Abstract
In most animal species, males are predicted to compete for reproductive opportunities, while females are expected to choose between potential mates. However, when males’ rate of reproduction is constrained, or females vary widely in ‘quality’, male mate choice is also predicted to occur. Such conditions exist in the promiscuous mating system of feral Soay sheep on St Kilda, Scotland, where a highly synchronized mating season, intense sperm competition and limitations on sperm production constrain males’ potential reproductive rate, and females vary substantially in their ability to produce successful offspring. We show that, consistent with predictions, competitive rams focus their mating activity and siring success towards heavier females with higher inclusive fitness. To our knowledge, this is the first time that male mate choice has been identified and shown to lead to assortative patterns of parentage in a natural mammalian system, and occurs despite fierce male–male competition for mates. An additional consequence of assortative mating in this population is that lighter females experience a series of unstable consorts with less adept rams, and hence are mated by a greater number of males during their oestrus. We have thus also identified a novel male-driven mechanism that generates variation in female promiscuity, which suggests that the high levels of female promiscuity in this system are not part of an adaptive female tactic to intensify post-copulatory competition between males.
Keywords: sexual selection, sperm competition, mate guarding, optimal insemination period, horn size, body size
1. Introduction
In most animal species, females make a greater investment in each offspring than males, and are thus more limited in the number of offspring that they can produce (Trivers 1972). As a result, the operational sex ratio (the number of sexually mature males/receptive females) is commonly male biased, leading to competition among males for reproductive opportunities, and females that choose between potential mates to gain resources or genetic benefits (Trivers 1972; Emlen & Oring 1977; Clutton-Brock & Vincent 1991; Clutton-Brock & Parker 1992). However, when male potential reproductive rate (PRR: the maximum rate at which independent offspring can be produced) is restricted, or where there is substantial variation in the ‘quality’ of females, male mate choice is also predicted to occur (Clutton-Brock & Vincent 1991; Clutton-Brock & Parker 1992; Owens & Thompson 1994).
There are a several factors that can impact on males’ PRR. A monogamous mating system and high paternal investment in offspring will both heavily constrain males’ PRR, and hence favour the evolution of male mate choice (Clutton-Brock & Vincent 1991; Clutton-Brock & Parker 1992). In seasonally breeding polygynous species, high synchronicity of females’ oestrus can favour male choice of mates because of constraints imposed by search and processing costs (Emlen & Oring 1977). Costs of searching may also have a negative impact on the probability of mate choice evolving, however, as when available mates are dispersed, or are few in number, the costs of sampling receptive females may counteract any fitness gains from choosing to mate with only those of highest quality (Parker 1983; Real 1990). The degree of male ‘choosiness’ is therefore predicted to vary with the relative density of receptive females.
When females mate with multiple males in a given oestrous, male PRR can be influenced by factors associated with sperm competition: the competition between the sperm of two or more males to fertilize a given female’s ova (Parker 1970, 1998). Sperm competition may, for example, promote the evolution of lengthy periods of mate guarding, or the frequent production of large and costly ejaculates, both of which can constrain males’ ability to produce large numbers of offspring (Dewsbury 1982; Møller & Birkhead 1989). The occurrence of sperm competition can also generate variation in the value of mammalian females within their oestrous period (Schwagmeyer & Parker 1990). Males that inseminate females during the optimal insemination period (OIP) will have a greater probability of siring than males that copulate either earlier or later in the same oestrus (Dziuk 1970; Jewell et al. 1986; Huck et al. 1989). Females close to their OIP are therefore of greater reproductive value, and males would be expected to prefer females at this time (Ginsberg & Huck 1989).
We investigate the promiscuous mating system of feral Soay sheep on St Kilda, Scotland. The onset of the Soay sheep rut is marked by an increase in male aggression, and by rams breaking away from home ranges as they begin to search for oestrous females. Once located, males will often fight to gain access to oestrous females, during which they butt the flanks of rivals and engage in violent head-on clashes (Preston et al. 2001). The ferocity of these fights is illustrated by a study showing that 60% of rams’ skeletons had evidence of fractures of the cervical vertebrae (Clutton-Brock et al. 1990), and fights have occasionally been observed to result in the death of one of the combatants (B. T. Preston, personal observation). The outcome of male contests appears to be heavily influenced by size, as large males with large horns are more successful at gaining access to, and subsequently guarding, receptive females (Preston et al. 2001, 2003b). In contrast to males, physical competition between females over access to mates has been observed on only one occasion during the entire course of our behavioural study (B. T. Preston, personal observation), and so appears to have little influence on this mating system.
Despite fierce competition between males, and an absence of paternal care, several features of this system make the evolution of male mate choice a theoretical likelihood. Female oestrus usually lasts 2 days, and is highly synchronized (ca. 90% of females enter oestrous over a three-week period), and the inability of rams to form harems means that their PRR is heavily limited (Grubb 1974; Jewell & Grubb 1974). Females are also highly promiscuous, and have been observed copulating with up to 10 different males within a single day of their 2 day oestrus (Preston 2001). The sperm competition that ensues reduces the PRR of males, and leads to substantial processing costs per female in the form of mate guarding. Furthermore, limitations on sperm production have been identified in Soay sheep, reducing the siring success of frequently copulating dominant males (Preston et al. 2001). Putative male preferences for females close to their OIP have already been identified in this species (Preston et al. 2003a).
Soay ewes vary widely in their ability to produce offspring successfully, in part owing to their ability to survive the periodic over-winter mortality episodes that occur during the gestation period, which can account for the deaths of more than 45% of adult females (Clutton-Brock et al. 1991). Body mass is known to be a key variable influencing female survival and their ability to raise offspring (Clutton Brock et al. 1996). Such variation in female quality is also predicted to promote the evolution of male mate choice (Owens & Thompson 1994).
To determine if constraints on male PRR and the observed variation in female quality have led to the evolution of male mate choice in Soay sheep, we investigated patterns of male mating behaviour and reproductive success testing the following predictions: (i) competitive males will focus their mate guarding, and hence paternity success, on females of highest body mass, and (ii) male choice of mates will be restricted to periods of highest female availability, when the costs associated with sampling receptive females are reduced. We also examined the potential consequences of male mate choice for female promiscuity.
2. Methodology
2.1 Study site and population
An unmanaged population of primitive Soay sheep have resided within the St Kilda archipelago (57°49′N, 08°34′W) for more than a millennium. Hirta is the largest island in the group at 638 ha (1 hectare (ha)=104 m2), and has a sheep population that fluctuates between 600 and 2000 individuals in size as a result of periodic population crashes. Our study focuses on a relatively distinct population that inhabit the Village Bay area (175 ha) to the southeast of the island, which comprises approximately one-third of the total Hirta population. From 1985, ca. 95% of lambs born in the Village Bay have been ear-tagged in each year, making them individually identifiable and of known age. Greater male mortality in population crashes results in a female-biased population sex ratio (males:females) of between 0.27 and 0.7. Adults (2 years or over) account for between 39% and 66% of the study population, and the adult sex ratio varies between 0.1 and 0.41. A detailed description of this population can be found elsewhere (see Jewell et al. 1974; Clutton-Brock & Pemberton 2003).
2.2 Morphometric data
Morphometric measurements were collected in a two-week period in August from 1986 to 1999, when a large proportion (mean=51%) of the study area population was captured in each year. Horn and hind-leg length measurements were collected from additional males after their capture in October and November of each year. Following previous studies, hind-leg lengths are taken to be a linear indicator of skeletal or body size (Clutton-Brock & Pemberton 2003), and were measured from the tubercalcis of the fibular tarsal bone to the distal end of the metatarsus. Horn lengths were measured from the base, along the outer curvature of the spiral, to the tip. Female body mass was measured using a carry net and hanging scale in August of all years. The body mass of adult females in August is highly correlated with their body mass in November (r=0.953, d.f.=9, p<0.001; data from 1987).
2.3 Behavioural observations
Daylight focal watches were performed by a team of observers, from the beginning of the rut in November to the end of the rut in mid-December in 1996 to 1999. During this period the study area was continually monitored for consorts throughout each day, with consorts defined as being a close spatial relationship between a male and female (typically within 5 m), with frequent male courtship and defence of receptive females. Adult females receiving elevated interest from males were watched if they had not previously been observed in consort, and thus were likely to be at the onset of their 2 day oestrus. In total, 55 females were watched on their first day of oestrus, and observations continued on 32 of these through their second day of oestrus. Nine females were watched in more than 1 year. Average duration of focal watches was 6 h on day 1, and 6.5 h on day 2 of oestrus. The identity of consorting males, the time of consort initiation and termination, and the occurrence of copulation attempts involving the focal ewe were recorded. We recorded 375 consorts involving 113 individually identifiable males. For consorts that had formed before the onset of focal watching, or continued after the watch had terminated, consort initiation or termination were estimated as the beginning or end of the watch, respectively. Copulation attempts were defined as mounts in which the male’s forelegs made contact with the female’s rump. Focal watches were terminated when females resisted all copulation attempts and received only transient interest from males.
2.4 Conceptions, births and paternities
Since 1987, daily searches of the study area have been conducted during March and April for lambing females. There is a close correspondence between conception and lambing dates (Jewell et al. 1974), and so the numbers of females lambing on each day also provides an accurate measure of the numbers of females conceiving 151 days earlier in the rut. Lambs were captured soon after birth, and tissue sampled for genetic analysis. Using microsatellite DNA genotypes of lambs, their mothers and possible sires, paternities could be assigned at the 80% or 95% confidence level using the paternity inference software Cervus 1.0 (Marshall et al. 1998). For further details of this approach see Pemberton et al. (1999).
2.5 Data analysis
For behavioural and paternity analyses we treat individual females as our independent unit of analysis. Behavioural data were analysed using mixed-effects models in Genstat 5 (release 3.22), which control for the repeated observations on females by fitting female identity as a random effect (Genstat 5 Committee 1993). Females were rarely recorded more than once in the longest consort duration and paternity analyses (less than 23% of observations), and so only one of the repeated observations was chosen at random for inclusion in analyses using general linear models (GLMs). In each analysis the minimal model was arrived at by a process of stepwise deletion (McCullagh & Nelder 1983), with significance being taken as p<0.05. The exclusion probabilities reported were obtained by adding the previously deleted explanatory term to the minimal model.
An index of ‘competitive ability’ was used in analyses to estimate males’ ability to successfully compete for access to receptive females. Competitive ability was predicted from male horn and hind-leg lengths according to the logistic regression equation: logit(y)=−13.78+0.0653x+0.0494z, where x is horn length and z is hind-leg length (Preston 2001). The importance and effect of male horn length and body size (as estimated by their hind-leg lengths) on their competitive ability was established by implementing a generalized linear mixed model (GLMM) that found male body size and horn length to be the principal phenotypic attributes that determined whether males were able to gain copulatory access to receptive females (Preston 2001). This analysis used 617 h of behavioural data from 34 males in the ruts of 1995 to 1999, and also examined male age, body mass, body condition, basal horn circumference and scrotal circumference for an influence on male competitive ability (Preston 2001; see also Preston et al. 2001, 2003a for use of this approach). Competitive ability increases with larger horn and body size, so we use ‘large’ and ‘competitive’ interchangeably. Competitive ability also reflects dominance standing, and correctly predicted the outcome of 71% of 54 dyadic dominance interactions between adults recorded in 1997 to 1999 (Preston 2001).
Yearly differences in adult female body mass, as determined by measurement of ca. 70% of adult females in the study population in each August, were corrected before analyses by subtracting the difference between the mean adult female body mass in 1986 and the mean adult female body mass for the year in question from each measurement of female body mass. By doing so, we precluded the possibility that yearly variation in female body mass contributed to relationships that are interpreted as occurring within years. Year was tested as a factor explaining differences in competitive ability of males or consort duration in models. The number of days from the onset of the rut, with the onset of the rut defined as the date on which the first oestrous female was observed, the number of oestrous (or conceiving) females, female body mass, age and hind-leg length were tested as continuous explanatory variables in models.
We use the definition of ‘behavioural oestrus’ of Jewell et al. (1986), in which oestrus begins from the first point at which a female does not try to evade the copulation attempts of a male. Females may have accepted copulations before the onset of focal watching in some cases; however, we are not aware of any systematic bias in our analyses. Female body mass is not correlated with either the time before onset of oestrous or the period of oestrous observed (linear regressions, time to oestrous onset: n=50, t=−0.24, p>0.8; period of oestrous observed: n=50, t=−1.35, p>0.15). Neither was the mean competitive ability of males in consort with females associated with the period of oestrous observed (n=39, t=−0.78, p>0.4), and previous analyses have failed to detect any association between the competitive ability of males and female evasion of copulation attempts (Preston et al. 2003a). Females also became increasingly receptive to male copulation attempts through their first day of oestrous, while their receptivity gradually declined over their second day of oestrous, suggesting that our estimates of oestrous onset are accurate (Preston et al. 2003a). The OIP was estimated to occur 12 h after the onset of oestrus, as this is the mid-point of the 9–15 h OIP that has been found in domestic sheep (Jewell et al. 1986). The minimum time from the OIP for each consort was used as an explanatory term when testing for an association between male competitive ability and the OIP. For males observed in consort on the first day of a female’s oestrus (when they had yet to reach their OIP), the time of consort termination was taken as the minimum time from the OIP. On the second day of oestrus, at which point the OIP had passed, the time of consort initiation was taken as the minimum time from the OIP.
All analyses were restricted to consorts (or paternities) involving adult males, except for analyses investigating female promiscuity, which aimed to examine variation in the total number of males that inseminated females, and so these analyses included all guarding males that copulated with females. Paternities that are assigned with 80% confidence can be expected to generate incorrect sires at a rate of one in five paternities assigned, and so statistical analyses were restricted to paternities assigned at the 95% confidence level, thereby minimizing the occurrence of spurious outliers. After restrictions, data from seventy-two offspring conceived between 1987 and 1998 were available for analyses. Behavioural analyses were restricted to consort males that had attempted to copulate with focal females. The competitive ability of 30 adult males could be calculated for consorts in which the body mass of guarded females was also known (seven males occurred in more than 1 year and so contributed different competitive abilities), and of 42 sires for which maternal body mass was known (six males sired offspring in more than 1 year). Each consort and/or paternity is treated as an independent event for males.
3. Results
3.1 Male mate choice
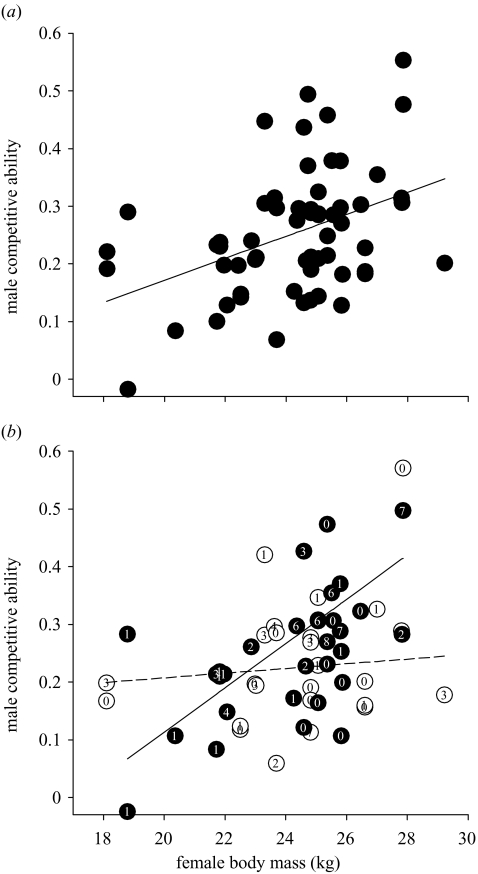
There was a highly significant positive association between the competitive ability of guarding rams (as determined by their horn and body size) and the body mass of defended females (table 1a; figure 1a), indicating that ‘large’ males focused their mating activity on females of greater body mass. This relationship remained strongly significant when only the most stable (i.e. longest) mate-guarding period a female experienced was considered in analyses (Spearman rank correlation: rs=0.640, t(approx)=3.724, d.f.=20, p<0.01), thus excluding the possibility that smaller males were able to gain only transient access to lighter females. After controlling for the effect of female body mass, our analysis also indicates that competitive rams focused their mate guarding to times closer to female OIPs (table 1a).
Table 1.
The competitive ability of guarding males and the stability of consorts.
(The aim of analyses was to establish the determinants of (a) the competitive ability of males (see § 2e) observed guarding and copulating with females; linear mixed model (REML), nfemales=35, nconsorts=60, constant=−0.00458 and (b) consort stability; linear model with females’ longest mate-guarding period as the dependent variable, n=45, constant=0.78. All other terms tested (see § 2) were excluded from models with p>0.05. All terms were also tested in interaction with year, but were excluded with p>0.1.)
| term | d.f. | effect | s.e.m. | Wald statistic (χ2) | p-value |
| (a) competitive ability of guarding male | |||||
| time from OIP | 1 | −0.0143 | 0.00503 | 8.12 | 0.004 |
| female body mass | 1 | 0.0191 | 0.00608 | 9.84 | 0.002 |
| year 1996 | 3 | 0 | 0 | 9.13 | 0.028 |
| 1997 | −0.0678 | 0.0464 | |||
| 1998 | −0.0674 | 0.0445 | |||
| 1999 | −0.128 | 0.0439 | |||
| (b) stability of consorts | t-statistic | ||||
| female body mass | 1 | −0.210 | 0.219 | −0.96 | 0.146 |
| number of oestrous females | 1 | −0.852 | 0.393 | −2.17 | 0.036 |
| female body mass: number of oestrous females | 1 | 0.0388 | 0.0162 | 2.39 | 0.021 |
Figure 1.
Female body mass as a predictor of guarding males’ competitive ability and the influence of female availability. The competitive ability of males (see § 2e) that were observed guarding females as a function of female body mass (a) across the entirety of the rut; the data are controlled for terms remaining in the minimal model and the fitted regression line is plotted from model predictions (see table 1a) and (b) at above (filled circles and solid line) and below (open circles and dashed line) average female availability; for illustrative purposes female availability is fitted here as a categorical term (linear mixed model (REML) analysis weighted for consort duration: female body mass : number of oestrous females interaction; χ2=12.02, d.f.=1, p=0.001, random effect=female identity). The exclusion of females below 22 kg leaves the results of these analyses qualitatively unchanged. Embedded numbers indicate the duration of consorts to the nearest hour.
The size of consorting males appears to have been independent of the number of females simultaneously in oestrus (female body mass:number of females in oestrus interaction, p>0.1). However, consorts varied from less than 1 min to more than 7 h in duration, and so would not equally reflect the strength of male mating decisions, or the ability of rams to defend oestrous females. When we weighted each consort in our analyses by its duration, we found that the positive correlation between guarding rams’ competitive ability and female body mass was present only when high numbers of oestrus females were available (weighted analyses: female body mass:number of females in oestrus interaction, coefficient=−0.00839, s.e.m.=0.00324, Wald statistic (χ2)=6.69, d.f.=1, p=0.01; figure 1b).
3.2 Female promiscuity
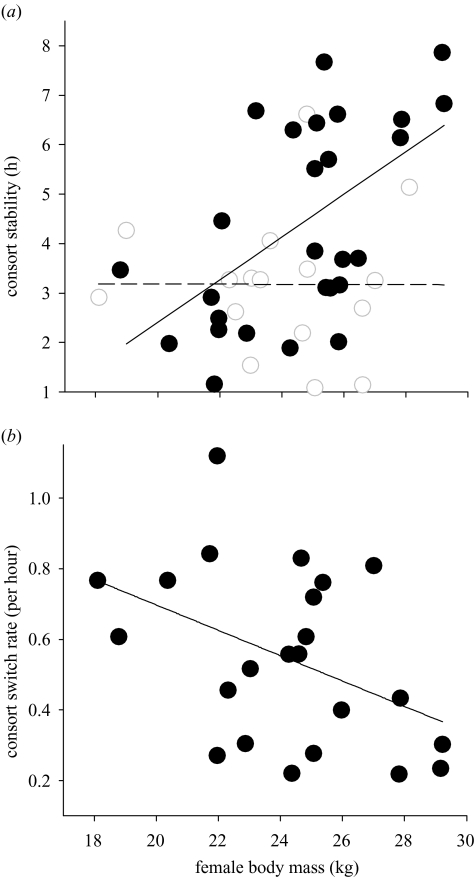
As larger Soay rams appear better able to defend consorts (Preston et al. 2003b), and also restrict their mate guarding to heavy females, our findings seem likely to have implications for levels of mate switching in this population. To investigate this possibility we examined the stability of consorts in relation to female body mass. As expected, we found that there was a significant positive correlation between female body mass and the duration of the most stable (i.e. longest) consort they experienced (linear regression: coefficient=0.287, s.e.m.=0.104, t(43)=2.77, p=0.008). However, consort stability also seems to be dependent on the number of females simultaneously in oestrus, as all females were guarded for only short periods when few other ewes were in oestrus (female body mass : number of oestrous females interaction; table 1b; figure 2a). The association between female body mass and consort stability appears to result in lighter females being inseminated by a greater number of consorting rams over their 2 days of oestrus, as there was a significant negative correlation between the body mass of females and their mate switching rate (linear regression: coefficient=−0.0359, s.e.m.=0.0165, d.f.=1, t(21)=−2.18, p=0.041; figure 2b).
Figure 2.
Female body mass as a predictor of consort stability and promiscuity. The association between female body mass and (a) consort stability at above (filled circles and solid line) and below (open circles and dashed line) average female availability; for illustrative purposes female availability is fitted here as a categorical term (linear model: female body mass:number of oestrous females interaction; t(41)=2.17, d.f.=1, p=0.036), (b) consort change rate each hour over 2 days of oestrus.
3.3 Size assortative mating and parentage
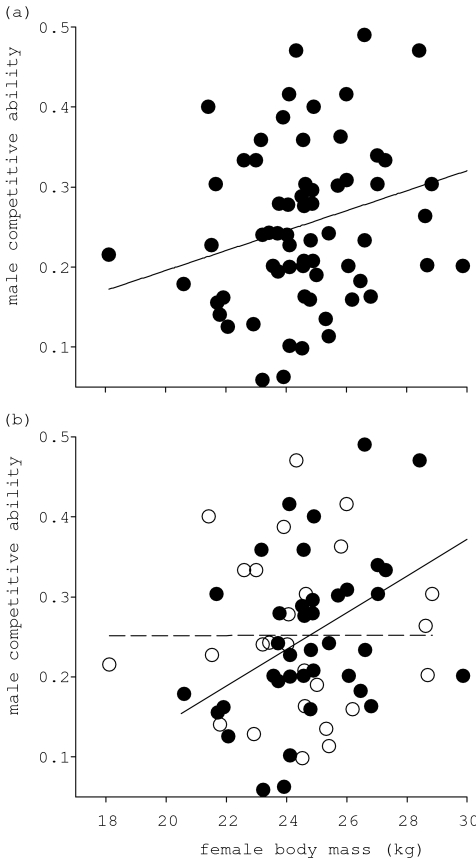
Apparent mate choice by rams led to assortative patterns of parentage, as large males sired the offspring of heavier females (linear regression: coefficient=0.0136, s.e.m.=0.00535, t(70)=2.54, p=0.013; figure 3a). Although we detected no immediate influence of female availability on this relationship (competitive ability of male:females conceiving interaction, p>0.1), bootstrapping this interaction term produced only marginally non-significant results (bootstrapped regression coefficient=0.00201, nbootstraps=1000, 95% confidence intervals=−0.00024, 0.005 05; figure 3b) suggesting that our failure to detect a significant association may have been due to a small number of influential males or relatively low sample size. This interaction indicates that the association between the competitive ability of the sire and female body mass may occur only as the number of oestrous females increases; at lower female availability the competitive ability of the sire is more variable. These patterns are consistent with those in our behavioural analyses.
Figure 3.
Female body mass as a predictor of siring rams’ competitive ability and the influence of female availability. The competitive ability of siring males (see § 2e) as a function of female body mass (a) across the entirety of the rut and (b) at above (filled circles and solid line) and below (open circles and dashed line) average female availability. There is a significant interaction between female body mass and the number of conceiving females (when female availability is treated as a categorical term for illustrative purposes) in determining the competitive ability of the sire (linear model: female body mass:number of conceiving females interaction; t(63)=2.07, d.f.=1, p=0.044). All other terms tested (see § 2) were excluded from the model with p>0.1. Excluding the outlying female of low body mass (less than 20 kg) leaves the results of these analyses qualitatively unchanged.
4. Discussion
4.1 Male mate choice and assortative parentage
As predicted, our analyses indicated that larger rams preferentially guarded heavier females, but that this relationship was dependent upon the number of females simultaneously in oestrus. When fewer females were in oestrus, competitive males appeared to invest mate-guarding effort in any female that was receptive. Mate choice by rams was reflected in assortative patterns of parentage, as competitive males sired the offspring of heavier females. This relationship also seems contingent upon there being high numbers of oestrous females in the study area, though the results of these analyses are less robust.
Body mass is a key determinant of female reproductive success, as heavier females are better able to survive to parturition and produce heavier offspring, who are in turn more likely to survive through their first winter (Clutton-Brock et al. 1991, 1996). Furthermore, the progeny of heavier females are more likely to produce offspring themselves in their first year of life (Preston 2001). Male preferences for females of higher body mass thus appear highly adaptive.
Our results are consistent with agricultural studies on domestic sheep, which have shown that rams exhibit consistent mate preferences for ‘woollier’ females (Tilbrook 1987; Tilbrook & Cameron 1989). Because bulky fleeces are a result of artificial selection for greater wool production (Ryder 1983), it seems likely that the results of these studies reflect male preferences for greater female size or condition, and that domestic rams are ‘misled’ in their assessment of females by an artificially selected thicker fleece.
Although female choice of mates has the potential to contribute to the observed association between female body mass and male competitive ability, previous analyses have been unable to detect an effect of rams’ age, horn length, body size or competitive ability on female willingness to copulate (Preston et al. 2003a), and in studies on domestic sheep, preferences exhibited by rams have been reported as being either unconnected or only weakly associated with the solicitation behaviour of ewes (Synnott & Fulkerson 1984; Tilbrook et al. 1987).
Another possible explanation for the association between the competitive ability of males and the body mass of defended females is assortative patterns of reproductive activity (Crespi 1989). Juvenile Soay females (less than 1 year old) come into oestrus towards the end of the five week rut (Jewell & Grubb 1974), and it could be that lighter adult females also enter oestrus later in the rutting season, when larger males may be physically exhausted by their prior rutting efforts. This kind of male ‘endurance rivalry’ may be important in determining patterns of paternity in red deer (Cervus elaphus) and elephant seals (Mirounga angustirostris) (Clutton-Brock 1988; Deutsch et al. 1990; Andersson 1994). In Soay sheep, however, larger rams continued to increase their reproductive activity relative to smaller males as the rut progressed (Preston et al. 2001; Stevenson et al. 2003), and there appears to be no effect of age or body mass on the oestrus dates of adult females (unpublished analysis). Instead, females enter oestrus on a similar date in each rut (Preston 2001), suggesting oestrus date may be under a degree of genetic control. These observations indicate that the association between the competitive ability of males and the body mass of consorting females cannot be explained by assortative patterns of reproductive activity.
Male choice of mates for fecundity benefits has been found in several other vertebrate taxa (e.g. fishes: Kraak & Baker 1998; Amundsen & Forsgren 2001; birds: Jones & Hunter 1993; reptiles: Olsson 1993), but empirical evidence of mate choice in mammals that translates into assortative patterns of parentage is scarce. Our study on Soay sheep is also unusual in that it is the competitive sex that appears to be choosy, despite the lack of paternal care, and an operational sex ratio (OSR) that is always heavily male biased. The requirement for periods of mate guarding and limited sperm reserves is probably the major constraint driving male choosiness, which emphasizes the importance of sperm competition in shaping this mating system.
Our analyses confirm previous findings on Soay rams’ behaviour in relation to female optimal insemination periods (Preston et al. 2003a). While controlling for the correlation between the size of guarding rams and female body mass, our results show that larger rams guarded females at periods closer to their OIP, thus bringing putative advantages in sperm competition (see Preston et al. 2003a for discussion).
4.2 Female availability
Although males may gain considerable reproductive benefits by siring the offspring of higher-quality females, male reproductive success will remain heavily influenced by the number of fertilizations per unit time they achieve. To maximize their siring success, optimality theory predicts that males will adjust their degree of paternity assurance with each female according to the availability of other oestrus females (Dewsbury 1982; Sherman 1989). When few females are in oestrus, males should invest relatively more per female, as the probability of finding another reproductive opportunity is low. However, when oestrous females are relatively abundant, males are predicted to sacrifice some level of paternity assurance with the current female in exchange for fertilizations with new females (Dewsbury 1982; Sherman 1989). Our results appear at odds with these predictions, because all Soay rams guarded females for relatively short periods of time when few other ewes were in oestrus. A probable explanation for this discrepancy is the intensity of overt male–male competition in this system. At the beginning and end of the rutting season, the OSR can be as high as 236:1, and thus male competition for access to females could be so intense that even the largest rams cannot defend consorts from take-overs and other interference from rivals. At the peak of the rut, there is a reduction in the intensity of overt competition for access to females (Preston et al. 2003b), which may allow competitive males to defend consorts effectively. Although competitive males may have been unable to increase paternity assurance when few females were in oestrus, they did invest mate-guarding effort in females of lower body mass only at these times. These results are consistent with the prediction that males will modify their rejection threshold of potential mates according to the costs associated with sampling receptive females (Parker 1983; Real 1990). In the freshwater amphipod Hyalella azteca, similar dynamics in both the occurrence of male mate choice and duration of mate guarding with changing OSR have been observed (Wen 1993), and plasticity in the strength of male mate choice with changing OSR has also been documented in experimental studies of several other taxa (e.g. bush crickets (Kawanaphila nartee), Kvarnemo & Simmons 1999; and pipefish (Syngnathus typhle), Berglund 1994).
Although we argue that the lack of assortative mating at low female availability was due to competitive males modifying their mate rejection thresholds, we are unable to discount the possibility that it was smaller rams that became less choosy. Smaller rams might prefer to invest in lower-quality females that are avoided by stronger competitors, if they offered a greater potential for successful reproduction than high-quality females that attract highly competitive males (Fawcett & Johnstone 2003). If such preferences existed, they would reinforce patterns of assortative mating at high female availability, but may be inhibited when few females are in oestrus, and the costs of choice are higher.
4.3 Female promiscuity
The benefits females might gain by seeking promiscuous matings is the subject of much current interest (e.g. Stockley 1997a, 2003; Gomendio et al. 1998; Hoogland 1998) because benefits are required to offset the putative costs of multiple mating, such as increased parasite transmission and predation (Thrall et al. 1997; Gomendio et al. 1998). However, it appears to be ram mating preferences and the asymmetry in their ability to defend consorts that generates variation in the levels of female promiscuity in the Soay sheep system. Females of low body mass are unable to attract sustained consorts from competitive males, and instead experience a series of unstable consorts from less competent rams, thus receiving inseminations from a greater number of males. Because this variation in mate-switching rate (and hence sperm competition intensity) appears to be generated by male-driven processes, it suggests that the extremes of promiscuity found in this system are driven by male competition for access to receptive ewes, and male preferences for higher female body mass, rather than being sought by females as part of an adaptive tactic.
It could be argued that the decline in promiscuity with female body mass is a consequence of conflict between males and females, as mate guarding by rams is an attempt to reduce the intensity of sperm competition, which may be in conflict with female benefits from multiple mating (Stockley 1997b). In support of this idea, heavier female Gunnison’s prairie dogs (Cynomys gunnisoni) are reported to be better at avoiding monopolization by males than are lighter females, and gain fertility benefits by soliciting copulations from a greater number of males during their oestrus (Hoogland 1998). Females of other taxa also avoid the attentions of guarding males while seeking copulations with additional males (e.g. dunnocks (Prunella modularis): Davies 1992), though variation in female success in this regard has received little attention. Hence, it could be that high levels of promiscuity are adaptive for Soay ewes, but guarding by competitive rams constrains the mating behaviour of preferred females. Sexual conflict between oestrous females and guarding rams seems unlikely to explain a lower re-mating rate of heavier Soay females, however, as stable consorts appear to require the cooperation of both ram and ewe (Grubb & Jewell 1973; Jewell & Grubb 1974). It is more likely that females trade copulations with consorting rams in exchange for defence from harassment by other rams. The costs of harassment for Soay females can be substantial, as male competition for reproductive opportunities has been observed to lead to the injury and even death of oestrous females (Stevenson et al. 2003). Male harassment of receptive females and coerced matings are common among other animal taxa (Clutton-Brock & Parker 1995). In the red-sided garter snake (Thamnophis sirtalis parietalis), even non-reproductive juvenile females appear to accept copulations from courting males, because male harassment of unmated females is thought to increase predation risk by impeding their dispersal from over-wintering dens (Shine et al. 2000). Whether females actively seek promiscuous matings or not, male mate choice and male competition may influence the extent of female promiscuity within populations. Hoogland (1998) concludes that Gunnison’s prairie dog males may be more ‘attracted’ to heavier females because of their higher fecundity, and so it is conceivable that the variation in levels of promiscuity with female quality in the Gunnison’s prairie dog mating system are also influenced by male mating preferences and competition. Consequences of male competition and male mate choice on female promiscuity may also emerge in wild baboons (Papio cynocephalus anubis), where males compete more intensely for females of higher reproductive value, as indicated by the size of females’ perineal swelling (Domb & Pagel 2001). If the occurrence of male mate choice alongside male reproductive competition proves to be more common than had been thought, as several authors have suggested (e.g. Owens & Thompson 1994; Andersson & Iwasa 1996; Johnstone et al. 1996), then variation in levels of promiscuity according to female quality may have consequences across a range of taxa.
In conclusion, it appears that in Soay sheep both male mate choice and intense male–male competition influence patterns of reproductive behaviour and subsequent paternity. This is likely to further increase the variability in reproductive success of males, the most competitive of which sire the offspring of the highest-quality females, and thus gain the associated advantages of increased offspring survival and early reproductive success. A further consequence of mate choice by competitive males is that light females experience a series of unstable consorts with less adept rams, and receive inseminations from a greater number of males over their oestrus. Variation in female promiscuity can thus be explained by male-driven processes in this system, suggesting that high levels of promiscuity per se are not part of an adaptive female tactic.
Acknowledgments
The authors thank Scottish Natural Heritage and the National Trust for Scotland for permission to undertake work on St Kilda. Royal Artillery Range Hebrides and Serco provided essential logistical support. Many thanks to other members of the Soay sheep project, especially Tim Clutton-Brock, Steve Albon and Bryan Grenfell who initiated and maintained the current phase of research, and Jill Pilkington who collected much of the core field data. The authors are also grateful for the help of many volunteers, particularly Ali Donald, Rusty Hooper, Owen Jones and Gill Telford. Tim Birkhead, Peter Hudson, Paula Stockley and two anonymous referees provided comments on the manuscript. This work was funded by The Royal Society, the University of Stirling and the Natural Environment Research Council.
References
- Amundsen T., Forsgren E. Male mate choice selects for female coloration in a fish. Proc. Natl Acad. Sci. USA. 2001;98:13155–13160. doi: 10.1073/pnas.211439298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson M. Sexual selection. Princeton University Press; Princeton, NJ: 1994. [Google Scholar]
- Andersson M., Iwasa Y. Sexual selection. Trends Ecol. Evol. 1996;11:53–58. doi: 10.1016/0169-5347(96)81042-1. [DOI] [PubMed] [Google Scholar]
- Berglund A. The operational sex-ratio influences choosiness in a pipefish. Behav. Ecol. 1994;5:254–258. [Google Scholar]
- Clutton-Brock J., Dennis-Bryan K., Armitage P.L. Osteology of the Soay sheep. Bull. Br. Mus. Nat. Hist. (Zool.) 1990;56:1–56. [Google Scholar]
- Clutton-Brock T.H. Reproductive success. University of Chicago Press; 1988. [Google Scholar]
- Clutton-Brock T.H., Parker G.A. Potential reproductive rates and the operation of sexual selection. Q. Rev. Biol. 1992;67:437–456. [Google Scholar]
- Clutton-Brock T.H., Parker G.A. Sexual coercion in animal societies. Anim. Behav. 1995;49:1345–1365. [Google Scholar]
- Clutton-Brock T.H., Pemberton J.M. Soay sheep: population dynamics and selection in an island population. Cambridge University Press; 2003. [Google Scholar]
- Clutton-Brock T.H., Vincent A.C.J. Sexual selection and the potential reproductive rates of males and females. Nature. 1991;351:58–60. doi: 10.1038/351058a0. [DOI] [PubMed] [Google Scholar]
- Clutton-Brock T.H., Price O.F., Albon S.D., Jewell P.A. Persistent instability and population regulation in Soay sheep. J. Anim. Ecol. 1991;60:593–608. [Google Scholar]
- Clutton-Brock T.H., Stevenson I.R., Marrow P., MacColl A.D., Houston A.I., McNamara J.M. Population fluctuations, reproductive costs and life-history tactics in female Soay sheep. J. Anim. Ecol. 1996;65:675–689. [Google Scholar]
- Crespi B.J. Causes of assortative mating in arthropods. Anim. Behav. 1989;38:980–1000. [Google Scholar]
- Davies N.B. Dunnock behaviour and social evolution. Oxford University Press; 1992. [Google Scholar]
- Deutsch C.J., Haley M.P., Le Boeuf B.J. Reproductive effort of male northern elephant seals: estimates from mass loss. Can. J. Zool. 1990;68:2580–2593. [Google Scholar]
- Dewsbury D.A. Ejaculate cost and male choice. Am. Nat. 1982;119:601–610. [Google Scholar]
- Domb L.G., Pagel M. Sexual swellings advertise female quality in wild baboons. Nature. 2001;410:204–206. doi: 10.1038/35065597. [DOI] [PubMed] [Google Scholar]
- Dziuk P. Estimation of optimum time for insemination of gilts and ewes by double-mating at certain times relative to ovulation. J. Reprod. Fertil. 1970;22:277–282. doi: 10.1530/jrf.0.0220277. [DOI] [PubMed] [Google Scholar]
- Emlen S., Oring L. Ecology, sexual selection, and the evolution of mating systems. Science. 1977;197:215–223. doi: 10.1126/science.327542. [DOI] [PubMed] [Google Scholar]
- Fawcett T.W., Johnstone R.A. Mate choice in the face of costly competition. Behav. Ecol. 2003;14:771–779. [Google Scholar]
- Genstat 5 Committee Genstat 5 reference manual. Clarendon Press; Oxford: 1993. [Google Scholar]
- Ginsberg J.R., Huck U.W. Sperm competition in mammals. Trends Ecol. Evol. 1989;4:74–79. doi: 10.1016/0169-5347(89)90152-3. [DOI] [PubMed] [Google Scholar]
- Gomendio M., Harcourt A.H., Roldan E.R.S. Sperm competition in mammals. In: Birkhead T.R., Møller A.P., editors. Sperm competition and sexual selection. Academic Press; San Diego, CA: 1998. pp. 667–756. [Google Scholar]
- Grubb P. The rut and behaviour in Soay rams. In: Jewell P.A., Milner C., Boyd J.M., editors. Island survivors: the ecology of the Soay sheep of St Kilda. Athlone Press; London: 1974. pp. 195–223. [Google Scholar]
- Grubb P., Jewell P.A. The rut and occurrence of oestrus in the Soay sheep of St Kilda. J. Reprod. Fertil. Suppl. 1973;19:491–502. [PubMed] [Google Scholar]
- Hoogland J.L. Why do female Gunnison’s prairie dogs copulate with more than one male? Anim. Behav. 1998;55:351–359. doi: 10.1006/anbe.1997.0575. [DOI] [PubMed] [Google Scholar]
- Huck U.W., Tonias B.A., Lisk R.D. The effectiveness of competitive male inseminations in golden hamsters, Mesocricetus auratus, depends on an interaction of mating order, time-delay between males, and the time of mating relative to ovulation. Anim. Behav. 1989;37:674–680. [Google Scholar]
- Jewell P.A., Grubb P. The breeding cycle, the onset of oestrus and conception in Soay sheep. In: Jewell P.A., Milner C., Boyd J.M., editors. Island survivors: the ecology of the Soay sheep of St Kilda. Athlone Press; 1974. pp. 224–241. [Google Scholar]
- Jewell P.A., Milner C., Boyd J.M. Island survivors: the ecology of the Soay sheep of St Kilda. Athlone Press; London: 1974. [Google Scholar]
- Jewell P.A., Hall S.J.G., Rosenberg M.M. Multiple mating and siring success during natural oestrus in the ewe. J. Reprod. Fertil. 1986;77:81–89. doi: 10.1530/jrf.0.0770081. [DOI] [PubMed] [Google Scholar]
- Johnstone R.A., Reynolds J.D., Deutsch J.C. Mutual mate choice and sex differences in choosiness. Evolution. 1996;50:1382–1391. doi: 10.1111/j.1558-5646.1996.tb03912.x. [DOI] [PubMed] [Google Scholar]
- Jones I., Hunter F. Mutual sexual selection in a monogamous seabird. Nature. 1993;362:238–239. [Google Scholar]
- Kraak S.B.M., Bakker T.C.M. Mutual mate choice in sticklebacks: attractive males choose big females, which lay big eggs. Anim. Behav. 1998;56:859–866. doi: 10.1006/anbe.1998.0822. [DOI] [PubMed] [Google Scholar]
- Kvarnemo C., Simmons L.W. Variance in female quality, operational sex ratio and male mate choice in a bush cricket. Behav. Ecol. Sociobiol. 1999;45:245–252. [Google Scholar]
- McCullagh P., Nelder J.A. Generalized linear models. Chapman & Hall; London: 1983. [Google Scholar]
- Marshall T.C., Slate J., Kruuk L.E.B., Pemberton J.M. Statistical confidence for likelihood-based paternity inference in natural populations. Mol. Ecol. 1998;7:639–655. doi: 10.1046/j.1365-294x.1998.00374.x. [DOI] [PubMed] [Google Scholar]
- Møller A.P., Birkhead T.R. Copulation behaviour in mammals: evidence that sperm competition is widespread. Biol. J. Linn. Soc. 1989;38:119–132. [Google Scholar]
- Olsson M. Male preference for large females and assortative mating for body size in the sand lizard (Lacerta agilis) Behav. Ecol. Sociobiol. 1993;32:337–341. [Google Scholar]
- Owens I.P.F., Thompson D.B.A. Sex differences, sex ratios and sex roles. Proc. R. Soc. Lond. B. 1994;258:93–99. doi: 10.1098/rspb.1994.0148. [DOI] [PubMed] [Google Scholar]
- Parker G.A. Sperm competition and its evolutionary consequences in insects. Biol. Rev. 1970;45:525–567. [Google Scholar]
- Parker G.A. Mate quality and mating decisions. In: Bateson P., editor. Mate choice. Cambridge University Press; 1983. pp. 141–166. [Google Scholar]
- Parker G.A. Sperm competition and the evolution of ejaculates: towards a theory base. In: Birkhead T.R., MØller A.P., editors. Sperm competition and sexual selection. Academic Press; San Diego, CA: 1998. pp. 1–54. [Google Scholar]
- Pemberton J.M., Coltman D.W., Smith J.A., Pilkington J.G. Molecular analysis of a promiscuous, fluctuating mating system. Biol. J. Linn. Soc. 1999;68:289–301. [Google Scholar]
- Preston, B. T. 2001 Sexual selection and sperm competition in Soay sheep. PhD thesis, University of Stirling, UK.
- Preston B.T., Stevenson I.R., Pemberton J.M., Wilson K. Dominant rams lose out by sperm depletion. Nature. 2001;409:681–682. doi: 10.1038/35055617. [DOI] [PubMed] [Google Scholar]
- Preston B.T., Stevenson I.R., Wilson K. Soay rams target reproductive activity towards promiscuous females’ optimal insemination period. Proc. R. Soc. Lond. B. 2003a;270:2073–2078. doi: 10.1098/rspb.2003.2465. doi:10.1098/rspb.2003.2465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston B.T., Stevenson I.R., Pemberton J.M., Coltman D.W., Wilson K. Overt and covert competition in a promiscuous mammal: the importance of weaponry and testes size to male reproductive success. Proc. R. Soc. Lond. B. 2003b;270:633–640. doi: 10.1098/rspb.2002.2268. doi:10.1098/rspb.2002.2268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Real L. Search theory and mate choice. 1. Models of single-sex discrimination. Am. Nat. 1990;136:376–405. [Google Scholar]
- Ryder M.L. Sheep and man. Duckworth; London: 1983. [Google Scholar]
- Schwagmeyer P.L., Parker G.A. Male mate choice as predicted by sperm competition in thirteen-lined ground squirrels. Nature. 1990;348:62–64. [Google Scholar]
- Sherman P.W. Mate guarding as paternity insurance in Idaho ground squirrels. Nature. 1989;338:418–420. doi: 10.1038/338418a0. [DOI] [PubMed] [Google Scholar]
- Shine R., O’Connor D., Mason R.T. Sexual conflict in the snake den. Behav. Ecol. Sociobiol. 2000;48:392–401. [Google Scholar]
- Stevenson I.R., Marrow P., Preston B.T., Pemberton J.M., Wilson K. Adaptive reproductive strategies. In: Clutton-Brock T.H., Pemberton J.M., editors. Soay sheep: population dynamics and selection in an island population. Cambridge University Press; 2003. [Google Scholar]
- Stockley P. No evidence of sperm selection by female common shrews. Proc. R. Soc. Lond. B. 1997a;264:1497–1500. doi: 10.1098/rspb.1997.0207. doi:10.1098/rspb.1997.0207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockley P. Sexual conflict resulting from adaptations to sperm competition. Trends Ecol. Evol. 1997b;12:154–159. doi: 10.1016/s0169-5347(97)01000-8. [DOI] [PubMed] [Google Scholar]
- Stockley P. Female multiple mating behaviour early reproductive failure, and litter size variation in mammals. Proc. R. Soc. Lond. B. 2003;270:271–278. doi: 10.1098/rspb.2002.2228. doi:10.1098/rspb.2002.2228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Synnott A.L., Fulkerson W.J. Influence of social-interaction between rams on their serving capacity. Appl. Anim. Ethol. 1984;11:283–289. [Google Scholar]
- Thrall P.H., Antonovics J., Bever J.D. Sexual transmission of disease and host mating systems: within-season reproductive success. Am. Nat. 1997;149:485–506. [Google Scholar]
- Tilbrook A.J. Physical and behavioral factors affecting the sexual attractiveness of the ewe. Appl. Anim. Behav. Sci. 1987;17:109–115. [Google Scholar]
- Tilbrook A.J., Cameron A.W.N. Ram mating preferences for woolly rather than recently shorn ewes. Appl. Anim. Behav. Sci. 1989;24:301–312. [Google Scholar]
- Tilbrook A.J., Cameron A.W.N., Lindsay D.R. The influence of ram mating preferences and social-interaction between rams on the proportion of ewes mated at field joining. Appl. Anim. Behav. Sci. 1987;18:173–184. [Google Scholar]
- Trivers R.L. Parental investment and sexual selection. In: Campbell B., editor. Sexual selection and the descent of man 1871–1971. Heinemann; London: 1972. pp. 136–179. [Google Scholar]
- Wen Y.H. Sexual dimorphism and mate choice in Hyalella azteca (Amphipoda) Am. Midl. Nat. 1993;129:153–160. [Google Scholar]