Abstract
Novel observations collected from video, acoustic and conductivity sensors showed that Antarctic fur seals consistently exhale during the last 50–85% of ascent from all dives (10–160 m, n>8000 dives from 50 seals). The depth of initial bubble emission was best predicted by maximum dive depth, suggesting an underlying physical mechanism. Bubble sound intensity recorded from one seal followed predictions of a simple model based on venting expanding lung air with decreasing pressure. Comparison of air release between dives, together with lack of variation in intensity of thrusting movement during initial descent regardless of ultimate dive depth, suggested that inhaled diving lung volume was constant for all dives. The thrusting intensity in the final phase of ascent was greater for dives in which ascent exhalation began at a greater depth, suggesting an energetic cost to this behaviour, probably as a result of loss of buoyancy from reduced lung volume. These results suggest that fur seals descend with full lung air stores, and thus face the physiological consequences of pressure at depth. We suggest that these regular and predictable ascent exhalations could function to reduce the potential for a precipitous drop in blood oxygen that would result in shallow-water blackout.
Keywords: marine mammal, otariid, diving, physiology, Antarctic fur seal, shallow-water blackout
1. Introduction
The diving physiology of marine vertebrates is governed by the dual constraints of maintaining body function in the absence of access to oxygen and avoiding physiological trauma caused by rapid changes in pressure (Kooyman 1989; Butler & Jones 1997). Most research on free-diving physiology has been performed on true seals (the phocids), whereas less is known about fur seals and sea lions (the otariids). The relatively large differences in oxygen stores between the two orders suggest they are likely to use different physiological strategies (Kooyman & Ponganis 1998). Approximately 30–35% of oxygen stores are sequestered in muscle in both orders, but only 5% of oxygen is stored in the lung of phocids compared with 60–65% in the blood (Kooyman 1985). Because there is little benefit to be gained from lung oxygen during dives, phocids tend to exhale before diving (Scholander 1940; Kooyman et al. 1970). By contrast, measurements to date suggest that a much larger proportion (19%) of oxygen is stored in the lung of otariids compared with 47% in the blood, and these seals dive with inflated lungs (Kooyman 1973, 1985). This sixfold difference in the proportion of oxygen in the lungs compared with the blood in the two orders (1 : 2.5 in otariids compared with 1 : 12 in phocids), might lead to substantial differences in diving strategy.
All marine mammals have strengthened airways which cause a graded compression of the lung with increasing depth, with air moving from the compliant alveoli into the rigid, non-exchanging upper airways (Kooyman et al. 1970; Denison & Kooyman 1973). Exhalation before diving therefore helps to promote alveolar collapse at relatively shallow depths (of 30–50 m) for phocids (Kooyman et al. 1972; Falke et al. 1985). This prevents gas exchange, and is thought to help avoid decompression sickness (‘the bends’), which might otherwise be a threat to these deep divers (Scholander 1940). Alveolar collapse does not occur until much greater depths in otariids, based on measurement of blood gases in the California sea lion (Zalophus californianus), in which 100% cessation of air exchange was estimated to occur at 160 m (Kooyman & Sinnett 1982). A depth of alveolar collapse of 180 m also corresponds to expectations based on full inhalation for the anatomy of a 40 kg grey seal (Halichoerus grypus) with 80 cm3 in bronchies, trachea and nose cavities, versus 1500 cm3 full capacity (Scholander 1940). Alveolar collapse at these depths would allow otariids to exploit their lung oxygen store while diving, as increased partial pressures increase gas absorption and partly counteract the pulmonary shunt caused by reduction in alveolar surface area (Scholander 1940). Exposure to lung gases at depth, however, would increase the risk of physiological trauma during ascent because of decompression sickness or shallow-water blackout. Decompression sickness is caused when elevated levels of dissolved nitrogen in the blood and tissue expand during ascent, causing bubbles and the formation of emboli (Kooyman 1989). Shallow-water blackout is caused by depletion of blood oxygen often associated with the declining partial pressure of lung oxygen during ascent. Consequently, the air in the lung no longer contributes oxygen to the blood, or there may even be a reversal of the oxygen gradient at the blood–lung interface, causing removal of oxygen from the blood (Craig 1961; Lanphier & Rahn 1963; Kooyman 1989).
The diving and foraging behaviour of lactating Antarctic fur seals (Arctocephalus gazella) has been well categorized by several previous studies (Croxall et al. 1985; Kooyman et al. 1986; Boyd & Croxall 1992; Boyd et al. 1995, 2002). However, recent observations from conductivity, temperature and depth (CTD) profilers carried by these fur seals showed previously unrecorded and highly anomalous behaviour consistently during the last portion of the ascent of all dives (Hooker & Boyd 2003; figure 1a). The inductive conductivity sensor used to collect these data measured the decay of an electromagnetic (EM) field. Discrepancies such as those recorded would therefore most likely be caused by the intrusion of material into the small field around the sensor (Hooker & Boyd 2003). The consistency with which these anomalies were observed during the later portion of all ascents, together with the lack of these anomalies during descent and at the bottom of dives, suggested the presence of a previously unrecorded behaviour associated with the ascent phase of dives in this species.
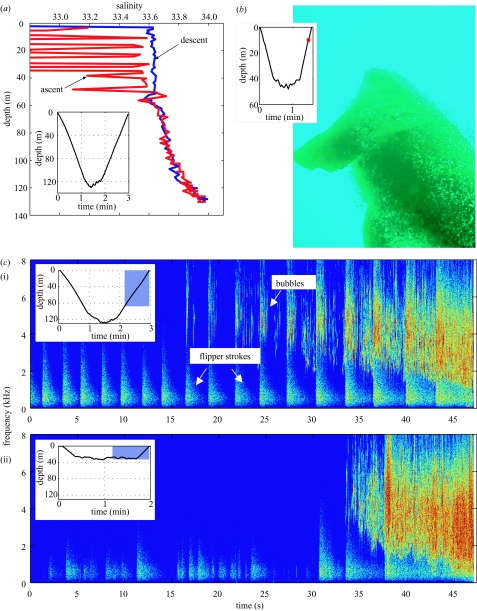
Figure 1.
Ascent exhalations recorded by three data sources. (a) Salinity record from a dive to 125 m, showing anomalies apparent in the results during the later portion of ascent. (b) Digital photograph taken from a neck-mounted camera showing another ascending seal. (c) Spectrograms showing the bubble sounds heard during ascent from a deep dive to 125 m (i), and a shallow dive to 35 m (ii). Insets show time and depth profiles for each dive from which data are shown, either for the whole dive (a), with position of record shown by dot (b), or section of dive highlighted from which spectrogram is plotted (c).
Images recorded by video systems attached to these seals showed the presence of bubbles during several dives (Hooker et al. 2002), suggesting that these may have been responsible for the discrepancies recorded. Among other marine mammal species, the descent and ascent phases of dives have been found to differ in thrusting patterns depending on buoyancy (Williams et al. 2000). Often, negatively buoyant animals will show a gliding phase during descent and an active swimming phase during ascent (e.g. Crocker et al. 1997; Biuw et al. 2003). Such an active swimming phase, with limbs or movement of the body and fur around the conductivity sensor was also a possible cause of the interference observed.
Here we show that air bubbles exhaled by the seals during ascent were indeed responsible for the conductivity anomalies previously recorded. Such ascent exhalation has not, to our knowledge, been described in any marine mammal so far. The present study explored how this behaviour related to the diving behaviour and the thrusting effort (buoyancy) of fur seals during ascent and descent, in order to understand its function.
2. Methods
This study was conducted on female Antarctic fur seals during the breeding season at Bird Island, South Georgia (54° S, 38° W). Instruments were deployed on lactating females for a single foraging trip (generally 5–7 days). Three sets of instrumentation were deployed during austral summer seasons in 2000, 2001 and 2002.
Conductivity and temperature sensors (model ACT-HR, Alec Electronics, Japan) were deployed alongside time–depth recorders (Mk7 or Mk9, Wildlife Computers, USA) to record salinity, temperature and depth data at 1 s intervals (n=29 seals, 2000–2001). The conductivity sensor generated and then measured the decay of an EM field (ca. 3 cm around the sensor), and this, together with temperature, was used to calculate water salinity. Any disturbance to this EM field from intrusion of material caused observable discrepancies in the recorded data (Hooker & Boyd 2003; figure 1a).
Custom-built underwater cameras linked to time–depth recorders were used to record video and still images (Wild Insight Ltd, UK). As part of a study of seal foraging, these were placed on the neck region of the seal facing forwards to take still images at 3 s intervals and 640×480 pixel resolution (n=16 seals, 2000–2002). The camera was activated below a user-defined depth (10–20 m) and used a near-infrared light source to provide illumination (see Hooker et al. (2002) for details). A modified version of this, with a blue light source (500 nm wavelength) was used to provide additional illumination for cameras facing backwards, which were deployed to investigate the cause of the anomalous CTD data. These cameras took movie images (5–12 frames per second at 160×120 pixel resolution, n=3 seals, 2002). A forward facing camera was also modified to record sound data (incorporating a hydrophone to the sound channel of the camera, n=1 seal, 2002).
A custom-built multi-sensor acoustic tag (D-tag, Woods Hole Oceanographic Institution, USA) was also used to record acoustic data at 16 kHz and sampled depth, acceleration and orientation at 23.5 Hz (see Johnson & Tyack (2003) for further details). These high-resolution data were recorded over a period of 6 h (n=1 seal, 2002), once triggered by diving deeper than 20 m.
Video and photographic observations demonstrated that ascent conductivity anomalies (figure 1a) were caused by the presence of bubbles, originating at the snout and coating the body of the seal during ascent (figure 1b). The quantity of visible bubbles clearly increased throughout the ascent (see electronic Appendix). Increasingly loud bubble sounds were also clearly audible in the acoustic recordings (figure 1c and electronic Appendix).
We derived a simple model based on Boyle’s law and the relationship between pressure and depth (d), to describe the excess air (ΔV) which would be released if a compressed volume (Vc) were maintained during ascent from depth, d+Δd, to depth, d (see Appendix A):
| (2.1) |
This model describes the volume of bubbles (ΔV) produced per increment of depth (Δd) during an ascent. Because ascent rates were found to vary little between seals and between dives, this model effectively predicts the rate of air release. We can further estimate Vc by the slope of this metric (ΔV/Δd) versus 1/(d+10). In addition, if surface (inhaled) lung volume is consistent across dives, Vc should be a linear function of 1/PB, where PB is the pressure at the depth at which bubbles were first heard (see Appendix A for details).
Inspection of the sound recording showed clearly that bubble sounds began at a low amplitude and increased as the animal moved into shallower depths (figure 1c and electronic Appendix). Close inspection of the waveform during the bubble-release period showed that the acoustic record was dominated by multiple decaying acoustic oscillations, created by the expansion and contraction of multiple bubbles at their resonant frequency (Minnaert 1933).
The acoustic intensity of a set of oscillating bubbles is determined by the number of bubbles present, and other factors such as their size, the amplitude of the oscillations, and their distance to the recording hydrophone (Leighton & Walton 1987). We used the increase in intensity (pressure amplitude squared) of the acoustic signal from 1–16 kHz (for which the hydrophone has a flat response) to create a relative index of bubble air released (ΔV) over the course of the ascents of this seal. This measure provides a rough, relative index of the change in air volume released at various points in an ascent. We have assumed that changes in bubble sizes, oscillation amplitude and range distribution from the hydrophone were minor relative to the change in the number of bubbles (see electronic Appendix). In fact, the central frequency of bubbles decreased during ascent (figure 1c), suggesting larger bubbles were formed later in ascent. However, because this central frequency change was consistent at similar depths across dives, our measure should be unbiased in comparing air release between dives. To minimize differences in background noise for different time periods, we calculated the average intensity between subsequent flipper strokes, and subtracted background noise intensity over one flipper-stroke interval just before the start of bubbles (figure 1c).
We used the acceleration sensors on the D-tag to estimate the intensity of thrusting movements made by the seal during descent and ascent in the upper 1–13 m of the water column. This was further split into thrusting intensity observed between 1–4 m, 4–7 m, 7–10 m, and 10–13 m. Fur seals generate forward thrust by stroking their large pectoral flippers vertically. Thus, thrusting movements by the seal are recorded as transient acceleration signals in the z-axis (dorsal–ventral) of the accelerometer in the D-tag. The z-axis accelerometer signal was first filtered at 0.1 Hz to remove slow changes caused by variation in animal orientation (e.g. Sato et al. 2003), and then the mean variance of the filtered accelerometer signal was calculated over each measurement period (see Miller et al. 2004). The mean variance was then divided by the distance travelled by the seal as δdepth/sin(pitch), to obtain a metric of thrusting intensity per metre travelled over each depth bin. One shallow dive for which bubbles began shallower than 13 m depth was excluded from analysis.
Occasional releases of bubbles could be heard or seen at the bottom of 15 dives in addition to the continuous exhalation during ascent. Because these ascent exhalations would no longer reflect inhaled lung volume, they were not included in analyses. Acoustic intensity values from one dive during which a loud external noise source was heard were also not included. Statistical analyses were conducted using Spss or Systat.
3. Results
Conductivity sensors were deployed on 29 fur seals, and recorded a total of 8029 dives deeper than 20 m depth (2–1022 dives per individual). Of these dives, over 99% showed anomalies in recorded salinity data (defined as a deviation of greater than 0.1 during ascent compared with descent). Results from all dives were consistent in form, such that after initial deviation, the magnitude of deviation increased until the seal reached the surface (figure 1a).
Rear-facing movies (122 dives from 3 seals) showed the appearance of bubbles during the final ascent phase of all dives (see electronic Appendix). These originated from a position anterior to the camera mounted between the shoulder blades, i.e. from the seal’s head. Bubbles were produced continuously during the last three-quarters of the ascent, increasing in frequency during this time. Still images of other passing seals showed that bubbles emitted at the head stream back and coat the body of the seal (figure 1b). Ascent rates of these seals were ca. 1.5−2 m s−1 so that an ascending animal would swim upwards faster than its exhaled bubbles. The camera with hydrophone (97 dives from one seal) and the D-tag hydrophone data (67 dives from one seal) provided a clearly audible record of bubble production (figure 1c and electronic Appendix). The continuous 6 h acoustic record from the D-tag demonstrated that although bubble emission could occasionally be heard during shallow dives before the ascent (15 of 67 dives, all of which were shallower than 30 m), these were isolated releases of a few bubbles only, rather than the continuous exhalation observed during the ascent.
The production of bubbles corresponded to the orientation of the seal towards the water surface, such that continuous bubble production was interrupted if the seal reversed orientation (head down towards the sea floor), as was observed during three ascents. This relationship between bubble sounds, pressure increase and orientation, together with video data, supports the conclusion that these bubbles originated from expanding air within the seal.
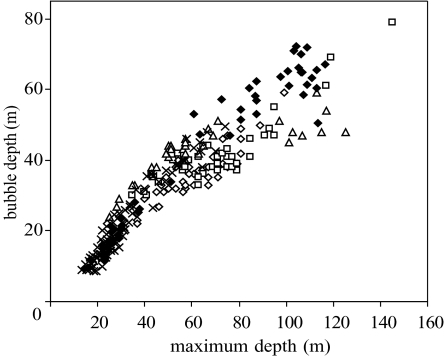
The depth of the seal at the first expiration of bubbles initiating the continuous ascent exhalation could be determined from all data sources (CTD, video and acoustic). Of the dive variables (maximum depth, duration, ascent rate and descent rate), the depth at which bubbles were first produced was best predicted by maximum depth of dive (figure 2; generalized linear mixed model: F1,8191=2656, p<0.001, 34 individuals).
Figure 2.
The depth of bubble production versus depth of dive for data recorded by camera and acoustic tags. Open diamonds, camera 1; squares, camera 2; triangles, camera 3; crosses, acoustic camera; closed diamonds, D-tag.
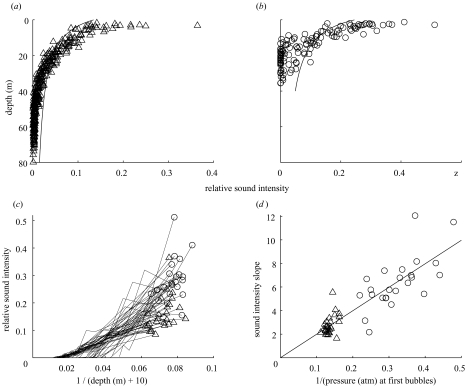
The intensity of bubble sounds followed general predictions of release of expanding gas above a fixed volume (equation (2.1); figure 3). However, fewer bubbles than expected were observed at first release of bubbles, suggesting partial reinflation of the lungs, and more air than expected near the surface, suggesting possible active exhalation. Comparing across dives, estimated Vc (the compressed volume maintained during ascent) was strongly linearly correlated with 1/PB, where PB is the pressure at which the first bubbles were released (r2=0.75, F1,49=149.5, p<0.001; figure 3). This supports our hypothesis that bubbles represent the release of air expanding above a fairly fixed (compressed) lung volume during ascent, and in addition, that surface (inhaled) lung volume is constant between dives.
Figure 3.
Intensity of bubble sounds (acoustic pressure squared) recorded by D-tag versus depth for (a) deep (more than 69 m) and (b) shallow (less than 44 m) dives. Note the strong pattern of increased bubble sound intensity as the seal ascended into shallower depths. The model described in equation (2.1) is shown (line) to fit observations at 20 m for deep dives and at 10 m for shallow dives. (c) The intensity of bubble sounds versus 1/(d+10). The slope of the linear regression for each dive was used to estimate Vc. (d) The slope of the increase in acoustic intensity, or estimate of Vc from (c), versus 1/PB, where PB is the pressure at which bubbles were first released. The strong linear relationship between these values is predicted from equation (2.1) if the seal inhaled equal amounts of air at the surface for all dives, and the rate of air release is determined by the maintenance of lung collapse (see text and Appendix A for details). trianges, deep dives; circles, shallow dives.
Acceleration sensors on the D-tag showed that flipper beating was maintained throughout both descent and ascent. The rate of flipper beating was rapid in the initial phase of descent (1.4 Hz), decreasing as the seal descended to depth (0.2 Hz at 100 m). A similar pattern was observed on ascent, with an initially high rate of flipper beating (0.6 Hz at 100 m), decreasing near the surface (0.3 Hz). No systematic change in orientation was apparent during descent or ascent.
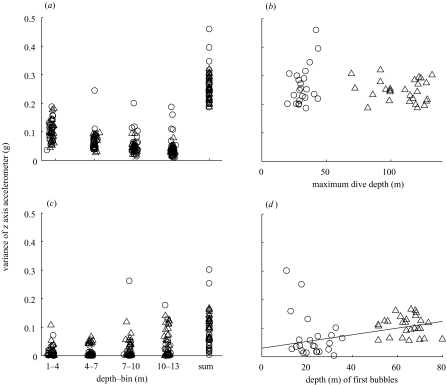
Thrusting intensity during descent and ascent was investigated in the upper 13 m of the water column (figure 4). Thrusting intensity was approximately three times greater for descent than for ascent through these depths (paired t49=16.4, p<0.0001), demonstrating that fur seals are positively buoyant near the water surface. Measured for each of four depth bins (1–4 m, 4–7 m, 7–10 m and 10–13 m), thrusting was strongest near the surface and decreased with depth during descent, and the reverse was true during ascent (figure 4a, c). This shows that much of the positive buoyancy of fur seals is a result of air carried to depth, which compresses rapidly with increasing pressure at depth. There was no correlation between ascent and descent thrusting intensity (Pearson r=0.06, p=0.67, n=50 dives).
Figure 4.
Intensity of thrusting movement, measured as variance in the z-axis accelerometer signal of the D-tag produced by the seal during (a) descent and (c) ascent in the top 13 m below the surface, calculated as the seal travelled through four depth bins (1–4, 4–7, 7–10 and 10–13 m) and also summed over the total depth range (1–13 m). The variance measure was normalized by the distance travelled in each depth bin. The overall variance in the z-axis of the accelerometer was greater for descent than ascent across this depth range. Note the decreasing thrusting intensity as the seal descends from the surface, and the converse for ascent. (b) Total variance in z-axis accelerometer (1–13 m) for descent is plotted against the maximum dive depth. Equal thrusting intensity regardless of dive depth suggests equal buoyancy (i.e. lung volume) for all dives. (d) Total variance in z-axis accelerometer (13–1 m) for ascent is plotted against the depth at which bubbles were first released. This metric of thrusting intensity was greater during ascents from dives in which bubbles were first produced at greater depths, reflecting the energetic cost of releasing air during ascent. Triangles, deep dives; circles, shallow dives.
The same thrusting intensity was used during all descents, regardless of the maximum depth obtained by the seal (F1,48=0.87, p=0.36; figure 4b). This lack of detectable variation in thrusting intensity suggests equal buoyancy (and thus equal inhaled surface lung volumes) for dives to varying depths. By contrast, thrusting during ascents in the upper 13 m was strongly affected by maximum dive depth obtained (F1,48=11.16, p=0.0016). Because the depth at which air is first released by the seal as bubbles was strongly related to the maximum dive depth, we tested this relationship against the depth of initial bubble release. There was a significant correlation between ascent thrusting energy and the depth at which bubbles were first heard (Pearson r=0.42, p=0.0022; figure 4d), supporting a probable energetic cost to ascent exhalations as a result of loss of positive buoyancy.
4. Discussion
Continuous exhalation of air during ascents from dives (figure 1, and electronic Appendix) has not, to our knowledge, previously been observed for any marine diver. However, only a few other species have been studied using instrumentation that would have recorded this behaviour if it were present. Although phocid seals and cetaceans have been recorded using either acoustic tags or rear-facing video cameras (Burgess et al. 1998; Williams et al. 2000; Miller et al. 2004), no other otariid species have, to our knowledge, been recorded with equipment that would have detected these bubbles.
The relationship between depth at which bubbles were first produced and maximum dive depth (figure 2), the increase in volume released as the seals ascend (figure 3a, b; electronic Appendix), and the higher rate of increase in acoustic intensity when bubbles are first released at lower pressures (figure 3c), all suggest that exhalation is associated with maintenance of lung collapse throughout the ascent phase of the dive. At shallow dive depths bubbles may be released before ascent, whereas during deep dives there is always a period of ascent before bubble release (figure 2). This suggests that there is some form of pressure threshold before which venting begins, possibly related to the need for higher buccal air pressure than ambient water pressure prior to opening the mouth. The fit of our proxy for bubble volume to the model was relatively good but not perfect (figure 3a, b). This is not surprising because our proxy provides only a rough index of air volume. So although less air appears to be released than predicted by our model during the early stages of bubble release and more air at the later stages (figure 3a, b), we suggest caution in interpreting this as retention of a portion of the expanding air initially and active release of remaining air near the surface. However, the proxy provided an unbiased method of comparing between dives, for which there was a strong relationship between the pressure at which bubbles were first produced and the estimated lung volume, Vc, maintained during ascent (figure 3d), suggesting that exhalation of expanding air is associated with maintenance of a relatively fixed lung volume. Furthermore, this strong linear fit (r2=0.75) suggests that seals carry a relatively constant volume of air from the surface for all dives.
That Antarctic fur seals inhale a fixed volume before each dive was independently supported by our observation that thrusting intensity during descent through the upper 13 m was not related to ultimate dive depth (figure 4b). The decrease in descent thrusting intensity with depth in the top 13 m (and the converse for ascent thrusting) implicates compressible gases as a substantial part of the source of positive buoyancy. These results provide support for previous suggestions that fur seals inhale before diving (Kooyman 1973, 1985), and suggest that they may take the maximum lung volume on each dive. In contrast to this, recent work on penguins, which are also breath-hold divers, has suggested that some penguins may regulate their air volume according to dive depth to optimize costs and benefits of buoyancy (Sato et al. 2002). Such a predictive strategy, although beneficial in terms of energy conservation, would allow less plasticity within a dive in terms of response to prey variability.
By diving after inhaling, fur seals face a buoyancy cost during the descent phase, which they do not recover on the ascent because of their exhalation (figure 4). Such inhalation would only benefit the seal if there were continued gas exchange during the dive (Kooyman et al. 1999). Although the pulmonary shunt increases with depth (for California sea lions at 60 m depth the shunt was ca. 50% of blood flow (Kooyman & Sinnett 1982)), the partial pressure of lung gases increases with depth (a sevenfold increase at 60 m), and therefore to some extent offsets this shunt. This would enable gas exchange throughout most fur seal dives (more than 90% of dives are to less than 100 m depth (Boyd & Croxall 1992)). However, in addition to increased oxygen uptake, this behaviour would also increase blood nitrogen uptake (although the diffusion rate of nitrogen is much lower than that of oxygen or carbon dioxide), implying that fur seals should face an increased risk of decompression sickness. However, their short-duration, shallow dives may result in nitrogen levels that are below threshold for bubble formation (Kooyman et al. 1973; Ponganis et al. 1999).
Having suffered its buoyancy cost during descent, it is not clear why fur seals then invariably expel this air during the ascent phase of dives, particularly when such behaviour is energetically costly (figure 4d). Bubbles were not associated with production of any vocalizations detected by hydrophone. Humpback whales (Megaptera novaeangliae) emit bubbles during dive ascents to concentrate prey swarms (Sharpe & Dill 1997), but no prey were observed associated with the fur seal bubbles. Camera observations demonstrated that prey were generally located at the bottom of fur seals’ dives (Hooker et al. 2002), whereas bubbles were emitted later during ascent. Based on the regularity of occurrence irrespective of dive depth, and the strong relationship between the depth at which bubbles were first emitted and the maximum pressure reached during a dive (figure 2), the most likely function for the observed exhalation appears to be physiological.
One potential explanation for this behaviour is that the lungs during ascent no longer serve as a useful oxygen store (Kooyman 1989), but may in fact present an oxygen sink. Continued gas exchange at depth means that otariid seals might be at risk from shallow-water blackout, the depletion of blood oxygen, which can be triggered as falling oxygen partial pressure in the lung during ascent causes removal of oxygen from the blood (Kooyman 1989). Blood oxygen concentrations at the end of fur seal dives have not been measured. However, our accelerometer results showed a high level of activity while foraging, agreeing with previous data suggesting relatively high metabolic foraging demands (Boyd et al. 1999), which would probably lead to low blood oxygen levels at the end of a dive. In addition, Ponganis et al. (1997) were unable to train California sea lions, a similar-sized otariid, to dive or swim submerged for longer than 3 min, suggesting that this is their aerobic limit to dive duration. By exhaling lung air during ascent, these seals would essentially extend the pulmonary shunt present at depth, maintaining collapse of the alveoli into shallower waters near the surface, preventing diffusion between lungs and blood during ascent. Thus any potential reversal of the oxygen gradient would be avoided, and oxygen would be locked in the blood until reaching the surface. This mechanism has previously been observed during measurement of blood nitrogen levels in a forced dive of a harbour seal (Phoca vitulina). The seal exhaled during decompression (the equivalent of ascent) from a simulated dive to ca. 130 m in a hyperbaric chamber, which resulted in delaying the removal of nitrogen from its blood until it surfaced and breathed (Kooyman et al. 1972). Fur seals may be doing the same to maintain elevated blood oxygen levels. Furthermore, this mechanism may be conservative and so would not necessarily require dangerously low oxygen levels on every dive. Among other seals there is evidence of relatively high tolerance to hypoxia (Elsner et al. 1970; Kerem & Elsner 1973). However, even if shallow-water blackout was a threat only occasionally to these divers, this could be fatal, and could provide the selective pressure for ascent exhalations.
Such a mechanism should also be important to other breath-hold divers. Another eared seal, the California sea lion, has been studied extensively in captive experimental situations but there is only one observation of such potential exhalation. During captive work using animals trained to dive and then exhale into a funnel after ascent, it was found that for dives deeper than 100 m, animals did not appear to have air left for such exhalation (Ridgway 1972), although it was not apparent at what stage of the dive air was lost. Many cetaceans (also breath-hold divers (Ridgway et al. 1969)) have a series of sphincter muscles in the terminal airway before the alveoli, the function of which is unknown (Kooyman 1973, 1985). These could be used to retain air in the alveoli during descent and/or to prevent their re-inflation during ascent. Bubbles have been observed from another group of breath-hold divers: the penguins (Kooyman 1975). Although most bubbles appear to originate from the feathers, a photograph of a surfacing Adélie penguin (Pygoscelis adeliae) shows a single bubble next to the neck which is ‘probably expirate’ (Kooyman 1975, p. 133).
It is clear that additional observation of other species will be required before the generality of this mechanism can be ascertained. However, these observations of costly ascent exhalations in diving Antarctic fur seals suggest a behavioural adaptation linked with breath-hold diving and the effects of pressure. We hypothesize that this counter-intuitive behaviour may function to mitigate the threat of shallow-water blackout, previously described as ‘one of the most puzzling and unsolved mysteries of deep diving’ (Kooyman 1989, p. 52). However, our speculation is largely based on inference as many studies of these aspects of diving physiology have been conducted for only one or two species. Alternative functional explanations are possible, but the regularity and predictability of this behaviour suggests that, whatever its function, venting air during the ascent phase of dives is a necessity for fur seals.
Acknowledgments
The fieldwork was assisted by staff at the British Antarctic Survey (BAS) Bird Island Field Station. Field methods were approved by BAS and conformed to British Home Office regulations for avoiding unnecessary suffering to the animals. S.K.H. received support from a Royal Society Dorothy Hodgkin fellowship; P.J.O.M. received support from a Royal Society USA fellowship. We are grateful to S. Smout for assistance with model derivation. This work has benefited from discussions and review from D. M. Denison, G. L. Kooyman, P. Ponganis, and colleagues at the Sea Mammal Research Unit.
Appendix A
At d (the depth of the seal below the surface in metres), pressure (in atm) is Pd=0.1(d+10), since pressure increases by 0.1 atm for every metre descent.
Let the seal ascend from (d+Δd) to d: at (d+Δd), the lung volume is Vc; at d, this air has expanded by ΔV, and to maintain lung volume Vc, the seal releases bubbles of total volume ΔV.
Boyle's law states that at constant temperature
or, in this case,
Now, Pd=0.1(d+10), and Pd+Δd=0.1(d+Δd+10).
Substituting these,
Therefore the incremental volume released at increments of depth is
Since the rate of ascent in these seals is constant, this represents the rate of air release.
For each dive, Vcis related to Vs, the surface (inhaled) lung volume, according to Boyle's Law, by the pressure at which bubbles are first heard, PB:
Thus, if the surface lung volume is constant between dives, then Vc should be a linear function of 1/PB.
References
- Biuw M., McConnell B.J., Bradshaw C.J.A., Burton H., Fedak M. Blubber and buoyancy: monitoring the body condition of free-ranging seals using simple dive char acteristics. J. Exp. Biol. 2003;206:3405–3423. doi: 10.1242/jeb.00583. [DOI] [PubMed] [Google Scholar]
- Boyd I.L., Croxall J.P. Diving behaviour of lactating Antarctic fur seals. Can. J. Zool. 1992;70:919–928. [Google Scholar]
- Boyd I.L., Reid K., Bevan R.M. Swimming speed and allocation of time during the dive cycle in Antarctic fur seals. Anim. Behav. 1995;50:769–784. [Google Scholar]
- Boyd I.L., Bevan R.M., Woakes A.J., Butler P.J. Heart rate and behavior of fur seals: implications for measurement of field energetics. Am. J. Physiol. 1999;276:H844–H857. doi: 10.1152/ajpheart.1999.276.3.H844. [DOI] [PubMed] [Google Scholar]
- Boyd I.L., Staniland I.J., Martin A.R. Distribution of foraging by female Antarctic fur seals. Mar. Ecol. Prog. Ser. 2002;242:285–294. [Google Scholar]
- Burgess W.C., Tyack P.L., Le Boeuf B.J., Costa D.P. A programmable acoustic recording tag and first results from free-ranging northern elephant seals. Deep Sea Res. II. 1998;45:1327–1351. [Google Scholar]
- Butler P.J., Jones D.R. Physiology of diving of birds and mammals. Physiol. Rev. 1997;77:837–899. doi: 10.1152/physrev.1997.77.3.837. [DOI] [PubMed] [Google Scholar]
- Craig A.B.J. Causes of loss of consciousness during underwater swimming. J. Appl. Physiol. 1961;16:583–586. doi: 10.1152/jappl.1961.16.4.583. [DOI] [PubMed] [Google Scholar]
- Crocker D.E., Le Boeuf B.J., Costa D.P. Drift diving in female northern elephant seals: implications for food processing. Can. J. Zool. 1997;75:27–39. [Google Scholar]
- Croxall J.P., Everson I., Kooyman G.L., Ricketts C., Davis R.W. Fur seal diving behaviour in relation to vertical distribution of krill. J. Anim. Ecol. 1985;54:1–8. [Google Scholar]
- Denison D.M., Kooyman G.L. The structure and function of the small airways in pinniped and sea otter lungs. Resp. Physiol. 1973;17:1–10. doi: 10.1016/0034-5687(73)90105-9. [DOI] [PubMed] [Google Scholar]
- Elsner R., Shurley J.T., Hammond D.D., Brooks R.E. Cerebral tolerance to hypoxemia in asphyxiated Weddell seals. Resp. Physiol. 1970;9:287–297. doi: 10.1016/0034-5687(70)90077-0. [DOI] [PubMed] [Google Scholar]
- Falke K.J., Hill R.D., Qvist J., Schneider R.C., Guppy M., Liggins G.C., Hochachka P.W., Elliott R.E., Zapol W.M. Seal lungs collapse during free diving: evidence from arterial nitrogen tensions. Science. 1985;229:556–558. doi: 10.1126/science.4023700. [DOI] [PubMed] [Google Scholar]
- Hooker S.K., Boyd I.L. Salinity sensors on seals: use of marine predators to carry CTD dataloggers. Deep Sea Res. I. 2003;50:927–939. [Google Scholar]
- Hooker S.K., Boyd I.L., Jessopp M., Cox O., Blackwell J., Boveng P.L., Bengtson J.L. Monitoring the prey-field of marine predators: combining digital imaging with datalogging tags. Mar. Mamm. Sci. 2002;18:680–697. [Google Scholar]
- Johnson M., Tyack P.L. A digital acoustic recording tag for measuring the response of wild marine mammals to sound. IEEE J. Oceanic Engng. 2003;28:3–12. [Google Scholar]
- Kerem D., Elsner R. Cerebral tolerance to asphyxial hypoxia in the dog. Am. J. Physiol. 1973;225:593–600. doi: 10.1152/ajplegacy.1973.225.3.593. [DOI] [PubMed] [Google Scholar]
- Kooyman G.L. Respiratory adaptations in marine mammals. Am. Zool. 1973;13:457–468. [Google Scholar]
- Kooyman G.L. Behaviour and physiology of diving. In: Stonehouse B., editor. The biology of penguins. Macmillan; London: 1975. pp. 115–137. [Google Scholar]
- Kooyman G.L. Physiology without restraint in diving mammals. Mar. Mamm. Sci. 1985;1:166–178. [Google Scholar]
- Kooyman G.L. Diverse divers. Springer; Berlin: 1989. [Google Scholar]
- Kooyman G.L., Ponganis P.J. The physiological basis of diving to depth: birds and mammals. A. Rev. Physiol. 1998;60:19–32. doi: 10.1146/annurev.physiol.60.1.19. [DOI] [PubMed] [Google Scholar]
- Kooyman G.L., Sinnett E.E. Pulmonary shunts in harbor seals and sea lions during simulated dives to depth. Physiol. Zool. 1982;55:105–111. [Google Scholar]
- Kooyman G.L., Hammond D.D., Schroeder J.P. Bronchograms and tracheograms of seals under pressure. Science. 1970;169:82–84. doi: 10.1126/science.169.3940.82. [DOI] [PubMed] [Google Scholar]
- Kooyman G.L., Schroeder J.P., Denison D.M., Hammond D.D., Wright J.M., Bergman W.P. Blood N2 tensions of seals during simulated deep dives. Am. J. Physiol. 1972;223:1016–1020. doi: 10.1152/ajplegacy.1972.223.5.1016. [DOI] [PubMed] [Google Scholar]
- Kooyman G.L., Schroeder J.P., Greene D.G., Smith V.A. Gas exchange in penguins during simulated dives to 30 and 68 m. Am. J. Physiol. 1973;225:1467–1471. doi: 10.1152/ajplegacy.1973.225.6.1467. [DOI] [PubMed] [Google Scholar]
- Kooyman G.L., Davis R.W., Croxall J.P. Diving behaviour of Antarctic fur seals. In: Gentry R.L., Kooyman G.L., editors. Fur seals: maternal strategies on land and at sea. Princeton University Press; 1986. pp. 115–125. [Google Scholar]
- Kooyman G.L., Ponganis P.J., Howard R.S. Diving animals. In: Lundgren C.E.G., Miller J.N., editors. The lung at depth. Marcel Dekker; New York: 1999. pp. 587–620. [Google Scholar]
- Lanphier E.H., Rahn H. Alveolar gas exchange during breath-hold diving. J. Appl. Physiol. 1963;18:471–477. doi: 10.1152/jappl.1963.18.3.471. [DOI] [PubMed] [Google Scholar]
- Leighton T.G., Walton A.J. An experimental study of the sound emitted from gas bubbles in a liquid. Eur. J. Phys. 1987;8:98–104. [Google Scholar]
- Miller P.J.O., Johnson M.P., Tyack P.L., Terray E.A. Swimming gaits, passive drag and buoyancy of diving sperm whales (Physeter catadon) J. Exp. Biol. 2004;207:1953–1967. doi: 10.1242/jeb.00993. [DOI] [PubMed] [Google Scholar]
- Minnaert M. On musical bubbles and the sounds of running water. Phil. Mag. Lett. 1933;16:235–248. [Google Scholar]
- Ponganis P.J., Kooyman G.L., Winter L.M., Starke L.N. Heart rate and plasma lactate response during submerged swimming and trained diving in California sea lions, Zalophus californianus. J. Comp. Physiol. B. 1997;167:9–16. doi: 10.1007/s003600050042. [DOI] [PubMed] [Google Scholar]
- Ponganis P.J., Kooyman G.L., Van Dam R., LeMaho Y. Physiological responses of king penguins during simulated diving to 136 m depth. J. Exp. Biol. 1999;202:2819–2822. doi: 10.1242/jeb.202.20.2819. [DOI] [PubMed] [Google Scholar]
- Ridgway S.H. Homeostasis in the aquatic environment. In: Ridgway S.H., editor. Mammals of the sea: biology and medicine. Charles C. Thomas; Springfield, IL: 1972. pp. 590–747. [Google Scholar]
- Ridgway S.H., Scronce B.L., Kanwisher J. Respiration and deep diving in the bottlenose porpoise. Science. 1969;166:1651–1654. doi: 10.1126/science.166.3913.1651. [DOI] [PubMed] [Google Scholar]
- Sato K., Naito Y., Kato A., Niizuma Y., Watanuki Y., Charrassin J.B., Bost C.-A., Handrich Y., Le Maho Y. Buoyancy and maximal diving depth in penguins: do they control inhaling air volume? J. Exp. Biol. 2002;205:1189–1197. doi: 10.1242/jeb.205.9.1189. [DOI] [PubMed] [Google Scholar]
- Sato K., Mitani Y., Cameron M.F., Siniff D.B., Naito Y. Factors affecting stroking patterns and body angle in diving Weddell seals under natural conditions. J. Exp. Biol. 2003;206:1461–1470. doi: 10.1242/jeb.00265. [DOI] [PubMed] [Google Scholar]
- Scholander P.F. Experimental investigations on the respiratory function in diving mammals and birds. Hvalradets Skrifter. 1940;22:1–131. [Google Scholar]
- Sharpe F.A., Dill L.M. The behavior of Pacific herring schools in response to artificial humpback whale bubbles. Can. J. Zool. 1997;75:725–730. [Google Scholar]
- Williams T.M., Davis R.W., Fuiman L.A., Francis J., Le Boeuf B.J., Horning M., Calambokidis J., Croll D.A. Sink or swim: strategies for cost-efficient diving by marine mammals. Science. 2000;288:133–136. doi: 10.1126/science.288.5463.133. [DOI] [PubMed] [Google Scholar]