Gaillard 1999) would predict in this situation that early conceived embryos should be males, which is opposite to our finding. In addition, later conceived, lightweight embryos might be less viable, which would bias the secondary sex ratio even more towards females.
Springbok do not, therefore, appear to fit the predictions of the Trivers & Willard (1973) model, assuming that sons are indeed more valuable to mothers in superior condition. Why might this assumption not be true? In some species, maternal quality is better correlated with that of daughters than sons if, for example, daughters inherit a higher dominance status from high-ranking mothers (Skogland 1986; Lloyd & Rasa 1989; Mendl et al. 1995). However, no female dominance hierarchies are apparent in springbok (Bigalke 1970; Estes 1991). When there is local resource competition and females are the dispersing sex (Silk 1983; Hewison & Gaillard 1996), daughters may be more valuable (Leimar 1996). However, there is no evidence so far for intersexual differences in springbok dispersal (Bigalke 1970; Estes 1991). Thus, neither local resource competition nor any effect of dominance-rank inheritance are likely to explain our results. Furthermore, sons do not seem to be more costly than daughters, a finding matched by that of Post et al. (1999) in red deer, so mothers should not be constrained in which sex of offspring they produce.
Daughters may benefit more than sons from being in good condition during their first year of life. Females can become pregnant by six months of age and can have two offspring in 1 year, a rate of reproduction unparalleled by any ungulate of comparable size (Bigalke 1970; Estes 1991). In contrast, males only become sexually mature at 24 months, when they start to defend a territory (Estes 1991). Although the argument of faster fitness returns via female progeny holds in principle for almost all polygynous ungulate species, the effects are probably most obvious in springbok. Closely related African antelope species (Thomson’s gazelle (Gazella rufifrons), Grant’s gazelle (G.granti), gerenuk (Litocranius walleri), Dorcas gazelle (G.dorcas), Soemmerring’s gazelle (G.soemmering), dibatang (Ammodorcas clarkei)), varying in body mass between 18 and 45 kg, have earliest conception dates ranging from 11 to 18 months (Kingdon 1982; Estes 1991). For springbok, with an average female body mass of ca. 25 kg, the earliest conception date is thus half that of comparable antelope species. The discrepancy between early fitness returns from producing daughters compared with late fitness returns from producing sons is likely, therefore, to be considerably larger in springbok than in any other ungulate species.
Whereas high-condition daughters can reproduce quickly and give their mothers an immediate fitness return in the unpredictable, harsh environment of the Kalahari, low-condition daughters cannot. This means that for a mother in superior condition, daughters might be of greater reproductive value than sons (Daan et al. 1996; Leimar 1996), because by the time a son can reproduce, a daughter may already have provided as many as four grand-offspring and two great grand-offspring. Mothers in poorer condition will benefit from producing sons, as males experience their growth spurt after lactation and the end of nutritional care, and none mate in their first two years (Estes 1991).
A word of caution might be warranted at this point. Although there was no systematic bias introduced by the culling regime, more males were shot than females and this could of course have affected our results. However, our analyses focused on individual optimization of offspring sex ratio rather than the interplay between population sex ratio and maternal inclusive fitness (Fisher 1930) and hence we believe that the results presented here are robust against the bias of greater male culling.
Since some springbok females breed in their first year, whereas males never breed before two years of age, conception date may have a greater influence on the probability of early breeding of females than males. The fitness benefits of producing female offspring are expected to decrease over the breeding season (see Daan et al. 1996 for a model), because later-born offspring will have less time to feed and build up condition prior to the next breeding season. Similar seasonal effects have been shown in kestrels (Falco tinnunculus; Dijkstra et al. 1990), great tits (Parus major; Visser & Verboven 1999) and crimson rosellas (Platycercus elegans; Krebs et al. 2002).
If daughters do benefit more than sons from maternal investment, the predictions made in the introduction concerning the effects of rainfall and population density should be reversed. Conditions of high nutritional stress, resulting from high population densities and/or low rainfall levels, should lead to a reduction in the production of daughters. Indeed, our results indicated a positive effect of rainfall on the production of daughters, thus supporting this hypothesis. However, there was no significant effect of population density. This might be because the population is operating below carrying capacity, which would be expected in a culled population (see also Flint et al. 1997).
With regard to the mechanism behind sex-ratio variation, previous investigators have been largely unable to identify whether sex-ratio skews are a consequence of females using sperm of different sex, or of differential probabilities of foetal reabsorption or mortality. Sex-specific foetal mortality is unlikely to be the mechanism behind the variation in offspring sex ratio between years in the springbok. Although we cannot categorically rule out the possibility that females classified as non-pregnant might have reabsorbed their embryo at a very early stage, the number of non-pregnant females in many years is simply too small to affect qualitatively the results of the population-level analysis. The results of a recent meta-analysis suggested that sex-ratio adjustment in mammals was most likely to occur at or near implantation (Cameron 2004), and our results would seem to confirm this: the biases in springbok sex production are likely to be the result of mothers preferentially utilizing X- or Y-carrying sperm, or of differential implantation. Both mechanisms provide a low-cost way to influence offspring sex (Krackow 1995).
We are indebted to De Beers Consolidated Mines (Ecology Division) for permission to work on their farm, and for logistic support, and to Tim Clutton-Brock, Tim Coulson, Nick Davies, Tim Fawcett, Jim Reynolds, Andy Russell, Fritz Trillmich and three anonymous referees for comments on earlier drafts of the manuscript. This work was funded by the Marie Curie programme of the EU, a Royal Society Research Fellowship and Churchill College and Girton College Junior Research Fellowships.
Acknowledgments
We are indebted to De Beers Consolidated Mines (Ecology Division) for permission to work on their farm, and for logistic support, and to Tim Clutton-Brock, Tim Coulson, Nick Davies, Tim Fawcett, Jim Reynolds, Andy Russell, Fritz Trillmich and three anonymous referees for comments on earlier drafts of the manuscript. This work was funded by the Marie Curie programme of the EU, a Royal Society Research Fellowship and Churchill College and Girton College Junior Research Fellowships.
References
- Anderson A.E., Bowden D.C., Medin D.E. Indexing the annual fat cycle in a mule deer population. J. Wildl. Mngmt. 1990;54:550–556. [Google Scholar]
- Bigalke R.C. Observations on springbok populations. Zool. Afr. 1970;5:59–70. [Google Scholar]
- Bigalke R.C. Observations on the behaviour and feeding habits of the springbok, Antidorcas marsupialis. Zool. Afr. 1972;7:333–359. [Google Scholar]
- Cameron E.Z. Facultative adjustment of mammalian sex ratios in support of the Trivers–Willard hypothesis: evidence for a mechanism. Proc. R. Soc. B. 2004;271:1723–1728. doi: 10.1098/rspb.2004.2773. doi:10.1098/rspb.2004.2773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassinello J., Gomendio M. Adaptive variation in litter size and sex ratio at birth in a sexually dimorphic ungulate. Proc. R. Soc. B. 1996;263:1461–1466. [Google Scholar]
- Charnov E. The theory of sex allocation. Princeton University Press; Princeton: 1982. [PubMed] [Google Scholar]
- Clutton-Brock T.H., Iason G.R. Sex ratio variation in mammals. Q. Rev. Biol. 1986;61:339–373. doi: 10.1086/415033. [DOI] [PubMed] [Google Scholar]
- Clutton-Brock T.H., Albon S.D., Guinness F.E. Parental investment in male and female offspring in polygynous mammals. Nature. 1981;289:487–489. [Google Scholar]
- Clutton-Brock T.H., Albon S.D., Guinness F.E. Maternal dominance, breeding success and birth sex ratios in red deer. Nature. 1984;308:358–360. [Google Scholar]
- Daan S., Dijkstra C., Weissing F.J. An evolutionary explanation for seasonal trends in avian sex ratios. Behav. Ecol. 1996;7:426–430. [Google Scholar]
- Dijkstra C., Daan S., Buker J.B. Adaptive seasonal variation in the sex ratio of kestrel broods. Funct. Ecol. 1990;4:143–147. [Google Scholar]
- Estes R.D. The behaviour guide to African mammals. University of California Press; Berkeley: 1991. [Google Scholar]
- Fisher R.A. The genetical theory of natural selection. Clarendon Press; Oxford: 1930. [Google Scholar]
- Flint A.P.F., Albon S.D., Jafar S.I. Blastocyst development and conceptus sex selection in red deer Cervus elaphus: studies of a free-living population on the Isle of Rum. Gen. Comp. Endocrinol. 1997;106:374–383. doi: 10.1006/gcen.1997.6879. [DOI] [PubMed] [Google Scholar]
- Hamilton W.D. Extraordinary sex ratios. Science. 1967;156:477–488. doi: 10.1126/science.156.3774.477. [DOI] [PubMed] [Google Scholar]
- Hewison A.J.M., Gaillard J.M. Birth-sex ratios and local resource competition in roe deer, Capreolus capreolus. Behav. Ecol. 1996;7:461–464. [Google Scholar]
- Hewison A.J.M., Gaillard J.M. Successful sons or advantaged daughters? The Trivers–Willard model and sex-biased maternal investment in ungulates. Trends Ecol. Evol. 1999;14:229–234. doi: 10.1016/s0169-5347(99)01592-x. [DOI] [PubMed] [Google Scholar]
- James W.H. Continuing confusion. Nature. 1993;365:8. doi: 10.1038/365008a0. [DOI] [PubMed] [Google Scholar]
- Kingdon J. East African mammals: an atlas of evolution in Africa. Academic Press; London: 1982. [Google Scholar]
- Krackow S. Potential mechanisms for sex ratio adjustment in mammals and birds. Biol. Rev. 1995;70:225–241. doi: 10.1111/j.1469-185x.1995.tb01066.x. [DOI] [PubMed] [Google Scholar]
- Krackow S. Why parental sex ratio manipulation is rare in higher vertebrates. Ethology. 2002;108:1041–1056. [Google Scholar]
- Krebs E.A., Green D.J., Double M.C., Griffiths R. Laying date and laying sequence influence the sex ratio of crimson rosella broods. Behav. Ecol. Sociobiol. 2002;51:447–454. [Google Scholar]
- Kruuk L.E.B., Clutton-Brock T.H., Albon S.D., Pemberton J.M., Guinness F.E. Population density affects sex ratio variation in red deer. Nature. 1999;399:459–461. doi: 10.1038/20917. [DOI] [PubMed] [Google Scholar]
- Leimar O. Life-history analysis of the Trivers–Willard sex-ratio problem. Behav. Ecol. 1996;7:316–325. [Google Scholar]
- Lindström J. Early development and fitness in birds and mammals. Trends Ecol. Evol. 1999;14:343–348. doi: 10.1016/s0169-5347(99)01639-0. [DOI] [PubMed] [Google Scholar]
- Lloyd P.H., Rasa O.A.E. Status, reproductive success and fitness in Cape mountain zebra (Equus zebra zebra) Behav. Ecol. Sociobiol. 1989;25:411–420. [Google Scholar]
- Mendl M., Zanella A.J., Broom D.M., Whittemore C.T. Maternal social status and birth sex ratio in domestic pigs: an analysis of mechanisms. Anim. Behav. 1995;50:1361–1370. [Google Scholar]
- Mysterud A., Yoccoz N.G., Stenseth N.C., Langvatn R. Relationships between sex ratio, climate and density in red deer: the importance of spatial scale. J. Anim. Ecol. 2000;69:959–974. [Google Scholar]
- Post E., Forchhammer M.C., Stenseth N.C., Langvatn R. Extrinsic modification of vertebrate sex ratios by climatic change. Am. Nat. 1999;154:194–204. doi: 10.1086/303224. [DOI] [PubMed] [Google Scholar]
- Rautenbach I.L. Ageing criteria in the springbok, Antidorcas marsupialis (Zimmermann 1780) (Artiodyctyla: Bovidae) Ann. Trans. Mus. 1971;27:83–133. [Google Scholar]
- Rice W.R. Analyzing tables of statistical tests. Evolution. 1989;43:223–225. doi: 10.1111/j.1558-5646.1989.tb04220.x. [DOI] [PubMed] [Google Scholar]
- Silk J.B. Local resource competition and facultative adjustment of sex ratios in relation to competitive abilities. Am. Nat. 1983;121:56–66. [Google Scholar]
- Skogland T. Sex ratio variation in relation to maternal condition and parental investment in wild reindeer Rangifer t. tarandus. Oikos. 1986;46:417–419. [Google Scholar]
- Sutherland W.J. From individual behaviour to population ecology. Oxford University Press; Oxford: 1996. [Google Scholar]
- Trivers R.L., Willard D.E. Natural selection of parental ability to vary the sex ratio of offspring. Science. 1973;179:90–92. doi: 10.1126/science.179.4068.90. [DOI] [PubMed] [Google Scholar]
- Visser M.E., Verboven N. Long-term fitness effects of fledging date in great tits. Oikos. 1999;85:445–450. [Google Scholar]
- West S.A., Sheldon B.C. Constraints in the evolution of sex ratio adjustment. Science. 2002;295:1685–1688. doi: 10.1126/science.1069043. [DOI] [PubMed] [Google Scholar]