Abstract
Background
Local delivery of carmustine (BCNU) via biodegradable polymers has been shown to improve survival in patients with glioblastoma multiforme (GBM). In the current study, we hypothesized that local delivery of an anthracycline antibiotic, doxorubicin (DOX), might act to improve the survival of animals bearing experimental intracranial glioma.
Materials and Methods
Polyanhydride polymers (PCPP-SA) containing either 3% or 5% ADR by weight were prepared using the mix-melt method. Forty male Fisher 344 rats received an intracranial challenge with a lethal dose of 9L gliosarcoma cells. Five days later, they received DOX or blank polymer. There were a total of four treatment groups: 1) blank polymer; 2) 3% DOX polymer; 3) 5% DOX polymer, and 4) control group with no polymer.
Results
Compared to control animals treated with no polymers or blank polymer, animals receiving DOX had significantly extended survival. The median survival for the control group was 21 days vs. 34 days (p<0.01) for the 3% DOX group and 45 days (p<0.0001) for the 5% DOX group.
Conclusion
Doxorubicin, when delivered locally, is an effective monotherapeutic agent against experimental intracranial glioma.
Keywords: Doxorubicin, biodegradable polymer, brain tumor, interstitial chemotherapy
The field of biodegradable polymer drug release has improved the treatment of malignant gliomas, and sparked interest in the treatment of metastatic brain tumors, by allowing the local delivery of chemotherapy to brain tumors (4, 5, 27, 29–31). Phase III clinical trials utilizing BCNU-polymers combinations have shown Gliadel® to be efficacious in the treatment of malignant gliomas, both in the recurrent setting as well as at the time of initial surgery (27–31). The delivery of chemotherapeutic agents directly to the tumor bypasses the blood brain barrier, thereby allowing a high concentration of drug at the site of interest while eliminating the toxicity associated with the traditional systemic delivery of chemotherapeutic drugs.
While different strategies to improve the efficacy of polymer-mediated chemotherapy against malignant gliomas or even metastatic brain tumors are currently being pursued, one potential strategy is to test drugs other than BCNU. One such drug is doxorubicin (DOX) which is presently used in the treatment of a wide variety of cancers, such acute lymphoblastic leukemias, lymphomas, multiple myeloma, sarcomas, mesotheliomas, germ cell tumors of the ovary or testis, carcinomas of the head and neck, breast. pancreas, stomach, liver, ovary, lung, prostate, uterus, and neuroblastomas (2). Doxorubicin is an anthracycline antibiotic produced by the Streptomyces peucetius varieta caesius. Doxorubicin blocks DNA and RNA synthesis by inhibiting topoisomerase II (3).
Despite doxorubicin’s clinical effectiveness in the treatment of many malignant tumors, clinical trials involving systemic administration of the drug have demonstrated very limited efficacy in the treatment of gliomas (11, 21). Very high doses of doxorubicin must be administered systemically to exert any therapeutic benefit and these doses are highly neurotoxic and therefore ineffective in treating CNS malignancies (15, 17). Doxorubicin’s limited efficacy when administered systemically can be explained by the poor penetration of the drug thru the blood-brain barrier and effect of p-glycoprotein-mediated efflux from cerebrospinal fluid (CSF) (15, 17, 18). The low lipophilicity and high molecular weight of doxorubicin essentially prevent the delivery of the agent across the blood-brain barrier.
Recent in vitro data demonstrate that doxorubicin is toxic to glioblastoma cell lines (1, 6, 24). Moreover, in vivo data in animal models of malignant glioma suggest that doxorubicin is an effective anti-glioma agent (13, 14, 16, 19, 20, 22, 24, 25). In fact, in a recently published clinical study, ten patients with grade III or IV gliomas were treated with direct doxorubicin infusion via an Ommaya reservoir (28). Fifty percent of the patients showed objective radiologic response. There were no clinically significant adverse reactions either in the brain or systematically. Intratumoral administration of doxorubicin therefore appears to be a safe and potentially effective treatment and should be further explored in the management of brain gliomas resistant to conventional forms of treatment.
In this study, we hypothesized that local intracerebral delivery of doxorubicin via a polymer matrix might be beneficial in the treatment of malignant glioma. Specifically, we investigated: 1) the efficacy of doxorubicin against an experimental brain tumor cell line in vitro; 2) the drug release kinetics of implantable wafers composed of doxorubicin and the biodegradable polymer pCPP:SA; 3) the toxicity of the doxorubicin-polymer system after implantation into the rat brain; 4) the ability of doxorubicin delivered via the polymer system in vivo to extend survival in an intracerebral malignant glioma model.
Materials and Methods
Tumor cell lines
The 9L gliosarcoma cell line was obtained from Dr. M Barker at the University of California at San Francisco Brain Tumor Research Center (San Francisco, CA, USA). The cells were maintained in tissue culture in Dulbecco’s minimum essential medium with 10% fetal bovine serum, streptomycin (80.5 Ìg/ml), penicillin (base; 80.5 units/ml), and 1% L-glutamine (all products from GIBCO laboratories, Grand Island, NY, USA). Cells were maintained in a humidified atmosphere of 5% CO2 at 37°C. The cells were grown to confluence, detached with 0.25% trypsin in Dulbecco’s phosphate-buffered saline, and resuspended in medium.
In vitro activity of doxorubicin. Inhibition of tumor proliferation was tested with rodent 9L glioma. Cells were plated at 10,000 cells/well in 24 well plates with increasing concentrations of doxorubicin, ranging from 1 ng/ml to 50 Ìg/ml. The cells were counted after 5-day exposure by using a cell counter and compared with control cells receiving no doxorubicin.
Wafer preparation
The matrix pCPP:SA had a 10:90 molar ratio. Doxorubicin was combined with pCPP:SA (10:90), methanol to dissolve doxorubicin and methylene chloride. Cylindrical wafers weighing 10 mg each (3 mm in diameter, 1 mm thick) were then manufactured using the steel molding press.
Drug release kinetics
The pCPP:SA wafers were placed in 1.5 ml vials containing 1 ml of 0.1 M phosphate buffered saline solution, ph 7.4. The vials were capped to prevent evaporation and placed in an incubator at 37ÆC. The saline solution was removed and replaced to approximate infinite sink conditions. The amount of doxorubicin released was determined by reading samples on the Bertramm Spectrophotometer at 480 nm and by calculating the drug concentration based on a standard curve.
Intracranial tumor implantation
Rats were anesthetized with an intraperitoneal injection of 2 to 4 ml/kg of a stock solution containing ketamine hydrochloride (25 mg/ml), xylazine (2.5 mg/ml), and ethanol in a sterile 0.9% NaCl solution. The heads were shaved and disinfected with a 70% ethanol and povidone-iodine solution. After a midline scalp incision, the galea overlying the left cranium was swept laterally. With the aid of an operating microscope, a 3-mm burr hole was made over the left parietal bone, with its center 2 to 3 mm posterior to the coronal suture and 3 to 4 mm lateral to the sagittal suture. Great care was taken to avoid injury to the dura mater. The rats were then placed in a stereotactic frame, and 1x102 9L glioma cells were implanted. After ensuring hemostasis, the wound was closed with surgical staples.
Wafer implantation
In animals not receiving tumor cells, following burr hole placement, the dura mater and underlying brain parenchyma were opened using a No. 11 surgical blade. Then, with the aid of an operating microscope, the wafer was placed into the brain parenchyma at a depth of approximately 1mm below the dura. After ensuring hemostasis, the skin was closed with surgical staples. In tumor-bearing animals, surgical wounds were reopened and wafers were implanted five days after tumor implantation.
In vivo doxorubicin wafer toxicity
To determine the maximally tolerated doxorubicin loading dose, 80 rats, evenly divided into 4 groups, underwent intracerebral implantation of wafers containing 10%, 7%, 5% and 3% doxorubicin pCPP:SA wafers. Animals were closely monitored for signs of toxicity, including wound healing problems, weight loss, failure to thrive, and neurological deficits. Survival was then assessed and autopsies performed on all animals.
Histological evaluation
In the toxicity study, the brains, lungs, hearts, kidneys, livers, spleens, and intestines were all examined when an animal died following implantation of a polymer. The same protocol was repeated when an animal died in the efficacy group. In addition, a representative animal from each efficacy group was euthanized at days 7 and 14 after polymer implant and the brains were removed and compared to non-treated controls. All tissue was fixed in 10% formalin, blocked in paraffin, and stained with hematoxylin and eosin (H&E).
Sources of supplies and equipment
The matrix pCPP:SA was supplied by Guilford Pharmaceuticals Corp. (Baltimore, MD, USA). Doxorubicin was purchased from Sigma-Aldrich Corp. (St. Loius, MO, USA). The ultraviolet/visible spectrophotometer (Genesys 5) used to determine the amount of doxorubicin released was acquired from Spectronic (Rochester, NY, USA).
Animals
A total of two hundred 10-week-old, female Fisher 344 rats weighing 180 to 220 g were purchased from Charles River Laboratories (Wilmington, MA, USA). Eighty animals were used for toxicity studies and the remaining one hundred twenty were used for efficacy studies (40 animals/study, repeat experiment three times). The animals were kept in standard animal facilities with 3 or 4 rats per cage, and given free access to rat chow and water. They were housed in accordance with the policies and principles of laboratory care of the Johns Hopkins University School of Medicine Animal Care and Use Committee.
Statistical methods
For in vitro studies, data was analyzed using the two-tailed Student’s t-test. For animal experiments, survival data were analyzed with log-rank (Mantel-Cox) test in a Kaplan-Meier nonparametric analysis performed using statistical software.
Results
In vitro efficacy of doxorubicin
Doxorubicin is a potent inhibitor of rodent glioma cell growth. At a concentration of 50 ng/ml (range 10–50 ng/ml), 9L glioma was inhibited by at least 97.5% after five days of incubation with DOX (Figure 1).
Figure 1.
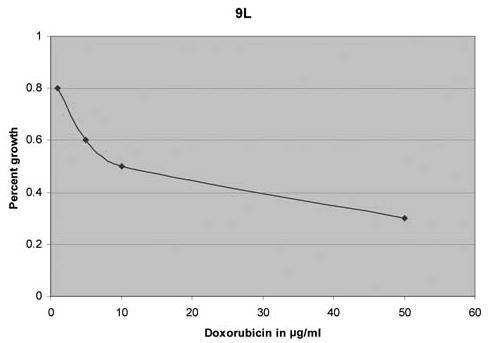
In vitro growth inhibition of rodent 9L glioma. The cell line was incubated with doxorubicin for five days and shows a 10–50 ng/ml DOX tumoricidal range.
Polymer formulation and drug release kinetics
Doxorubicin was loaded 1–10% by weight into pCPP:SA controlled release polymer and drug release was assayed by ultraviolet spectroscopy. Spectroscopy showed that while the 1% loaded polymer released 6.5% (65 Ìg) of drug over 200 hours, 10% loaded polymer released 21% (210 Ìg) of drug over 200 hours, a concentration well in excess of the tumoricidal range (Figure 2).
Figure 2.
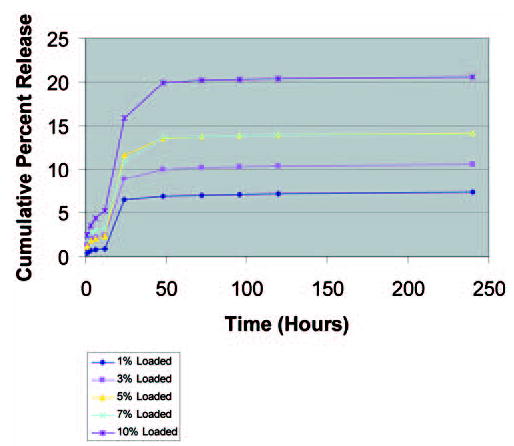
In vitro release kinetics for 1–10% DOX in pCPP:SA polymer.
In vivo doxorubicin wafer toxicity
None of the animals exhibited any problems with wound healing or weight loss. Animals which developed toxicity uniformly exhibited failure to thrive as manifested by inability to reach food and water. In the 10% and 7% doxorubicin groups, the majority of animals (>70%) also developed hemiparesis. Animals which were symptomatic for more than 24 hours were then euthanized. When 10% doxorubicin pCPP:SA polymers were administered to F344 rats, all animals died by day 40. Lowering the dosage to 7% doxorubicin polymers, we found that 11 of 20 rats died by day 100, while the remaining 9 survived to day 350. Five rats loaded with 5% doxorubicin polymers died by day 150, while the remaining 15 survived to day 350. Of the twenty rats loaded with 3% doxorubicin polymer, no toxicity related deaths were seen. These data are presented in Table I.
Table I.
Toxicity study.
| Polymer % Dox | Animals alive (#, %, N=20) | Early deaths (1–14 days) (#, %, N=20) | Late deaths (14 days+) (#, %, N=20) |
|---|---|---|---|
| 10% | 0 | 3, 15% | 17, 85% |
| 7% | 9, 45% | 1, 5% | 10, 50% |
| 5% | 15, 75% | 1, 5% | 4, 20% |
| 3% | 20, 100% | 0, 0% | 0, 0% |
Dox: Doxorubicin
Histopathological findings in toxicity study
All animals which died during the toxicity study were subjected to autopsies and their organs (brain, lung, heart, kidney, liver, spleen, and intestine) were removed and examined. Analysis of brains during the time of early death (1–14 days after polymer implant) was remarkable for the presence of hemorrhage and edema. There was minimal amount of necrosis. However, the majority of late deaths (14 days +) appeared to be secondary to tissue necrosis and local edema (Figure 3). This data is summarized in Table II. The lungs, heart, liver, spleen, kidneys, and intestine were normal without any associated hemorrhage, necrosis, or edema.
Figure 3.
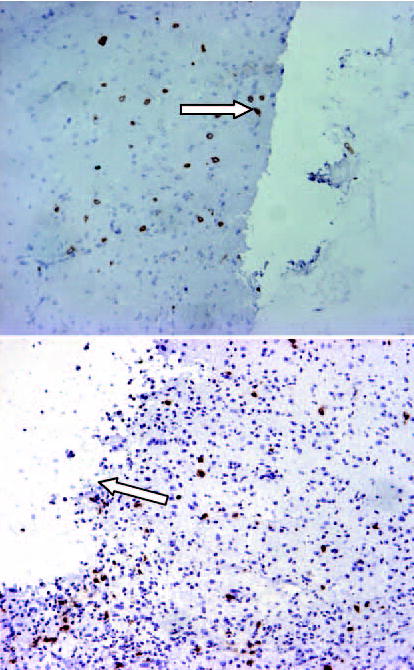
Histological analysis of animal brains obtained in the toxicity study. The figure illustrates the presence of necrosis (arrow) which was frequently seen as a late (14 days +) cause of death following DOX-polymer implant.
Table II.
Histopathological analysis of toxicity associated with doxorubicin.
| Polymer (%Dox) | Hemorrhage | Necrosis | Edema |
|---|---|---|---|
| Early Deaths (1–14 days) | |||
| 10% (n=3) | +++ | + | ++ |
| 7% (n=1) | ++ | + | ++ |
| 5% (n=1) | ++ | + | ++ |
| Late Deaths (14+ days) | |||
| 10% (n=17) | + | +++ | +++ |
| 7% (n=10) | + | +++ | +++ |
| 5% (n=4) | – | + | ++ |
Dox: Doxorubicin
Present >5 mm from tumor/polymer
Present <5 but >2 mm from tumor/polymer
Present <2 mm from tumor/polymer
In vivo intracranial efficacy
Of the 40 F344 rats which received intracranial 9L implantation, the animals receiving empty pCPP:SA polymer showed a median survival time of 21 days. Those treated with Doxorubicin polymers showed prolonged survival time: for those loaded with 5% DOX-pCPP:SA polymer, median survival time was 45 days (p<0.0001), compared to 34 day (p<0.01) survival for those loaded with 3% DOX-pCPP:SA polymers (Figure 4).
Figure 4.
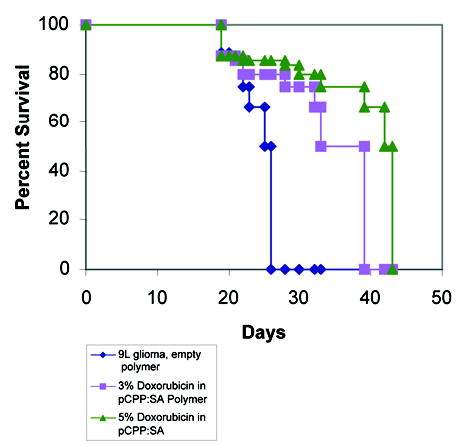
Results of in vivo intracranial efficacy trials. Compared to control animals treated with no polymers or blank polymer, animals receiving DOX had significantly extended survival. The median survival for the control groups was 21 days vs. 34 days (p<0.01) for the 3% DOX group and 45 days (p<0.0001) for the 5% DOX group.
Histopathological findings in efficacy study
In the efficacy study, all animals which died were subjected to autopsy and histological analysis. In the control group as well as DOX treated groups, the cause of death was a large intracranial tumor. Representative animals from the DOX groups were also sacrificed at days 7 and 14 after polymer implantation. The brains treated with DOX showed significantly decreased tumor burden accompanied by local tissue necrosis. While the tissue necrosis occurred within the tumor, there was also evidence of necrosis in the surrounding brain (Figure 5). This necrosis was most evident in the 5% DOX group, and extended to within 4 mm of the tumor margin in 4/10 treated animals. Only one animal in the 3% DOX group showed evidence of brain necrosis, and this was limited to 2 mm of the tumor margin.
Figure 5.
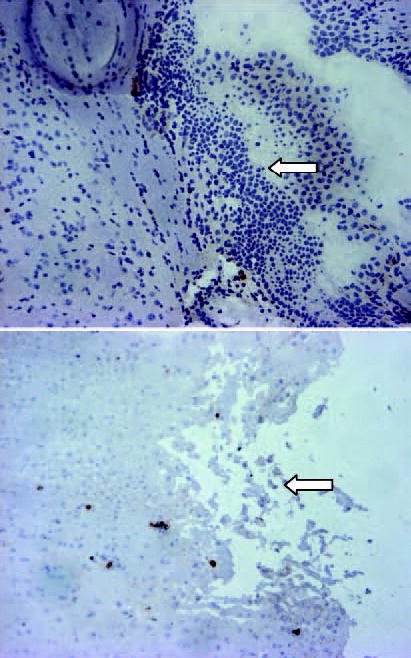
Histological analysis of animal brain treated with DOX. Representative section from (A) control animal and (B) DOX treated animal shows the presence of tumor cells (arrow in A) and tissue necrosis (arrow in B).
Discussion
Doxorubicin has been shown to inhibit tumor cell growth in numerous tumor lines and is currently used to treat a host of cancers from multiple myeloma to carcinoma of the head and neck. Previous studies have shown that doxorubicin is toxic to glioblastoma cell lines (1, 24). We have shown that doxorubicin is a potent inhibitor of rodent glioma in vitro and in vivo.
While the in vitro potency of doxorubicin is remarkable and its current indications in treating peripheral tumors have proven efficacious, doxorubicin has yet to be used successfully to treat malignant gliomas. Systemically administered doxorubicin has shown poor penetration of the blood-brain barrier and attempts to improve drug delivery, via cerebrospinal infusion, have not been very successful (15). Underlying the failure of these attempts has been the fundamental limitation in achieving therapeutic concentrations of doxorubicin in the CNS while minimizing systemic adverse side effects.
To achieve a therapeutic concentration of doxorubicin, we devised a strategy of delivering doxorubicin locally and in a controlled manner. Since the majority of malignant brain tumors recur very close to the original tumor site and given that these tumors are rarely metastatic, local delivery of anti-tumor agents represents an attractive approach (7, 12, 23). The strategy of local delivery is used clinically with carmustine-loaded pCPP:SA wafers and has demonstrated efficacy in patients with both recurrent and newly diagnosed malignant gliomas (7).
In the preceding experiments, we first confirmed that doxorubicin is an inhibitor of glioma cell lines. We found that doxorubicin antagonized the growth of all cell lines when delivered at 10–50 ng/ml. We then chose to use the 9L line for our in vivo studies. We manufactured an implantable disk made from doxorubicin and the biodegradable polymer pCPP:SA and measured its release profile. We found that anywhere from 6.5% to 21% of the doxorubicin loaded into the polymer was released over several days, depending on the percent of DOX in the polymer. The inability of doxorubicin to penetrate the blood-brain barrier made us confident that no doxorubicin was able to leave the CNS and enter systemic circulation. Furthermore, our in vitro release kinetics study may have overestimated the rate of doxorubicin release from the polymer matrix by approximating the volume of distribution of the rodent brain as an infinite sink condition (7). We can therefore conclude that the in vivo release of doxorubicin from the pCPP:SA matrix is slower and longer in duration than our in vitro data suggests. Indeed, pharmacokinetic studies of various drug-polymer combinations utilizing different animal and human models support this notion (8–10, 26) and further confirm that the release kinetics are a function of the polymer matrix rather than the chemotherapeutic agent.
Our in vivo toxicity studies indicated that polymers loaded with 3% doxorubicin were well tolerated by F344 rats. We then used these low dose doxorubicin wafer formulations for our in vivo efficacy studies and found that both the 3% and the 5% treatment groups significantly extended the survival of subjects implanted with 9L gliosarcoma intracranially. Specifically, we observed a dose-dependent trend in survival of animals treated with doxorubicin wafers. When 3% doxorubicin wafers were administered, survival extended by 52.6% compared to controls. When 5% doxorubicin wafers were administered, survival was extended by 137%. However, given the higher rate of toxicity seen with 5% polymers, we favor further development of lower concentrations of DOX-polymer wafers.
While these results are rather promising, we caution that our experimental model has key limitations in application to high-grade brain tumors in humans. First, the 9L gliosarcoma is not a perfect model of human glioma because it less invasive. Secondly, we administered treatment to our animals 5 days after tumor implantation, when the tumor was arguably smaller than those seen in most clinical scenarios.
In spite of these qualifications, the clinical prospects of local delivery of doxorubicin are promising. Currently, some patients diagnosed with malignant glioblastoma receive BCNU wafers at the time of surgery. Intraoperative delivery of the BCNU wafer has been shown to increase 6-month survival by 50% and to prolong overall survival from 23 to 31 weeks (5). While these results are significant, the prognosis for patients diagnosed with GBM remains poor. The elegance of local delivery system with a novel chemotherapeutic agent is therefore an attractive prospect.
In these studies, we have shown that doxorubicin shows robust tumoricidal activity against human glioma tumor cells lines. However, doxorubicin’s clinical use has been severely limited by its known toxicity. Furthermore, doxorubicin has not been shown to reach therapeutic levels interstitially due to difficulty penetrating the BBB. These limitations further suggest that doxorubicin may be best used when delivered locally as an adjunct to current therapeutic modalities.
Conclusion
In these studies, we have designed a successful means of delivering doxorubicin locally, which minimizes systemic side effects and delivers doxorubicin in a controlled, sustained fashion at therapeutic concentrations. We have shown that doxorubicin, when delivered in this manner, significantly prolongs survival of rodents bearing malignant brain tumors. While these initial findings point to clinical application of doxorubicin in the treatment of malignant gliomas, further work must first be done to investigate CNS toxicity associated with local delivery in more complex animal models.
Acknowledgments
This work was supported by the National Cooperative Drug Discovery Group of the National Cancer Institute (Grant U01-CA 52857), as well as a gift from the Lawrence Lomax Gift Fund. We thank Dr. Pamela Talalay for assistance in the preparation of the manuscript.
References
- 1.Abe T, Hasegawa S, Taniguchi K, Yokomizo A, Kuwano T, Ono M, Mori T, Hori S, Kohno K, Kuwano M. Possible involvement of multidrug-resistance-associated protein (MRP) gene expression in spontaneous drug resistance to vincristine, etoposide and doxorubicin in human glioma cells. Int J Cancer. 1994;58(6):860–864. doi: 10.1002/ijc.2910580619. [DOI] [PubMed] [Google Scholar]
- 2.Benjamin RS, Riggs CE, Jr, Bachur NR. Pharmacokinetics and metabolism of doxorubicin in man. Clin Pharmacol Ther. 1973;14(4):592–600. doi: 10.1002/cpt1973144part1592. [DOI] [PubMed] [Google Scholar]
- 3.Benjamin RS. Pharmacokinetics of doxorubicin (NSC-123127) in patients with sarcomas. Cancer Chemother Rep. 1974;58(2):271–273. [PubMed] [Google Scholar]
- 4.Brem H, Mahaley MS, Jr, Vick NA, Black KL, Schold SC, Jr, Burger PC, Friedman AH, Ciric IS, Eller TW, Cozzens JW, et al. Interstitial chemotherapy with drug polymer implants for the treatment of recurrent gliomas. J Neurosurg. 1991;74(3):441–446. doi: 10.3171/jns.1991.74.3.0441. [DOI] [PubMed] [Google Scholar]
- 5.Brem H, Piantadosi S, Burger PC, Walker M, Selker R, Vick NA, Black K, Sisti M, Brem S, Mohr G, Muller P, Morawetz R, Schold SC. Placebo-controlled trial of safety and efficacy of intraoperative controlled delivery of biodegradable polymers of chemotherapy for recurrent gliomas. Lancet. 1995;345(8956):1008–1012. doi: 10.1016/s0140-6736(95)90755-6. [DOI] [PubMed] [Google Scholar]
- 6.Darling JL, Thomas DG. Response of short-term cultures derived from human malignant glioma to aziridinylbenzoquinone, etoposide and doxorubicin: an in vitro phase II trial. Anticancer Drugs. 2001;12(9):753–760. doi: 10.1097/00001813-200110000-00007. [DOI] [PubMed] [Google Scholar]
- 7.DiMeco F, Li KW, Tyler BM, Wolf AS, Brem H, Olivi A. Local delivery of mitoxantrone for the treatment of malignant bran tumors in rats. J Neurosurg. 2002;97:1173–1178. doi: 10.3171/jns.2002.97.5.1173. [DOI] [PubMed] [Google Scholar]
- 8.Fleming AB, Saltzman WM. Pharmacokinetics of the carmustine implant. Clin Pharmacokinet. 2002;41(6):403–419. doi: 10.2165/00003088-200241060-00002. [DOI] [PubMed] [Google Scholar]
- 9.Fung LK, Ewend MG, et al. Pharmacokinetics of interstitial delivery of carmustine, 4-hydroperoxycyclophosphamide, and paclitaxel from a biodegradable polymer implant in the monkey brain. Cancer Res. 1998;58(4):672–684. [PubMed] [Google Scholar]
- 10.Fung LK, Shin M, et al. Chemotherapeutic drugs released from polymers: distribution of 1,3-bis(2-chloroethyl)-1-nitrosourea in the rat brain. Pharm Res. 1996;13(5):671–682. doi: 10.1023/a:1016083113123. [DOI] [PubMed] [Google Scholar]
- 11.Hau P, Fabel K, et al. Pegylated liposomal doxorubicin-efficacy in patients with recurrent high-grade glioma. Cancer. 2004;100(6):1199–1207. doi: 10.1002/cncr.20073. [DOI] [PubMed] [Google Scholar]
- 12.Liang BC, Thornton AF, Jr, Sandler HM, et al. Malignant astrocytomas: focal tumor recurrence after focal external beam radiation therapy. J Neurosurg. 1991;75:559–563. doi: 10.3171/jns.1991.75.4.0559. [DOI] [PubMed] [Google Scholar]
- 13.Lin R, Shi Ng L, et al. In vitro study of anticancer drug doxorubicin in PLGA-based microparticles. Biomaterials. 2005;26(21):4476–4485. doi: 10.1016/j.biomaterials.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 14.Mamot C, Drummond DC, et al. Epidermal growth factor receptor (EGFR)-targeted immunoliposomes mediate specific and efficient drug delivery to EGFR- and EGFRvIII-overexpressing tumor cells. Cancer Res. 2003;63(12):3154–3161. [PubMed] [Google Scholar]
- 15.Merker PC, Lewis MR, Walker MD, Richardson EP., Jr Neurotoxicity of doxorubicin (doxorubicin) perfused through the cerebrospinal fluid spaces of the rhesus monkey. Toxicol Appl Pharmacol. 1978;44(1):191–205. doi: 10.1016/0041-008x(78)90298-3. [DOI] [PubMed] [Google Scholar]
- 16.Muldoon LL, Neuwelt EA. BR96-DOX immunoconjugate targeting of chemotherapy in brain tumor models. J Neurooncol. 2003;65(1):49–62. doi: 10.1023/a:1026234130830. [DOI] [PubMed] [Google Scholar]
- 17.Neuwelt EA, Pagel M, Barnett P, Glassberg M, Frenkel EP. Pharmacology and toxicity of intracarotid doxorubicin administration following osmotic blood-brain barrier modification. Cancer Res. 1981;41(11 Pt 1):4466–4470. [PubMed] [Google Scholar]
- 18.Ohnishi T, Tamai I, Sakanaka K, Sakata A, Yamashima T, Yamashita J, Tsuji A. In vivo and in vitro evidence for ATP-dependency of P-glycoprotein-mediated efflux of doxorubicin at the blood-brain barrier. Biochem Pharmacol. 1995;49(10):1541–1544. doi: 10.1016/0006-2952(95)00082-b. [DOI] [PubMed] [Google Scholar]
- 19.Pavillard V, Kherfellah D, et al. Effects of the combination of camptothecin and doxorubicin or etoposide on rat glioma cells and camptothecin-resistant variants. Br J Cancer. 2001;85(7):1077–1083. doi: 10.1054/bjoc.2001.2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saito R, Bringas JR, et al. Distribution of liposomes into brain and rat brain tumor models by convection-enhanced delivery monitored with magnetic resonance imaging. Cancer Res. 2004;64(7):2572–2579. doi: 10.1158/0008-5472.can-03-3631. [DOI] [PubMed] [Google Scholar]
- 21.Sakai N, Kondo H, et al. Postoperative treatment for malignant intracranial tumors – especially concerning intermittent intra-carotid administration of adriamycin. No Shinkei Geka. 1984;12(3 Suppl):237–243. [PubMed] [Google Scholar]
- 22.Saul JM, Annapragada A, et al. Controlled targeting of liposomal doxorubicin via the folate receptor in vitro. J Control Release. 2003;92(1–2):49–67. doi: 10.1016/s0168-3659(03)00295-5. [DOI] [PubMed] [Google Scholar]
- 23.Sneed PK, Gutin PH, Larson DA, et al. patterns of recurrence of glioblastoma multiforme after external irradiation followed by implant boost. Int J Radiat Oncol Biol Phys. 1994;29:719–727. doi: 10.1016/0360-3016(94)90559-2. [DOI] [PubMed] [Google Scholar]
- 24.Stan AC, Casares S, Radu D, Walter GF, Brumeanu TD. Doxorubicin-induced cell death in highly invasive human gliomas. Anticancer Res. 1999;19(2A):941–950. [PubMed] [Google Scholar]
- 25.Steiniger SC, Kreuter J, et al. Chemotherapy of glioblastoma in rats using doxorubicin-loaded nanoparticles. Int J Cancer. 2004;109(5):759–767. doi: 10.1002/ijc.20048. [DOI] [PubMed] [Google Scholar]
- 26.Strasser JF, Fung LK, et al. Distribution of 1,3-bis(2-chloroethyl)-1-nitrosourea and tracers in the rabbit brain after interstitial delivery by biodegradable polymer implants. J Pharmacol Exp Ther. 1995;275(3):1647–1655. [PubMed] [Google Scholar]
- 27.Valtonen S, Timonen U, Toivanen P, Kalimo H, Kivipelto L, Heiskanen O, Unsgaard G, Kuurne T. Interstitial chemotherapy with carmustine-loaded polymers for high-grade gliomas: a randomized double-blind study. Neurosurgery. 1997;41(1):44–48. doi: 10.1097/00006123-199707000-00011. discussion 48–49. [DOI] [PubMed] [Google Scholar]
- 28.Voulgaris S, Partheni M, et al. Intratumoral doxorubicin in patients with malignant brain gliomas. Am J Clin Oncol. 2002;25(1):60–64. doi: 10.1097/00000421-200202000-00013. [DOI] [PubMed] [Google Scholar]
- 29.Walter KA, Tamargo RJ, Olivi A, Burger PC, Brem H. Intratumoral chemotherapy. Neurosurgery. 1995;37:1128–1145. [PubMed] [Google Scholar]
- 30.Walter KA, Cahan MA, Gur A, Tyler B, Hilton J, Colvin OM, Burger PC, Domb A, Brem H. Interstitial taxol delivered from a biodegradable polymer implant against experimental malignant glioma. Cancer Res. 1994;54(8):2207–2212. [PubMed] [Google Scholar]
- 31.Westphal M, Hilt DC, Bortey E, Delavault P, Olivares R, Warnke PC, Whittle IR, Jaaskelainen J, Ram Z. A phase 3 trial of local chemotherapy with biodegradable carmustine (BCNU) wafers (Gliadel wafers) in patients with primary malignant glioma. Neuro-oncol. 2003;5(2):79–88. doi: 10.1215/S1522-8517-02-00023-6. [DOI] [PMC free article] [PubMed] [Google Scholar]


