Abstract
Heat shock protein 90 (Hsp90) is an emerging therapeutic target of interest for the treatment of cancer. Its role in protein homeostasis and the selective chaperoning of key signaling proteins in cancer survival and proliferation pathways has made it an attractive target of small molecule therapeutic intervention. 17-Allylamino-17-demethoxygeldanamycin (17-AAG), the most studied agent directed against Hsp90, suffers from poor physical-chemical properties that limit its clinical potential. Therefore, there exists a need for novel, patient-friendly Hsp90-directed agents for clinical investigation. IPI-504, the highly soluble hydroquinone hydrochloride derivative of 17-AAG, was synthesized as an Hsp90 inhibitor with favorable pharmaceutical properties. Its biochemical and biological activity was profiled in an Hsp90-binding assay, as well as in cancer-cell assays. Furthermore, the metabolic profile of IPI-504 was compared with that of 17-AAG, a geldanamycin analog currently in clinical trials. The anti-tumor activity of IPI-504 was tested as both a single agent as well as in combination with bortezomib in myeloma cell lines and in vivo xenograft models, and the retention of IPI-504 in tumor tissue was determined. In conclusion, IPI-504, a potent inhibitor of Hsp90, is efficacious in cellular and animal models of myeloma. It is synergistically efficacious with the proteasome inhibitor bortezomib and is preferentially retained in tumor tissues relative to plasma. Importantly, it was observed that IPI-504 interconverts with the known agent 17-AAG in vitro and in vivo via an oxidation-reduction equilibrium, and we demonstrate that IPI-504 is the slightly more potent inhibitor of Hsp90.
The heat shock response, first identified in 1962 by Ritossa (1), was initially characterized as the induction of select polypeptides in response to an acute cellular heat shock. These polypeptides were proteins that bound to partially unfolded proteins to prevent their aggregation and assist in their refolding (2, 3), and were termed chaperones. Of the heat shock proteins, heat shock protein 90 (Hsp90) in particular has been the subject of intense investigation. Work over the last decade has revealed not only a general protein chaperone role for Hsp90, but also a specific chaperone role in the binding of select conformations or metastable forms of signaling proteins (clients), thereby attenuating their signaling activity (4–6). Client proteins include the targets of key cancer survival and proliferation pathways, including Akt, Bcr-Abl, Her-2, mutant EGFR, and c-Kit, many of which are the subject of individual investigation for points of therapeutic intervention. Therefore, two functions of Hsp90 exist: (i) a general protein chaperone function (protein homeostasis) and (ii) a specific function to modulate the integrity of cell-signaling pathways through the proper folding of pathway members that are Hsp90 clients.
Multiple myeloma (MM) is a neoplasm of terminally differentiated B cells (plasma cells) (7). Because of the high protein secretory load of the myeloma cell, it is highly dependent on proper protein homeostasis – protein chaperones (e.g., cytoplasmic Hsp90 and its endoplasmic reticulum homolog Grp94) as well as other protein homeostatic mechanisms (e.g., ubiquitin-proteasome pathway, whose role is to degrade damaged or unfolded proteins) play key roles in this process. The recent regulatory approvals of the proteasome inhibitor bortezomib for the treatment of MM validates the clinical utility of disrupting protein homeostasis in treating myeloma (8). Work has also demonstrated the importance of the NF-κB pathway for the growth and survival of myeloma cells (9). The Hsp90 client and NF-κB pathway member IkB kinase (IKK) is a key regulator of the signaling in this pathway (10). Therefore myeloma is an attractive candidate for therapeutic Hsp90 inhibition (11).
Geldanamycin is a natural product inhibitor of both Hsp90 and Grp94, with potent and broad anti-cancer properties. Its mechanism was unknown for many years until it was characterized by Whitesell et al. (12) as a direct inhibitor of the ATPase function of Hsp90. The natural product was deemed too toxic to treat patients and subsequent modifications were made to geldanamycin to improve its therapeutic index. In particular, the 17-allylamine derivative, 17-allylamino-17-demethoxygeldanamycin (17-AAG) has received considerable attention because of its more favorable pharmacologic properties. As a potent reversible inhibitor of Hsp90, it has demonstrated efficacy in both hematologic as well as solid tumor animal models of cancer and is now the subject of multiple clinical trials (13). Notwithstanding its improved pharmacologic properties, 17-AAG has poor pharmaceutical properties, namely water insolubility. Its solubility in aqueous solution is ≈50 μg/ml and requires the addition of organic excipients such as DMSO or polyoxyl castor oil (Cremophor) (13). DMSO is known to cause adverse events when administered to patients (nausea, vomiting, mal-odor) and to be potentially hepatotoxic and cardiotoxic (14, 15). Cremophor is known to induce hypersensitivity reactions and anaphylaxis and requires pretreatment with anti-histamines and steroids (16). Clinical trials with 17-AAG in these excipients may confound the true maximum tolerated dose of 17-AAG and identification of the optimal dosing regimen in patients. Therefore extensive effort has been directed toward the development of novel, water-soluble Hsp90 inhibitors.
The work contained in this article will describe the synthesis of 17-allylamino-17-demethoxygeldanamycin hydroquinone hydrochloride (IPI-504), a water-soluble Hsp90 inhibitor, and its in vitro and in vivo biological characterization in MM.
Results and Discussion
Discovery of IPI-504.
In an effort to synthesize water-soluble analogs of 17-AAG, it was recognized that the benzoquinone of 17-AAG could be chemically reduced to its hydroquinone analog. Literature precedence suggested hydroquinone derivatives of geldanamycin were prone to air oxidation and readily converted back to their quinone forms (17, 18). However, it was realized that protonation of the aniline nitrogen of 17-AAG hydroquinone decreases electron density in the aromatic ring, thus reducing the oxidative potential of the hydroquinone (Fig. 5, which is published as supporting information on the PNAS web site). The hydroquinone hydrochloride salt (IPI-504) can be isolated in high purity as a solid and is less prone to air oxidation than the free base hydroquinone (19). As a stable hydrochloride salt, IPI-504 exhibits dramatically different physical-chemical properties compared with 17-AAG. IPI-504 is readily soluble in water (>250 mg/ml) compared with 17-AAG (≈50 μg/ml), thereby enabling aqueous delivery formulations of IPI-504 that do not require organic solubilizing agents which have their own limitations.
IPI-504 and 17-AAG Interconvert in vitro and in Vivo.
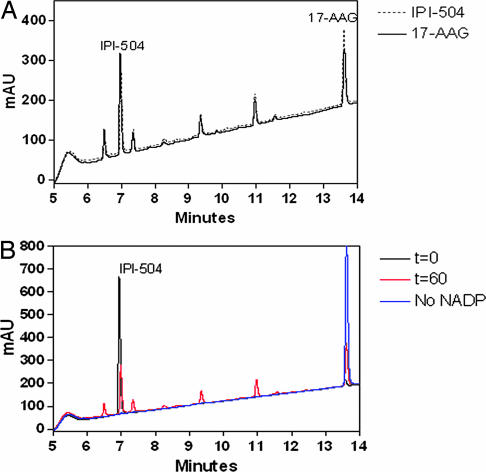
To compare the metabolic profile of the water-soluble Hsp90 inhibitor IPI-504 with 17-AAG, human liver microsomes were incubated for 1 h at 37°C with 50 μg/ml IPI-504 or 17-AAG. To stabilize the hydroquinone, the reactions were quenched with a mixture of methanol and an acidic buffer containing ascorbic acid as an antioxidant. The overlay of the HPLC chromatograms (Fig. 1A) shows that IPI-504 and 17-AAG exhibit the same metabolic profile with respect to the major metabolites, of which some have already been previously identified for 17-AAG (20). Comparison of the chromatogram for the 60 min incubation in the presence or absence of the enzymatic cofactor NADPH and the chromatogram for the incubation at t = 0 min shows that all of the metabolites are formed in a NADP/NADPH-dependent manner (Fig. 1B).
Fig. 1.
Representative HPLC chromatograms of a 60-min time point of IPI-504 or 17-AAG incubated with human liver microsomes. (A) IPI-504 (50 μg/ml) (dashed line) or 17-AAG (solid line) was incubated in human liver microsomes. The reactions were quenched at 60 min with an equal volume of 2:1 methanol:270 mM citrate (pH 3), 0.5% (wt/vol) EDTA, and 0.5% (wt/vol) ascorbate to preserve IPI-504 and analyzed by HPLC (λ = 230 nm). (B) IPI-504 (50 μg/ml) was incubated with 0.8 mg/ml human liver microsomes for 0 (black line) and 60 min (red line), as well as for 60 min without NADP as a control reaction (blue line). The reaction was quenched by addition of an equal volume of 2:1 methanol:270 mM citrate (pH 3), 0.5% (wt/vol) EDTA, and 0.5% (wt/vol) ascorbate to preserve IPI-504 and analyzed by HPLC (λ = 230 nm).
A surprising result, however, was the appearance of IPI-504, the hydroquinone form of 17-AAG, in the incubation of 17-AAG with human liver microsomes and in the incubation reaction with IPI-504 (Fig. 1A), even though it was expected to oxidize to 17-AAG under the neutral pH conditions of a microsome assay.
To further investigate this potential physiological equilibrium between IPI-504 and 17-AAG, both compounds were incubated with human liver microsomes, the reactions quenched at incremental time points and the peak areas for IPI-504 and 17-AAG determined (Fig. 2). The results clearly demonstrate that incubation of 17-AAG with human liver microsomes leads to a hydroquinone-quinone equilibrium after ≈30 min in human liver microsomes at 50 μg/ml substrate concentration. IPI-504 initially oxidizes to 17-AAG as expected under physiological conditions. After ≈10 min, however, the amount of IPI-504 increases again because of an enzymatic reduction in the microsomal preparation of hepatocytes, which is likely to be performed by oxidoreductases (21). As in the case of 17-AAG, an enzymatic reaction produces the hydroquinone form (i.e., the free base of IPI-504) after 10 min, reaching equilibrium at 30 min with the oxidation of IPI-504 to 17-AAG. The further decrease in the abundance of both compounds is due to further metabolism.
Fig. 2.
Percentage of total peak area of IPI-504 and 17-AAG at different time points of an incubation of IPI-504 (A) or 17-AAG (B) in human liver microsomes. IPI-504 or 17-AAG (50 μg/ml) was incubated with 0.8 mg/ml human liver microsomes and quenched at incremental time points by addition of an equal volume of 2:1 methanol:270 mM citrate (pH 3), 0.5% (wt/vol) EDTA, and 0.5% (wt/vol) ascorbate. The levels of IPI-504 and 17-AAG were analyzed by HPLC and plotted as percentage of total peak area. Each point is the mean ± range for two samples.
In light of the fact that IPI-504 and 17-AAG interconvert because of oxidation of the hydroquinone and enzymatic reduction of the quinone, we wanted to investigate the assumption that this interconversion should lead to similar pharmacokinetic profiles for IPI-504 and 17-AAG in vivo. Therefore, IPI-504 or 17-AAG were administered intravenously to BALB/c mice at 50 mg/kg. At selected time points, plasma was collected and immediately quenched with an acidic buffer containing antioxidant to stabilize the hydroquinone. The samples were then analyzed by online extraction liquid chromatography–tandem mass spectrometry (LC-MS/MS) without further sample preparation to determine the plasma concentration-time profile of IPI-504, 17-AAG and the major metabolite 17-(amino)-17-demethoxygeldanamycin (17-AG). Fig. 3 shows that i.v. administration of either IPI-504 or 17-AAG in BALB/c mice leads to very similar plasma concentration-time profiles for IPI-504, 17-AAG and 17-AG. In both cases, a rapid interconversion between IPI-504 and 17-AAG was observed which leads to an equilibrium between the hydroquinone (IPI-504) and the quinone (17-AAG) that is established by 5 min postinjection (the first time point at which blood is sampled). Similar experiments have been conducted in rats and cynomolgus monkeys with the same conclusions (M. H. Abdelhameed, M. Guirguis, J. Carpenter, R. Poole, J.R.S., and J.A.B., unpublished data, personal communication), supporting that IPI-504 and 17-AAG likely interconvert in all species, including humans. The important implication of this finding is that patients who have been administered 17-AAG have previously been exposed to IPI-504.
Fig. 3.
Comparison of pharmacokinetics of IPI-504 and 17-AAG in mice. (A) Plasma Concentration-Time Profiles of IPI-504, 17-AAG, and 17-AG in BALB/c mice after i.v. administration of IPI-504 at 50 mg/kg. (B) Plasma concentration-time profiles of IPI-504, 17-AAG, and 17-AG in BALB/c mice after i.v. administration of 17-AAG at 50 mg/kg. Test articles were administered via the tail vein, plasma was collected at selected time points and quenched to stabilize the hydroquinone IPI-504, and samples were analyzed by online extraction LC-MS/MS (see Materials and Methods).
We were therefore able to demonstrate that IPI-504, the hydroquinone, and 17-AAG, the quinone, interconvert in vitro and in vivo, demonstrating that IPI-504 is a reduction product of 17-AAG. This enzyme-mediated quinone reduction process has been reported for other quinone-containing molecules, including a number of quinone-based anti-cancer agents (22–24). In addition, it has also been proposed that 17-AAG is a substrate for NADPH-quinone oxidoreductase 1 (NQO1) (25, 26). It is, therefore, likely that 17-AAG exists in an equilibrium with its hydroquinone state.
The development of IPI-504 required analytical methods in which the hydroquinone is stabilized to allow its quantitative detection. An acidic citrate quench buffer containing ascorbate as an antioxidant allowed the stabilization of the hydroquinone in plasma and other solutions and therefore enabled the detection of not only IPI-504, but also discover the hydroquinone as a previously unidentified major metabolite of 17-AAG. The incubation of either IPI-504 or 17-AAG with human liver microsomes clearly demonstrated that both compounds exist in a dynamic equilibrium because of an enzymatic reduction of 17-AAG and an oxidation of IPI-504 to 17-AAG under physiological conditions (Fig. 1).
Different human cancer and normal cell lines were incubated with IPI-504 and 17-AAG and the same equilibrium between IPI-504 and 17-AAG was observed in the cellular fraction (data not shown). This redox equilibrium therefore seems to be mediated by multiple oxidoreductases including the NADPH-quinone oxidoreductase NQO1 which is expressed in a variety of tissues and has previously been proposed to affect 17-AAG and also other quinone drugs (21, 26, 27).
Whether the hydroquinone-quinone equilibrium of IPI-504 and 17-AAG leads to other cytotoxic effects independent of Hsp90 inhibition is unclear at this point. Some work suggests that NQO1 as an oxidoreductase increases the sensitivity of cells to 17-AAG (25, 26), but it is not clear whether this effect is due only to an increased affinity of the hydroquinone for Hsp90 or to other cytotoxic mechanisms that are caused by a redox equilibrium.
Biochemical Characterization of IPI-504.
To assess the intrinsic Hsp90-binding activity of IPI-504 and to determine the active component in the IPI-504:17-AAG dynamic equilibrium, a fluorescence polarization-based Hsp90 competition binding assay was developed. This assay measures the affinity of IPI-504 or 17-AAG for purified human Hsp90 protein by their ability to compete with a fluorescent probe-labeled geldanamycin analogue. Because of the conversion of IPI-504 to 17-AAG at neutral pH in the presence of oxygen, the experiments were conducted in a nitrogen environment to prevent oxidation of IPI-504. IPI-504 reproducibly bound to Hsp90 with an apparent affinity ≈2-fold higher than that of 17-AAG (EC50 = 63 ± 13 nM and 119 ± 23 nM, respectively) (Fig. 6A, which is published as supporting information on the PNAS web site). Time-dependent binding studies also support the reversible nature of IPI-504 binding to Hsp90 (data not shown). These findings demonstrate that whereas IPI-504 and 17-AAG are both active, IPI-504 is ≈2-fold more potent. Based on previously solved crystal structures of Hsp90 with geldanamycin analogs, a model may be proposed to explain the tighter binding of IPI-504 relative to 17-AAG (28). Conversion of the quinone moiety in 17-AAG to hydroquinone in IPI-504 should allow better hydrogen-bonding interactions between 17-NH, phenol OH groups at C18 and C-21 positions, and hydrophilic residues (e.g., D54 and K112) in the binding pocket of Hsp90.
Further experiments were conducted to test the binding affinity of IPI-504 for Grp94, a closely related Hsp90 homolog that functions in the endoplasmic reticulum. The EC50 values for binding of IPI-504 and 17-AAG to Grp94 were 119 ± 18 nM and 124 ± 53 nM, respectively (Fig. 6B). These results suggest that inhibition of Grp94 may also be involved in the mechanism of cytotoxicity by IPI-504 and 17-AAG. One potential caveat is that the inhibition of Hsp90 and Grp94 by IPI-504/17-AAG may be altered in the cellular environment by the presence of cochaperones and client proteins, as Kamal et al. (29) suggested the existence of a high affinity Hsp90 chaperone complex in tumor cells. However, recent work by Gooljarsingh et al. (30) does not support a different affinity between Hsp90 chaperone complex and 17-AAG relative to Hsp90 alone.
In summary, our findings suggest that tissues with a strong reducing environment may lead to an equilibrium favoring IPI-504, the more potent inhibitor of Hsp90, over 17-AAG. It is well known that cancers generally produce hypoxic environments (31). It has also been reported that some cancers also have increased levels of NQO1, further favoring the IPI-504 hydroquinone (32). We believe that the reversible equilibrium between IPI-504 and 17-AAG may provide one of several sources of their therapeutic selectivity against cancer versus normal cells, as supported by recent work of Guo et al. (26).
Cytotoxicity of IPI-504.
Loss of Hsp90 function through pharmacologic inhibition results in both the disruption of general protein homeostasis as well as the destabilization and ultimate degradation of Hsp90 client proteins. In many cancer cells, general protein homeostasis and the integrity of Hsp90 client-signaling pathways are essential for the survival and proliferation of the cell. Therefore, experiments were conducted in myeloma cancer and normal cells to characterize the cytotoxicity of IPI-504 for these cell types. In incubations with normal human peripheral blood leukocytes (PBLs) and normal human diploid fibroblasts (NHDFs), the EC50 for cytotoxicity was multimicromolar or higher (Fig. 7A, which is published as supporting information on the PNAS web site). In contrast, when two human MM cell lines, MM1.s and RPMI-8226, were treated with increasing concentrations of IPI-504 and 17-AAG and the effect on viability evaluated by an Alamar Blue assay, they were both significantly more sensitive than the non-cancer cells. Both IPI-504 and 17-AAG affected cell viability with a similar potency in these cancer-cell lines (EC50 = 307 ± 51 nM and 306 ± 38 nM, respectively, in MM1.s and RPMI-8226 cells) (Fig. 7B). This finding demonstrates that IPI-504 is selectively cytotoxic to myeloma cancer cells when compared with normal cells.
Proteasome inhibition also disrupts general protein homeostasis. It was therefore hypothesized that IPI-504 may be synergistic with the proteasome inhibitor bortezomib in its cytotoxicity against the myeloma cell, which is heavily dependent on proper protein homeostasis for secretion of Ig. MM1.s cells were treated with varying combinations of bortezomib and IPI-504 and analyzed for cytotoxicity 72 h later. At combinations where the individual agents were at subeffective concentrations, the combination of bortezomib and IPI-504 was highly synergistic, as earlier suggested for a combination of bortezomib and Hsp90 inhibitors (33–35). Both compounds alone at 4 nM killed <10% of the cells, whereas the combination killed ≈50% of the MM1.s cells (Fig. 8, which is published as supporting information on the PNAS web site). This synergistic effect could be explained in part by the accumulation of ubiquitinated and cytotoxic proteins resulting from the destabilization of Hsp90 client proteins by IPI-504 and the inhibition of their degradation by bortezomib (34). Another combination of interest has recently emerged, demonstrating that the hyperacetylation of Hsp90 disrupted its chaperone function (36), and that the combination of histone deacetylase inhibitors with Hsp90 inhibitors exerted synergistic apoptotic effects (37), further supporting the rationale for combination studies.
in vivo Efficacy in MM and Tumor Pharmacokinetics.
The combined biochemical and cellular data on IPI-504 supported further investigation of this compound in in vivo models of MM. Before determination of in vivo efficacy, we investigated the tumor pharmacokinetics of IPI-504 in RPMI-8226 tumor-bearing mice after an i.v. bolus injection of 50 mg/kg. Tumor concentrations of IPI-504, 17-AAG, and the major and pharmacologically active metabolite 17-AG were determined by online extraction LC-MS/MS after homogenization in an acidic quench buffer containing ascorbic acid as an antioxidant. In all analyzed samples, IPI-504, 17-AAG and 17-AG were detected in tumor up to 48 h after i.v. administration of IPI-504. Fig. 4A shows a histogram of the combined concentrations of IPI-504, 17-AAG and 17-AG in the tumor tissue at 4, 24 and 48 h posttreatment. Comparison to the plasma pharmacokinetics (Fig. 3) also shows a preferred tumor retention of IPI-504, 17-AAG and 17-AG. At 48 h, all three compounds persist in tumor tissue at pharmacologically active concentrations (720 nM) (Fig. 4A). The specific mechanism for drug retention is unknown, but may be attributed to larger amounts of Hsp90 in tumor cells or to the higher affinity of activated Hsp90 in tumor cells for IPI-504, 17-AAG, and 17-AG (29). The rapid clearance of IPI-504, 17-AAG and 17-AG from the vascular compartment coupled with the retention of drug at biologically active levels in the tumor for at least 48 h may contribute to the therapeutic window for IPI-504.
Fig. 4.
Tumor retention and efficacy in MM xenograft models. (A) Selective tumor retention of IPI-504 in RPMI-8226 tumor-bearing mice after i.v. administration of 50 mg/kg IPI-504. RPMI-8226 tumor-bearing mice were administered with test article via the tail vain, and tumor samples were collected at selected time points. Tumor samples were homogenized, and the homogenate supernatants were analyzed by online extraction LC-MS/MS. (B) In vivo efficacy of IPI-504 in an RPMI-8226 xenograft model as a single agent and in combination with bortezomib. Tumor-bearing mice received either (i) IPI-504 and bortezomib vehicle (♦), (ii) IPI-504 at 100 mg/kg (300 mg/m2), twice weekly (▴), (iii) bortezomib 0.3 mg/kg (0.9 mg/m2), twice weekly (○), or (iv) IPI-504 at 75 mg/kg (225 mg/m2) and bortezomib at 0.3 mg/kg (0.9 mg/m2), twice weekly (■), and tumor dimensions were measured twice weekly, as well as λ light chain concentrations 4 h after the last dose. (Inset) Point A, IPI-504 75 mg/kg and bortezomib 0.3 mg/kg; point B, bortezomib 0.3 mg/kg; point C, IPI-504 100 mg/kg; and point D, vehicle).
Based on the tumor pharmacokinetics and the cancer-selective cytotoxicity of IPI-504, an in vivo efficacy study was designed with twice weekly administration of IPI-504 to further explore the in vivo anti-tumor activity of IPI-504. The in vivo experiment was designed to test two hypotheses; first that IPI-504 as a single agent would be active in myeloma, a disease dependent on general protein homeostasis; second, that IPI-504 would be synergistic with the proteasome inhibitor bortezomib. RPMI-8226 tumor-bearing mice were randomly assigned to one of four treatment arms: (i) vehicle, (ii) IPI-504 alone, (iii) bortezomib alone, or (iv) IPI-504 plus bortezomib. As a single agent, IPI-504 delayed tumor progression (total growth inhibition = 73%) when administered intravenously to mice at 100 mg/kg, twice per week (Fig. 4B). Similarly, bortezomib delayed tumor progression (total growth inhibition = 62%) when administered intravenously at 0.3 mg/kg, twice per week. The combination of 75 mg/kg IPI-504 and 0.3 mg/kg bortezomib resulted in complete and durable tumor regression. After 30 days of dosing, drug administration was stopped and animals were monitored for tumor regrowth. Animals were monitored for an additional 42 days, and no tumor regrowth was seen after cessation in the animals treated with IPI-504 and bortezomib.
Multiple myeloma cells are known to secrete large amounts of immunoglobulins which have been used as surrogate markers for efficacy in clinical trials (38, 39). RPMI-8226 cells, in particular, secrete large amounts of λ light chain. To correlate the in vivo efficacy results with plasma λ light chain concentrations as a systemic surrogate marker of efficacy, we determined its concentration in the plasma of treated animals 4 h after the last dose (Fig. 4B Inset). The results clearly demonstrate that the reduction in tumor burden directly correlates with λ light chain concentration in the plasma. Both IPI-504 and bortezomib treatment lead to a reduction of the plasma concentration. The combination treatment with both drugs, however, leads to a complete loss of λ light chain in the plasma.
These data provide strong preclinical rationale for the exploration of IPI-504 as a single agent in treating patients with MM and for the exploration of combinations of IPI-504 with bortezomib. In addition, it is important to note that clinical investigations of Hsp90 and proteasome inhibitor combinations are currently underway in Phase 1b trials.
In summary, we have described the development of IPI-504, an anti-cancer agent currently in Phase I clinical trials whose therapeutic window is maximized by preferentially exerting its effects on cancer versus normal cells at the biochemical, cellular, and in vivo level. The combined selectivity at the biochemical, cellular, and whole-organism level serve to maximize the therapeutic window for IPI-504.
Within the broad range of potential cancers that can be treated by an Hsp90 inhibitor, this paper specifically characterizes the effect of IPI-504 in MM. It is demonstrated that myeloma can be effectively treated both in vitro and in in vivo murine models by agents that disrupt protein homeostasis such as IPI-504 and bortezomib. Furthermore, the combination of these two agents is synergistic, suggesting an opportunity for the clinical treatment of MM.
Lastly, it was revealed that IPI-504 is a water-soluble analog of 17-AAG that allows a more favorable formulation for clinical development. Unknowingly it has been previously administered to humans through the administration of its quinone analog 17-AAG and its subsequent interconversion. Whereas 17-AAG has been the subject of extensive investigation over the last several years, its potential as a drug has been limited by its unfavorable pharmaceutical properties. Herein, we demonstrated how the pharmaceutical liabilities of 17-AAG have been solved through the synthesis of IPI-504, the hydroquinone hydrochloride of 17-AAG, which is highly soluble in aqueous formulations and does not require the addition of organic solvents or emulsification agents. In the process of characterizing the interconversion between 17-AAG and IPI-504, it was revealed that the slightly more potent component of 17-AAG is manifested in the unexpected formation of IPI-504.
Materials and Methods
Materials.
Pooled human liver microsomes were obtained from Xenotech (Lenexa, KS). NADP and glucose-6-phosphate, glucose-6-phosphate dehydrogenase, MgCl2, potassium phosphate, citric acid, and ascorbic acid were all purchased from Sigma. Water (HPLC grade) was obtained from J. T. Baker (Philipsburg, NJ). Acetonitrile (HPLC grade) was purchased from EMD Chemicals (Gibbstown, NJ), and formic acid was supplied by Fluka Chemie (Buchs, Switzerland). Calcium disodium EDTA was obtained from Spectrum (New Brunswick, NJ).
Synthesis of IPI-504.
For details regarding the synthesis, please refer to Supporting Materials and Methods, which is published as supporting information on the PNAS web site, or to Ge et al. (19).
Interconversion of IPI-504 and 17-AAG in vitro in Human Liver Microsomes.
A 0.5-ml reaction mixture containing 0.8 mg/ml liver microsomes, 1 mM NADP, 5 mM glucose-6-phosphate, 0.5 units/ml glucose-6-phosphate dehydrogenase, 3 mM magnesium chloride, 1 mM EDTA, 50 mM potassium phosphate (pH 7.4), and 80 μM compound was incubated at 37°C for 0, 5, 10, 15, 30, 45, and 60 min. Control reactions without NADP were also performed. After incubation, the reaction was stopped by the addition of 0.5 ml of the quench buffer [2:1 (vol/vol) mixture of methanol and 270 mM citrate (pH 3), 0.5% (wt/vol) EDTA, 0.5% (wt/vol) ascorbate]. The reaction was centrifuged for 3 min at 18,000 × g at 4°C. Twenty-five microliters of the supernatant was analyzed by HPLC on an Atlantis 2.1 × 150 mm dC18 column (Waters, Milford, MA) at a flow rate of 0.3 ml/min. The mobile phase initially started at 95% H2O, 0.1% (vol/vol) formic acid, and 5% acetonitrile 0.1% (vol/vol) formic acid, increasing to 30% organic over 1 min and then to 95% organic over 13 min. The peak area for the hydroquinone form of 17-AAG was determined by its absorbance at 230 nm and the quinone form at 335 nm. The chromatographic overlays were performed at 230 nm.
Comparative Pharmacokinetics and Interconversion of IPI-504 and 17-AAG in Mice.
BALB/c mice were randomly assigned to one of two treatment groups, and 50 mg/kg IPI-504 (in 50 mM citrate, 50 mM ascorbate, and 2.4 mM calcium disodium EDTA, pH 3.0) or 50 mg/kg 17-AAG (in N,N-dimethyl acetamide, Cremophor EL, and 0.9% saline at a ratio 5:7.5:87.5) were administered i.v. via the tail vein. At 5, 15, and 30 min and 1, 2, 4, 6, and 24 h postdose, mice (n = 3 per time point) were euthanized according to an institutional animal care and use committee protocol with carbon dioxide, and blood was collected by cardiac puncture. Plasma was immediately separated and quenched with ice-cold 75 mM citrate, 0.1% (wt/vol) EDTA, 0.1% (wt/vol) ascorbate at a ratio of 1:9 plasma:quench buffer and stored at −80°C.
Quenched plasma samples were thawed at room temperature and immediately transferred on ice after thawing. A 1:1 dilution was performed with ice cold, nitrogen-sparged 75 mM citrate, 0.1% (wt/vol) EDTA, 0.1% (wt/vol) ascorbate, pH 3 quenching buffer, containing 25 ng/ml deuterated 17-AAG as an internal standard, and samples were immediately analyzed by LC-MS/MS.
A standard curve was prepared in quenched plasma on ice from 15.6 ng/ml to 2 μg/ml (plasma concentration) and diluted in the same way with internal standard solution as the samples. For concentrations higher than 2 μg/ml plasma, a standard curve at higher concentrations was prepared, and both samples and standard curve were diluted at a higher ratio.
Sample analysis was performed on an Aria 2300 system from Cohesive Technologies (Franklin, MA) with a 4000 Q Trap mass spectrometer from Applied Biosystems (Foster City, CA). Samples were injected on a Cyclone-P turbulent flow column for extraction and then transferred to the analytical column (Symmetry IS, 2.1 × 20 mm, 5 μm from Waters) with 80% methanol in H2O, 0.1% (vol/vol) formic acid. Compounds were eluted from the analytical column with a 5-min gradient from 0–100% acetonitrile in H2O, 0.1% (vol/vol) formic acid. Mass spectrometric detection of IPI-504, 17-AAG, and 17-AG, as well as deuterated 17-AAG as the internal standard, was performed by multiple reaction monitoring (MRM) in negative mode with the following transitions for each compound: 17-AAG (584.5→541.3), 17-AG (544.5→501.3), IPI-504 (586.5→543.3), deuterated 17-AAG (589.5→546.3). The data were acquired and processed by using the software Analyst 1.4 (Applied Biosystems). The pharmacokinetics of IPI-504 and 17-AAG were analyzed by WinNonlin 4.1 (Pharsight, Mountain View, CA). The plasma concentration-time data were computed by the noncompartmental method, and PK parameters were generated for IPI-504, 17-AAG, and 17-AG.
Competition Binding Assay for Hsp90.
For details regarding the synthesis, please refer to Ge et al. (19).
Cytotoxicity Experiments.
For details regarding the synthesis, please refer to Supporting Materials and Methods.
Tumor Retention of IPI-504 in RPMI-8226 Tumor-Bearing Mice.
IPI-504 was formulated in a buffer containing 50 mM sodium citrate, 50 mM ascorbate, and 2.4 mM disodium EDTA, adjusted to pH 3.3 to achieve a final concentration of 10 mg/ml.
RPMI-8226 cells were harvested from cultures grown in vitro in RPMI medium 1640 supplemented with heat-inactivated 10% (wt/vol) FBS and 100 units/ml penicillin/streptomycin at 37°C under a humidified 95%/5% (vol/vol) mixture of air and CO2. Cells were washed twice by using sterile Hepes-buffered saline (HBS) and suspended in HBS to a concentration of 1 × 108 viable cells per ml. Twelve female Nu/Nu nude mice (≈20 g) were purchased from Charles River Laboratories (Worcester, MA). RPMI-8226 cells (1 × 107 cells per mouse) were implanted in the right flank. When tumor volume reached ≈200–500 mm3 (≈4 weeks postimplantation), animals received a single i.v. dose of 50 mg/kg IPI-504 via the tail vein. At 4, 24, and 48 h posttreatment, the animals were killed with carbon dioxide, and tumors were removed and stored at −80°C until analyzed. Four animals were used for each time point. Tumor samples were homogenized in an ice-cold, nitrogen-sparged 1:1 solution of MeOH:150 mM citrate, 0.2% (wt/vol) EDTA, and 0.2% (wt/vol) ascorbate (pH 3.0) for 1 min in an ice/water bath with a homogenizer at 17,500 rpm (Ultra-Turrax, T25, IKA Werke, Staufen, Germany). Samples were centrifuged for 5 min at 4°C at 18,000 × g. The supernatants were diluted 1:1 with ice-cold, nitrogen-sparged 75 mM citrate, 0.1% (wt/vol) EDTA, and 0.1% (wt/vol) ascorbate (pH 3) containing 25 ng/ml deuterated 17-AAG as internal standard and analyzed by LC-MS/MS analysis. The standard curve was prepared for IPI-504, 17-AAG, and 17-AG in 1:1 MeOH:150 mM citrate, 0.2% (wt/vol) EDTA, and 0.2% (wt/vol) ascorbate (pH 3.0); diluted 1:1 with ice-cold, nitrogen-sparged 75 mM citrate, 0.1% (wt/vol) EDTA, and 0.1% (wt/vol) ascorbate (pH 3.0) containing 25 ng/ml deuterated 17-AAG as internal standard; and analyzed by LC-MS/MS.
Efficacy Study in Human RPMI-8226 MM Xenograft Model.
Human MM RPMI-8226 cells were cultured in RPMI medium 1640 supplemented with 10% FBS plus 2 mM l-glutamine plus 100 μg/ml penicillin/streptomycin. Cells were incubated at 37°C in a humidified incubator with 5% CO2, trypsinized from the monolayer cultures, washed twice with DMEM and 10% FBS, counted, and resuspended in serum-free DMEM Ham's F12.
Four- to six-week-old male SCID/nonobese diabetic (NOD) mice (Taconic Laboratories, Hudson, NY) were inoculated s.c. with RPMI-8226 cells (1 × 107 cells) into the dorsal side of the right rear leg by using a 25-gauge needle. When tumors reached an average volume of ≈50 mm3, animals were randomly assigned to one of the treatment groups. Animals were divided into four groups (n = 6–8 mice per group) as follows: (i) IPI-504 and bortezomib vehicle, (ii) IPI-504 at 100 mg/kg (300 mg/m2), twice weekly (n = 7), (iii) bortezomib 0.3 mg/kg (0.9 mg/m2), twice weekly (n = 8), and (iv) IPI-504 at 75 mg/kg (225 mg/m2) and bortezomib at 0.3 mg/kg (0.9 mg/m2), twice weekly (n = 8). Each animal was administered the test article by using the schedule outlined above via the tail vein in a volume of 0.1 ml over ≈10 s. Tumor dimensions were measured twice weekly with digital calipers, and tumor volumes were calculated with the formula (width2 × length)/2.
Plasma from the tumor-bearing mice treated with IPI-504 and/or bortezomib was collected 4 h after the last dose, and λ light chain production was measured by ELISA. Ninety-six-well microtiter plates (Maxisorp, NUNC) were coated with 0.5 μg of goat F(ab)2 anti-human Ig (IgM + IgG + IgA, H + L) (Southern Biotechnology) overnight. The plates were then washed twice with PBS and 0.1% Tween 20, blocked for 2 h at room temperature with PBS and 20% FBS, and washed again. One hundred microliters of mouse plasma was then added to the coated wells at serial 2-fold dilutions for 2 h at room temperature and washed, and the bound human Ig was detected with goat anti-human lambda-HRP (Southern Biotechnology, Birmingham, AL). After washing, 50 μl of tetramethylbenzidine (TMB) substrate (Amersham, Piscataway, NJ) and 1 M sulfuric acid were added for 15 min, and color development was measured at 450 nm. A standard curve was constructed by using purified human λ chain (Southern Biotechnology).
Supplementary Material
Abbreviations
- Hsp90
heat shock protein 90
- MM
multiple myeloma
- IPI-504
17-allylamino-17-demethoxygeldanamycin hydroquinone hydrochloride
- NQO1
NADPH-quinone oxidoreductase 1
- 17-AGG
17-allylamino-17-demethoxygeldanamycin
- 17-AG
17-(amino)-17-demethoxygeldanamycin
- LC-MS/MS
liquid chromatography–tandem mass spectrometry.
Footnotes
The authors declare no conflict of interest.
References
- 1.Ritossa F. Experientia. 1962;18:571–573. [Google Scholar]
- 2.Hartl FU. Nature. 1996;381:571–579. doi: 10.1038/381571a0. [DOI] [PubMed] [Google Scholar]
- 3.Hayes SA, Dice JF. J Cell Biol. 1996;132:255–258. doi: 10.1083/jcb.132.3.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goetz MP, Toft DO, Ames MM, Erlichman C. Ann Oncol. 2003;14:1169–1176. doi: 10.1093/annonc/mdg316. [DOI] [PubMed] [Google Scholar]
- 5.Workman P. Curr Cancer Drug Targets. 2003;3:297–300. doi: 10.2174/1568009033481868. [DOI] [PubMed] [Google Scholar]
- 6.Whitesell L, Lindquist SL. Nat Rev Cancer. 2005;5:761–772. doi: 10.1038/nrc1716. [DOI] [PubMed] [Google Scholar]
- 7.Kuehl WM, Bergsagel PL. Nat Rev Cancer. 2002;2:175–187. doi: 10.1038/nrc746. [DOI] [PubMed] [Google Scholar]
- 8.Richardson PG, Barlogie B, Berenson J, Singhal S, Jagannath S, Irwin D, Rajkumar SV, Srkalovic G, Alsina M, Alexanian R, et al. N Engl J Med. 2003;348:2609–2617. doi: 10.1056/NEJMoa030288. [DOI] [PubMed] [Google Scholar]
- 9.Hideshima T, Chauhan D, Richardson P, Mitsiades C, Mitsiades N, Hayashi T, Munshi N, Dang L, Castro A, Palombella V, et al. J Biol Chem. 2002;277:16639–16647. doi: 10.1074/jbc.M200360200. [DOI] [PubMed] [Google Scholar]
- 10.Broemer M, Krappmann D, Scheidereit C. Oncogene. 2004;23:5378–5386. doi: 10.1038/sj.onc.1207705. [DOI] [PubMed] [Google Scholar]
- 11.Mitsiades CS, Mitsiades NS, McMullan CJ, Poulaki V, Kung AL, Davies FE, Morgan G, Akiyama M, Shringarpure R, Munshi NC. Blood. 2006;107:1092–1100. doi: 10.1182/blood-2005-03-1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Whitesell L, Mimnaugh EG, De Costa B, Myers CE, Neckers LM. Proc Natl Acad Sci USA. 1994;91:8324–8328. doi: 10.1073/pnas.91.18.8324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sausville EA, Tomaszewski JE, Ivy P. Curr Cancer Drug Targets. 2003;3:377–383. doi: 10.2174/1568009033481831. [DOI] [PubMed] [Google Scholar]
- 14.Davis JM, Rowley SD, Braine HG, Piantadosi S, Santos GW. Blood. 1990;75:781–786. [PubMed] [Google Scholar]
- 15.Zambelli A, Poggi G, Da Prada G, Pedrazzoli P, Cuomo A, Miotti D, Perotti C, Preti P, Robustelli della Cuna G. Anticancer Res. 1998;18:4705–4708. [PubMed] [Google Scholar]
- 16.Dye D, Watkins J. Br Med J. 1980;6228:1353. doi: 10.1136/bmj.280.6228.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schnur RC, Corman ML, Gallaschun RJ, Cooper BA, Dee MF, Doty JL, Muzzi ML, Moyer JD, DiOrio CI, Barbacci EG, et al. J Med Chem. 1995;38:3806–3812. doi: 10.1021/jm00019a010. [DOI] [PubMed] [Google Scholar]
- 18.Schnur RC, Corman ML, Gallaschun RJ, Cooper BA, Dee MF, Doty JL, Muzzi ML, DiOrio CI, Barbacci EG, Miller PE, et al. J Med Chem. 1995;38:3813–3820. doi: 10.1021/jm00019a011. [DOI] [PubMed] [Google Scholar]
- 19.Ge J, Normant E, Porter JR, Ali J, Dembski MS, Gao Y, Georges AT, Grenier L, Pak RH, Patterson J, et al. J Med Chem. 2006;49:4606–4615. doi: 10.1021/jm0603116. [DOI] [PubMed] [Google Scholar]
- 20.Egorin M, Rosen DM, Wolff JH, Callery PS, Musser SM, Eiseman JL. Cancer Res. 1998;58:2385–2396. [PubMed] [Google Scholar]
- 21.Ross D. Drug Metab Rev. 2004;36:639–654. doi: 10.1081/dmr-200033465. [DOI] [PubMed] [Google Scholar]
- 22.Thor H, Smith MT, Hartzell P, Bellomo G, Jewell SA, Orrenius S. J Biol Chem. 1982;257:12419–12425. [PubMed] [Google Scholar]
- 23.Talcott RE, Rosenblum M, Levin VA. Biochem Biophys Res Commun. 1983;111:346–351. doi: 10.1016/s0006-291x(83)80158-2. [DOI] [PubMed] [Google Scholar]
- 24.Chesis PL, Levin DE, Smith MT, Ernster L, Ames BN. Proc Natl Acad Sci USA. 1984;81:1696–1700. doi: 10.1073/pnas.81.6.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kelland LR, Sharp SY, Roger PM, Myers TG, Workman P. J Natl Cancer Inst. 1999;91:1940–1949. doi: 10.1093/jnci/91.22.1940. [DOI] [PubMed] [Google Scholar]
- 26.Guo W, Reigan P, Siegel D, Zirrolli J, Gustafson D, Ross D. Cancer Res. 2005;65:10006–10015. doi: 10.1158/0008-5472.CAN-05-2029. [DOI] [PubMed] [Google Scholar]
- 27.Ross D, Siegel D. Methods Enzymol. 2004;382:115–144. doi: 10.1016/S0076-6879(04)82008-1. [DOI] [PubMed] [Google Scholar]
- 28.Jez JM, Chen JC, Rastelli G, Stroud RM, Santi DV. Chem Biol. 2003;10:361–368. doi: 10.1016/s1074-5521(03)00075-9. [DOI] [PubMed] [Google Scholar]
- 29.Kamal A, Thao L, Sensintaffar J, Zhang L, Boehm MF, Fritz LC, Burrows FJ. Nature. 2003;425:407–410. doi: 10.1038/nature01913. [DOI] [PubMed] [Google Scholar]
- 30.Gooljarsingh LT, Fernandes C, Yan K, Zhang H, Grooms M, Johanson K, Sinnamon RH, Kirkpatrick RB, Kerrigan J, Lewis T, et al. Proc Natl Acad Sci USA. 2006;103:7625–7630. doi: 10.1073/pnas.0602650103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sartorelli AC. Cancer Res. 1988;48:775–778. [PubMed] [Google Scholar]
- 32.Belinsky M, Jaiswal AK. Cancer Metastasis Rev. 1993;12:103–117. doi: 10.1007/BF00689804. [DOI] [PubMed] [Google Scholar]
- 33.Mitsiades N, Mitsiades CS, Poulaki V, Chauhan D, Fanourakis G, Gu X, Bailey C, Joseph M, Libermann TA, Treon SP, et al. Proc Natl Acad Sci USA. 2002;99:14374–14379. doi: 10.1073/pnas.202445099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mimnaugh EG, Xu W, Vos M, Yuan X, Isaacs JS, Bisht KS, Gius D, Neckers L. Mol Cancer Ther. 2004;3:551–566. [PubMed] [Google Scholar]
- 35.Duus J, Bahar HI, Venkataraman G, Ozpuyan F, Izban KF, Al-Masri H, Maududi T, Toor A, Alkan S. Leuk Lymphoma. 2006;47:1369–1378. doi: 10.1080/10428190500472123. [DOI] [PubMed] [Google Scholar]
- 36.Bali P, Pranpat M, Bradner J, Balasis M, Fiskus W, Guo F, Rocha K, Kumaraswamy S, Boyapalle S, Atadja P, Seto E, Bhalla K. J Biol Chem. 2005;280:26729–26734. doi: 10.1074/jbc.C500186200. [DOI] [PubMed] [Google Scholar]
- 37.George P, Bali P, Annavarapu S, Scuto A, Fiskus W, Guo F, Sigua C, Sondarva G, Moscinski L, Atadja P, Bhalla K. Blood. 2005;105:1768–1776. doi: 10.1182/blood-2004-09-3413. [DOI] [PubMed] [Google Scholar]
- 38.Durie BG, Bataille R. Eur J Haematol Suppl. 1989;51:111–116. doi: 10.1111/j.1600-0609.1989.tb01502.x. [DOI] [PubMed] [Google Scholar]
- 39.Blade J, Rosinol L. Haematologica. 2004;89:517–519. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.