Abstract
Global energy consumption is projected to increase, even in the face of substantial declines in energy intensity, at least 2-fold by midcentury relative to the present because of population and economic growth. This demand could be met, in principle, from fossil energy resources, particularly coal. However, the cumulative nature of CO2 emissions in the atmosphere demands that holding atmospheric CO2 levels to even twice their preanthropogenic values by midcentury will require invention, development, and deployment of schemes for carbon-neutral energy production on a scale commensurate with, or larger than, the entire present-day energy supply from all sources combined. Among renewable energy resources, solar energy is by far the largest exploitable resource, providing more energy in 1 hour to the earth than all of the energy consumed by humans in an entire year. In view of the intermittency of insolation, if solar energy is to be a major primary energy source, it must be stored and dispatched on demand to the end user. An especially attractive approach is to store solar-converted energy in the form of chemical bonds, i.e., in a photosynthetic process at a year-round average efficiency significantly higher than current plants or algae, to reduce land-area requirements. Scientific challenges involved with this process include schemes to capture and convert solar energy and then store the energy in the form of chemical bonds, producing oxygen from water and a reduced fuel such as hydrogen, methane, methanol, or other hydrocarbon species.
The supply of secure, clean, sustainable energy is arguably the most important scientific and technical challenge facing humanity in the 21st century. Energy security, national security, environmental security, and economic security can likely be met only through addressing the energy problem within the next 10–20 yr. Meeting global energy demand in a sustainable fashion will require not only increased energy efficiency and new methods of using existing carbon-based fuels but also a daunting amount of new carbon-neutral energy. The various factors that conspire to support the above far-reaching conclusions and the basic science needed for the development of a large-scale cost-effective carbon-neutral energy system are the focus of this paper.
The Global Energy Perspective
In 2001, worldwide primary energy consumption was 425 × 1018 J, which is an average energy consumption rate of 13.5 terawatt (TW) (1). Eight-six percent of this energy was obtained from fossil fuels, with roughly equal parts from oil, coal, and natural gas. Nuclear power accounted for ≈0.8 TW of primary (thermal) energy, and the remainder of the energy supply came mostly from unsustainable biomass, with a relatively small contribution from renewable sources (1).
Future energy demand is projected to increase considerably relative to that in 2001. The most widely used scenarios for future world energy consumption have been those developed by the Intergovernmental Panel on Climate Change, an organization jointly established by the World Meteorological Organization and the United Nations Environment Program (after Scenario B2 in ref. 2; Ė = (869 EJ/yr)·(106 TJ/EJ)/(60·60·24·365 s/yr) = 27.54 TW (TJ, terajoule; and EJ, exajoule). The scenario outlined in the last two columns of Table 1 is based on “moderate” assumptions and hence is reasonably viewed as neither overly conservative nor overly aggressive.
Table 1.
World energy statistics and projections
| Quantity | Definition | Units | 2001* | 2050† | 2100‡ |
|---|---|---|---|---|---|
| N | Population | B persons | 6.145 | 9.4 | 10.4 |
| GDP | GDP§ | T $/yr | 46 | 140¶ | 284‖ |
| GDP/N | Per capita GDP | $/(person-yr) | 7,470 | 14,850 | 27,320 |
| Ė/GDP | Energy intensity | W/($/yr) | 0.294 | 0.20 | 0.15 |
| Ė | Energy consumption rate | TW | 13.5 | 27.6 | 43.0 |
| C/E | Carbon intensity | KgC/(W·yr) | 0.49 | 0.40 | 0.31 |
| Ċ | Carbon emission rate | GtC/yr | 6.57 | 11.0 | 13.3 |
| Ċ | Equivalent CO2 emission rate | GtCO2/yr | 24.07 | 40.3 | 48.8 |
*Ė = (403.9 Quads/yr)·(33.4 GWyr/Quad)·(10–3 TW/GW) = 13.5 TW; and Ċ = (24.072 GtCO2/yr)·(12/44 GtC/GtCO2) = 6.565 GtC (adapted from ref. 1).
†Ė = (869 EJ/yr)·(106 TJ/EJ)/(60·60·24·365 s/yr) = 27.5 TW [adapted from ref. 2 (Scenario B2), pp. 48–55].
‡Ė = (1,357 EJ/yr)·(106 TJ/EJ)/(60·60·24·365 s/yr) = 43.0 TW [adapted from ref. 2 (Scenario B2), pp. 48–55].
§All in year 2000 U.S. dollars, using the inflation-adjusted conversions: $2000 = 1/0.81590 $1990 (adapted from ref. 1), and ′purchasing power parity′ exchange rates.
¶In year 2000 U.S. dollars: (113.9 T$1990)·(1/0.81590 $2000/$1990) = 139.6 T$2000.
‖In year 2000 U.S. dollars: (231.8 T$1990)·(1/0.81590 $2000/$1990) = 284.1 T$2000.
To better understand this scenario, the top half of Table 1 breaks down the rate of energy consumption, Ė, into three fundamental factors (3):
where N is the global population, GDP/N is the globally averaged gross domestic product (GDP) per capita, and Ė/GDP is the globally averaged energy intensity (i.e., the energy consumed per unit of GDP). The world population was ≈6.1 billion in 2001, and in the scenario represented in Table 1, the global population is projected to increase by 0.9% yr−1 to ≈9.4 billion by 2050. World per capita GDP was ≈$7,500 per capita in 2001. In the Table 1 scenario, GDP/N is projected to increase at the historical average rate of 1.4% yr−1 to ≈$15,000 per capita by 2050. No country has a policy against economic growth, so this increase in GDP/N seems quite reasonable and in fact may well be modest given the rapid economic growth being experienced by China and India at present. With no changes in the globally averaged energy intensity, the world energy consumption rate would grow, due to population growth and economic growth, by 2.3% yr−1, from 13.5 TW in 2001 to ≈40.8 TW in 2050. However, the global average energy intensity has declined continuously over the past 100 yr, due to improvements in technology throughout the energy production, distribution, and end-use chain. In anticipation of continued improvements in technology, the global average energy intensity in the Table 1 scenario is projected to decrease at approximately the historical average rate of 0.8% yr−1, from 0.29 W/($ yr−1) in 2001 to 0.20 W/($ yr−1) by 2050. This decrease offsets somewhat the projected increases in population and per capita GDP, so that the world energy consumption rate is instead projected to grow by 2.3% yr−1 − 0.8% yr−1 = 1.5% yr−1, from 13.5 TW in 2001 to ≈27 TW by 2050. Hence, even factoring in a decrease in energy intensity, the world energy consumption rate is projected to double from 13.5 TW in 2001 to 27 TW by 2050 and to triple to 43 TW by 2100 (4).
The Global Energy Challenge Presented by Consumption of Fossil Fuels
Many sources indicate there are ample fossil energy reserves, in one form or another, to supply this energy at some reasonable cost. The World Energy Assessment Report estimates of the total reserves (i.e., 90% confidence that the reserves exist) and of the global resource base (5), including both conventional and unconventional sources, provide a benchmark for evaluating the total available global fossil energy base. Based on 1998 consumption rates, 40–80 yr of proven conventional and unconventional oil reserves exist globally, and 50–150 yr of oil are available if the estimated resource base is included. Sixty to 160 yr of reserves of natural gas are present, and between 207 and 590 yr of gas resources, not including the natural gas potentially available as methane clathrates in the continental shelves, are in the estimated resource base. Similarly, a 1,000- to 2000-yr supply of coal, shales, and tar sands is in the estimated resource base. Hence the estimated fossil energy resources could support a 25- to 30-TW energy consumption rate globally for at least several centuries.
Consumption of fossil energy at that rate, however, will produce a potentially significant global issue. Historically, the mean carbon intensity (kg of C emitted to the atmosphere as CO2 per year per W of power produced from the fuel) of the global energy mix has been declining. In the past two centuries, the energy mix has shifted from being dominated by wood to coal to oil and now more to natural gas. This shift has produced a decrease in the average carbon intensity of the energy mix, because oil and gas have higher H/C ratios and hence upon combustion produce more water and less CO2 per unit of heat released than does coal. If the carbon intensity were to remain at the year 2001 value (approximately equal parts coal, oil, and natural gas), the world carbon emission rate would grow due to the projected growth in the energy consumption from 6.6 billion metric tons of carbon (GtC) yr−1 in 2001 to 13.5 GtC yr−1 by 2050. The Intergovernmental Panel on Climate Change “business as usual” scenario of Table 1 projects, arguably optimistically, that the historical trend of mean carbon intensity decline with time will continue through 2050, producing an energy mix continually favoring cleaner-burning fuels from a carbon emissions viewpoint, until the average in 2050 is below that of the least carbon-intensive fossil energy source, natural gas. This decrease in carbon intensity would offset somewhat the increase in the rate of energy consumption. But even with this projected decrease in carbon intensity, the world carbon emissions rate in this scenario is projected to nearly double from 6.6 GtC yr−1 in 2001 to 11.0 GtC yr−1 by 2050 (2).
On the timescale of many centuries, CO2 emissions are essentially cumulative in the atmosphere. The CO2 equilibrates on an ≈10- to 30-yr timescale between the atmosphere and the near-surface layer of the oceans (6), which accounts for why only ≈50% of the anthropogenic CO2 emissions remain in the atmosphere (the remainder partitioning into the biosphere and the oceans). Because there are no natural destruction mechanisms of CO2 in the atmosphere, the long-term removal of atmospheric CO2 must occur by convection. The relevant mixing time between the near-surface ocean layer and the deep oceans is between 400 and several thousand years (6, 7). Hence, in the absence of geoengineering or active intervention, whatever environmental effects might be caused by this atmospheric CO2 accumulation over the next 40–50 yr will persist globally for the next 500–2,000 yr or more.
Although the precise future effects of such anthropogenic CO2 emissions are still somewhat uncertain, the emission levels can certainly be viewed rigorously within a historical perspective. The data from the Vostok ice core indicate that the atmospheric CO2 concentration has been between 210 and 300 ppm for the past 420,000 yr (8), and more recent studies of Dome Concordia ice cores have extended this time period to 650,000 yr (9). Over this same time period, the atmospheric CO2 concentration has been highly correlated with, but is not necessarily the cause of, temperature swings that have repeatedly caused ice ages on the planet. The CO2 concentrations in the past 50 yr have been rising because of anthropogenic CO2 emissions from fossil fuel consumption, and they are now in excess of 380 ppm. Without intervention, even the Table 1 scenario produces, within the 21st century, atmospheric CO2 concentrations that are more than double the preanthropogenic values (4, 6). The exact levels vary depending on the assumed composition of energy sources, the efficiency of energy production and consumption, the global economy, and different intervention scenarios to control CO2 levels. Modestly stringent interventions are based on stabilizing atmospheric CO2 in the 550- to 650-ppm range, with substantially higher values projected (>750 ppm) if the Table 1 scenario is followed. Climate models predict a variety of different global responses to levels of CO2 at or in excess of 550 ppm in the atmosphere. In some models, moderate changes are predicted, whereas in others, relatively serious sea level rises, changes in the hydrological cycle, and other effects are predicted (10). Tipping points involving positive feedback, such as the accelerated loss of permafrost, which could release further CO2 which then could accelerate still further permafrost loss, are of substantive concern. What can be said with certainty is that the atmospheric CO2 concentrations are being increased and without severe intervention will continue to increase, because of anthropogenic sources, to levels that have not been present on the planet in at least the past 650,000 yr and probably in the past 20 million yr.
Carbon-Neutral Fuel Sources and the Solar Opportunity
To meet the (arguably optimistic) Intergovernmental Panel on Climate Change projection in the Table 1 scenario for the average carbon intensity in 2050, the projected carbon intensity in 2050 is ≈0.45 kg of C yr−1 W−1, which is lower than that of any of the fossil fuels. The only way one can reach this value of the mean carbon intensity is through a significant contribution of carbon-free power to the total energy mix. This conclusion holds for an economy entirely based on natural gas; to the extent that the mix of consumed fossil fuels is not 100% natural gas but is roughly also equal parts oil and coal, even more carbon-free energy is required to maintain the average of the energy mix at the 0.45 kg of C yr−1 W−1 value. In fact, the amount of carbon-free power required in 2050 to meet these carbon intensity targets is >10 TW and is much greater than 10 TW if emissions are to be lowered such that CO2 can be stabilized at 550 ppm. Even more carbon-free power will be required later in the 21st century if CO2 levels are to be kept below 550 ppm or if a lower atmospheric CO2 target level is desired. By almost any reasonable estimate, stabilization of atmospheric CO2 levels at 550 ppm or lower will require as much carbon-neutral power by approximately the year 2050 as the amount of power produced at present from all energy sources combined (4). Furthermore, because CO2 emissions are cumulative on a century-level timescale, even higher levels of carbon-neutral power are required by 2050 if their introduction does not start immediately with a constant rampup but instead are delayed by 20 yr for their commissioning while awaiting technology development and/or policy and socioeconomic interventions.
Three general routes are available to produce such large amounts of carbon-neutral power.
Nuclear fission is one method, but it would require widespread implementation of breeder reactors (11). Estimated terrestrial U resources are sufficient to produce ≈100 TW-yr of electricity using conventional once-through U reactor technology. Hence, if 10 TW of power were obtained from conventional nuclear fission, the terrestrial U resource base would be exhausted at that level in less than a decade (in fact, it would be exhausted after the first 30 yr of reactor construction because of the fuel consumed during the rampup phase). Moreover, construction of nuclear power plants would need to proceed at a very rapid rate by historical standards (one 1-GWe (gigawatt-electric) power plant every 1.6 days for the next 45 yr). The international tokamak (magnetic confinement fusion) experiment (ITER) is now scheduled to demonstrate an energy breakeven point in 35 yr for a few minutes of operational time. Although fusion might possibly provide significant commercial energy late in the 21st century, the ITER time line is much too far in the future to provide a credible option to make a significant contribution to the amount of cost-effective carbon-neutral energy production needed to meet any reasonable atmospheric CO2 concentration target in the next 40–50 yr.
Carbon capture and storage comprise a second general approach (12). In this approach, the carbon dioxide is dissolved in the underground aquifers. To be a viable option technically, the CO2 must not leak at a globally averaged rate of 1% for a timescale of centuries. Otherwise, the emitted flux will be greater than or equal to that intended to be mitigated initially. Experiments at scale are needed, along with extensive modeling, simulation, monitoring, and validation, to ascertain with >99% confidence that the leak rate will be acceptably low for a 500- to 1,000-yr period. Furthermore, each reservoir is different geologically, so proof that sequestration works technically at one reservoir is not general proof that the process will work at the required level globally. The global reservoir capacity has been estimated to be equivalent to ≈100–150 yr of carbon emissions. Hence, sequestration could buy time if it works technically and is so validated within the next 10–20 yr. An additional condition is that the energy distribution and end-use chain must be transformed to handle massive quantities of carbon-free fuels (hydrogen) or electricity on the needed timescale to mitigate carbon emissions.
The third general approach is to use renewable energy. Of the various renewable energy sources, by far the largest resource is provided by the sun. More energy from sunlight strikes the earth in 1 hr (4.3 × 1020 J) than all of the energy currently consumed on the planet in 1 yr (4.1 × 1020 J in 2001) (5). Yet, in 2001, only <0.1% of electricity and <1.5% of fuels (mostly from biomass) were provided by a solar source (1). Against the backdrop of the daunting carbon-neutral energy needs of our global future, the large gap between our present use of solar energy and its enormous undeveloped potential defines a compelling imperative for science and technology in the 21st century.
Solar Energy Utilization
Solar energy utilization requires solar (i) capture and conversion and (ii) storage. Solar capture and conversion may be accomplished by photovoltaics (PVs). The challenge here is to dramatically reduce the cost per W of delivered solar electricity. Compared with fossil energy, solar energy is diffuse, and hence materials costs must be very inexpensive to make a solar-based process economical. Knowing the insolation striking an area of the earth for a 30-yr period, it is relatively simple to calculate the sale price of the converted energy that is needed to pay back at least the initial cost that is required to cover that area with the solar energy conversion system. At 10% efficiency, and a cost of $300 m−2, both typical of current Si-based solar electricity modules, along with a balance of systems cost of $3 Wp−1 (peak W), an electricity price of $0.35 [kW-hr]−1 is required to cover the initial system costs (13). By comparison, fossil-derived electricity (high-value energy) now costs approximately $0.02–0.05 [kW-hr]−1, and that cost includes storage and distribution costs. To reach a cost point near that of fossil-derived energy will thus require improvements in efficiency but additionally will require large decreases in cost, into a range below $100 m−2. For comparison, the cost of paint is about $1 m−2, so the solar energy conversion system can cost ≈10 times more than the cost of paint, but not much more if it is to provide cost-effective primary energy.
In the absence of cost-effective storage, solar electricity can never be a primary energy source for society, because of the diurnal variation in local insolation. In principle, storage of electricity could be obtained using batteries, but at present no battery is inexpensive enough, when amortized over the 30-yr lifetime of a solar device, to satisfy the needed cost per W targets for the whole system. A second method is to store the electrical energy mechanically. For instance, electricity could be used to drive turbines to pump water uphill. This approach is relatively inexpensive for storing large amounts of energy at modest charge and discharge rates, but is not well matched to being charged and discharged every 24 h to compensate for the diurnal cycle. For example, buffering the day/night cycle in the U.S. energy demand by this approach would require a pumping capacity equivalent of >5,000 Hoover Dams, filling and emptying reservoirs every day and every night. Currently, the cheapest method of solar energy capture, conversion, and storage is solar thermal technology, which can cost as little as $0.10–0.15 per kW-hr for electricity production. Advances in this potentially very important approach to solar energy utilization will require new materials for the focusing and thermal capture of the energy in sunlight, as well as new thermochemical cycles for producing useful fuel from the captured solar energy. The possibility of integrated capture, conversion, and storage functions makes solar thermal technology an option that should be vigorously pursued to exploit the large untapped solar energy resource for carbon-neutral energy production
A third method of storage is to borrow the design of nature, in which chemical bonds are broken and formed to produce solar fuels in an artificial photosynthesis process. Photosynthesis itself is relatively inefficient, when measured on a yearly average basis per unit area of insolation. For example, switchgrass, one of the fastest-growing crops, yields energy stored in biomass at a yearly averaged rate of <1 W/m2 (5). Because the averaged insolation at a typical midlatitude is 200–300 W/m2 (5), the yearly averaged energy conversion and storage efficiency of the most rapidly growing large area crops is currently <0.5%. Even if this efficiency could be reached with no energy inputs into the farm or any energy losses due to outputs from the utilization of the biomass, growth of energy crops on all of the naturally irrigated cultivatable land on earth that is not currently used for food crops would yield perhaps 5–10 TW of total power. Whereas biofuels derived from existing plants could provide a potentially significant contribution to liquid fuels for transportation uses (if cellulosic conversion technology can be successfully developed and deployed economically) increased energy conversion and storage efficiency are highly desirable to remove land area as a serious constraint on the amount of energy that can be obtained from the sun and stored in chemical bonds. One approach is to develop an artificial photosynthetic process with an average efficiency significantly higher than plants or algae.
The primary steps of photosynthesis involve the conversion of sunlight into a “wireless current.” In all cases, to form a useful fuel, O2 must be evolved, so it can be released into our oxygen-containing atmosphere and used elsewhere as an oxidation reagent for fuel consumption. The reduced fuel could be either hydrogen from water reduction, or it could be an organic species, such as methanol or methane, that is derived from the fixation of atmospheric CO2. Recombination of the reduced fuel with released O2 would then regenerate the original species, closing the cycle in a carbon-neutral fashion.
In natural photosynthesis, the anodic charge of the wireless current is used at the oxygen-evolving complex to oxidize water to oxygen, with the concomitant release of four protons. The cathodic charge of the wireless current is captured by Photosystem I to reduce the protons to “hydrogen,” with the reduced hydrogen equivalents stored through the conversion of NADP to NADPH. Thus, the overall primary events of photosynthesis store sunlight by the rearrangement of the chemical bonds of water, to form oxygen and Nature's form of hydrogen.
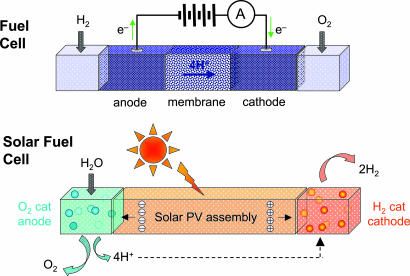
An artificial photosynthetic system could be realized by spatially separating solid-state or molecular water reduction and oxidation catalysts and connecting them to a light collection and charge separation system. In one such construct, the spatially separated electron–hole pairs provided by a photovoltaic assembly based on a solid-state junction, on either the macroscale or the nanoscale, are captured by the catalysts, and the energy is stored in the bond rearrangement of water to H2 and O2. Other concepts involve more intimate integration of the charge separation and chemical bond-forming functions, to avoid costs and system constraints associated with electrical contacts, wires, inverters, etc., involved with converting 1-eV photons into 1-eV chemical bonds through electricity as a discrete intermediary. One approach to this type of system is depicted in Fig. 1, in which the tightly integrated system is modeled after natural photosynthesis and serves as a model for the artificial photosynthetic systems that are discussed below.
Fig. 1.
H2 and O2 are combined in a fuel cell to generate a flow of electrons and protons across a membrane, producing electrical energy. The solar fuel cell uses light to run the electron and proton flow in reverse. Coupling the electrons and protons to catalysts breaks the bonds of water and makes the bonds H2 and O2 to effect solar fuel production.
The Basic Science Needs for PVs
One of the key issues in solar capture and conversion is how to separate charge efficiently over macroscopic distances without using expensive, highly pure, semiconductor materials. This effort requires the development of new chemical and materials methods to make polycrystalline and nanocrystalline semiconductors perform as if they were expensive single crystals. Numerous research approaches are being pursued (13). Materials consisting of a network of interpenetrating regions can facilitate effective charge separation and collection, thus relaxing the usual constraint in which the photogenerated carriers must exist long enough to traverse the entire distance of the cell. Present photon conversion devices based on a single-bandgap absorber, including semiconductor PV, have a theoretical thermodynamic conversion efficiency of 32% in unconcentrated sunlight. However, the conversion efficiency can be increased, in principle, to 45–65% if carrier thermalization can be prevented (by overcoming the so-called Shockley–Queisser limit). Multiple-bandgap absorbers in a cascaded junction configuration can result in high photoconversion efficiencies, particularly when cells are designed to sustain the operating conditions (e.g., elevated temperatures) associated with highly concentrated sunlight. It is expected that mature high-concentration PV systems can provide 10–20% more energy than standard PV systems with the same installed power rating.
In addition to making evolutionary changes to existing PV technologies, new materials for next-generation PVs are needed. Building upon the recent success in developing efficient molecular organic PVs and the recent advances in the controlled assembly of hybrid organic/inorganic nanostructures, organic and hybrid PV cells could possibly exceed 10% energy conversion efficiency, while offering a potentially inexpensive manufacturing paradigm (e.g., casting from emulsions, printing, and use of flexible substrates for production of large-area thin-film cells; ref. 14). To guide the PV nanostructure assembly, biologically derived and/or genetically engineered systems might be used to control the crystal structure, phase, orientation, and nanostructural regularity of inorganic materials. Genetically modified photosynthetic complexes from plants and bacteria can also convert incident light into photocurrent. Although the present energy conversion efficiencies of such systems are low, the projected maximum could be possibly as high as 10%. Finally, the Shockley–Queisser limit may be overcome by using multilayer junctions of semiconductor quantum dots, quantum wells and related nanostructures, and new inorganic materials and photoassemblies. Such materials could channel the excess energy of electron/hole pairs into photovoltages and photocurrents, with the design guided by a refined detailed understanding of photon absorption, charge creation, and charge separation processes.
The Basic Science Needs for Solar Fuels
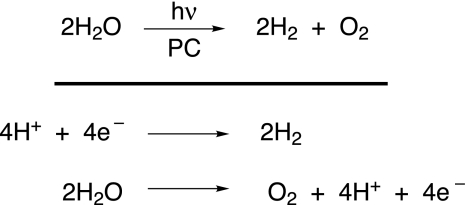
As described above, an important storage approach involves conversion of the energy captured in the charge-separated states of a solar capture and conversion system into chemical bonds. Water splitting is an example of a more general conversion to a solar fuel cycle that involves evolution of oxygen as one component and formation of a reduced fuel as the other. Unexplored basic science issues are immediately confronted when the problem is posed in the simplest chemistry framework (see Scheme 1).
Scheme 1.
The overall transformation is a multielectron process promoted by photocatalyst and light. Elucidation of the fundamental principles of single electron-transfer reactions represented such an important milestone in chemistry that two Nobel Prizes were awarded for such work (15, 16). Although dramatic advances have occurred in our understanding of single electron-transfer reactions, especially those in biology (17), a similar level of understanding of multielectron redox reactions has yet to be realized. Moreover, to ensure charge neutrality in the system, proton transfer must accompany electron transfer (i.e., proton-coupled electron transfer; ref. 18); hence, electron and proton inventories both need to be managed (19). Water splitting additionally presents sizable thermodynamic and kinetics barriers to making and breaking the bonds required to facilitate the desired chemical reactions. This is especially pertinent to the water-splitting problem, because the byproduct of water activation at the catalyst, whether molecular or solid, will invariably yield species that have strong metal–oxygen bonds. To close a catalytic cycle, these stable bonds need to be activated by the captured solar energy either directly or indirectly. More generally, the activation of all small molecules of consequence to carbon-neutral solar energy storage, including CO2, O2, and H2O, share the reaction commonalities of bond-making and -breaking processes that require multielectron transfers coupled to proton transfer.
The Reaction Chemistry of Solar Energy Storage in Chemical Bonds
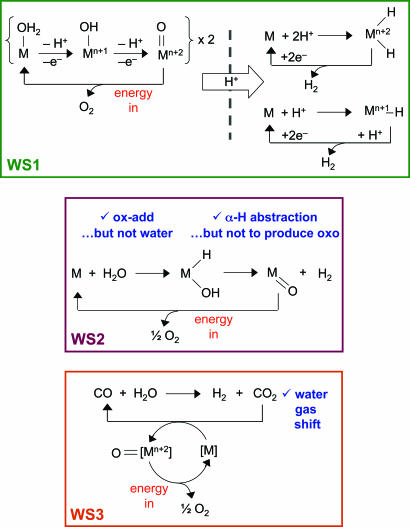
Perhaps the most straightforward water-splitting scheme is to have catalysts act directly on water, as exemplified by the two half-reactions denoted as WS1 (WS1, water-splitting strategy 1) in Scheme 2. The spatial separation of the catalysts requires that the charge-separation function be imbedded in some type of membrane, so that the protons generated on the anodic side of the cell are transported to the cathodic side of the cell for reduction. In effect, the system must be run in the opposite direction of a fuel cell, with sunlight providing the thermodynamic impetus to drive the cell in the desired fuel-forming direction.
Scheme 2.
The preparation of hydrogen-producing catalysts constitutes an intense area of study. Fe-only hydrogenases, comprised of small dithiolate-bridged bimetallic iron cofactors coordinated by CO and cyanide ligands, provide a benchmark for the efficient evolution of H2 in molecular systems (20, 21). Structural, and in some cases, functional, analogues of such enzymatic active sites have been prepared (22–27); however, none of these Fe-only hydrogenase biomimics yet produce H2 efficiently at low overpotential. Synthetic catalysts compare favorably to, and in some cases exceed, the efficiency of the biomimetic models. In the presence of sacrificial chemical reductants, mononuclear and binuclear metal complexes of Co, Ni, and Rh are known to effect catalytic hydrogen evolution electrochemically or photochemically (28–36). Intimate mechanistic details, however, are known in only a few cases (37), and the different possibilities, such as protonation of a hydride vs. uni- or bimolecular reductive elimination (right side, WS1, Scheme 2), in general have not yet been unraveled.
Other water-splitting cycles can also be developed. The water-splitting schemes WS2 and WS3 presented in Scheme 2 use basic reaction types that are common to organometallic catalysis. However, for the water-splitting problem, O, as opposed to C or N, needs to be managed. Every reaction, however, does have a precedent for carbon or nitrogen. In WS2 in Scheme 2, oxidative addition across X H (X = C, N) bonds is a basic reaction of organometallic chemistry but is not yet well established for water (38–43). If this reaction can be achieved cleanly, hydrogen may be generated by α-H abstraction, which is a common reaction in organometallic chemistry and is used to generate metal–ligand multiple bonds. For instance, the α-H abstraction of metal alkylidenes produces alkylidynes (44). But α-H abstraction to produce metal-oxo species, and H2 is uncommon for well defined hydroxo–hydrido complexes. In the case of WS3, the water–gas shift reaction produces H2 from H2O using CO as the reductant. An intense research effort, beginning in the 1970s and ending in the 1980s, provided the basic science for the development of catalysts to effect the water–gas shift reaction (45). However, the reaction must be closed by the conversion of CO2 to CO. On this front, little is known. Some inroads to CO2 reduction have been made on photo– (46, 47) and electro– (48–50) catalytic fronts, but generally the precise path to CO2 reduction is ill-defined, making it difficult to improve these systems by design. A recent report of CO2 reduction by a well defined homogeneous metal complex operating at high turnover number and frequency (51) is a harbinger of the promise that basic science holds for the design of efficient CO2 reduction catalysts.
H (X = C, N) bonds is a basic reaction of organometallic chemistry but is not yet well established for water (38–43). If this reaction can be achieved cleanly, hydrogen may be generated by α-H abstraction, which is a common reaction in organometallic chemistry and is used to generate metal–ligand multiple bonds. For instance, the α-H abstraction of metal alkylidenes produces alkylidynes (44). But α-H abstraction to produce metal-oxo species, and H2 is uncommon for well defined hydroxo–hydrido complexes. In the case of WS3, the water–gas shift reaction produces H2 from H2O using CO as the reductant. An intense research effort, beginning in the 1970s and ending in the 1980s, provided the basic science for the development of catalysts to effect the water–gas shift reaction (45). However, the reaction must be closed by the conversion of CO2 to CO. On this front, little is known. Some inroads to CO2 reduction have been made on photo– (46, 47) and electro– (48–50) catalytic fronts, but generally the precise path to CO2 reduction is ill-defined, making it difficult to improve these systems by design. A recent report of CO2 reduction by a well defined homogeneous metal complex operating at high turnover number and frequency (51) is a harbinger of the promise that basic science holds for the design of efficient CO2 reduction catalysts.
As in WS1, WS2 and WS3 cycles are closed by oxygen production, providing a further imperative for the development of reactions of the type described by Schemes 3–5. However, very few catalysts are known to oxidize water near the thermodynamic potential. Again, the most notable system is in biology, specifically involving the oxygen-evolving complex (OEC) of Photosystem II. The OEC comprises a cluster of four Mn centers and a Ca center (52–54), but no functional or structural models of the catalytically active site are yet available (55). At present, the ruthenium dimer [(bpy)2(OH2)RuIIIORuIII(OH2)(bpy)2]4+ (bpy = 2,2′-bipyridine) (56) and its relatives (57–59) represent the only unequivocally established molecular electrocatalysts for generating O2 from H2O. However, at present, this reaction proceeds at a high overpotential and with modest turnover numbers.
Scheme 3.
Scheme 4.
Scheme 5.
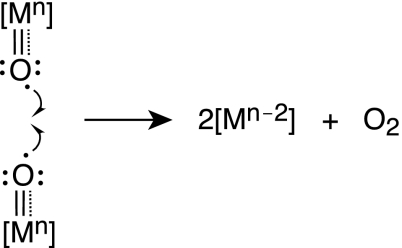
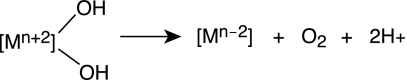
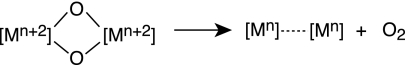
The success of WS1, WS2, and WS3 and other yet-undefined water-splitting schemes is predicated on systems that promote the conversion of oxygen from metal oxos. Many mechanistic possibilities for this conversion await discovery. They include the following.
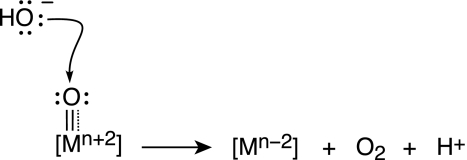
(i) Nucleophilic Attack of Hydroxide on High-Valent Metal Oxos (Scheme 3).
This basic reaction type is the foundation on which oxidation catalysts have been developed in the disciplines of organometallic and organic chemistry (60). Here the olefin bond attacks a metal oxo species to form two carbon–oxygen bonds. The replacement of the two-electron bond of the olefin by the lone pair of hydroxide would lead to the oxygen–oxygen bond-forming reaction that is critical for water oxidation. The substitution, however, is not trivial. OH− is thermodynamically more difficult to oxidize than are olefins. Also, the overall reaction to produce oxygen involves a four-electron change at the metal, so there may be benefits to examining reductive elimination from more than one metal center, in which the multielectron equivalency can be shared by metal centers working in concert.
(ii) Radical Coupling of Two Oxos (Scheme 4).
As shown, the oxygen radical may be delivered directly from a high-valent metal. Alternatively, the oxo species could be delivered from a multiply bonded metal–ligand species (61). This latter approach represents a paradigm shift in oxygen chemistry, because the strong metal–oxo double and triple bonds may be avoided, potentially lowering the activation barrier for oxygen atom delivery from a reactive multiply bonded metal–ligand center.
(iii) Reductive Elimination of bis Hydroxos or bis μ-Oxos (Scheme 5).
These unknown reaction types encompass a four-electron change to make oxygen. A shared electron equivalency among a multimetallic center may expedite the reaction, such as that shown for Scheme 6.
Scheme 6.
In Scheme 2, the WS cycles are completed by the same parent metal complex. This does not have to be the case. As has recently been demonstrated, metal complexes working in tandem can promote reactions of energy consequence (62). Accordingly, the water-splitting schemes may be accomplished by two different metal complexes working in concert. Regardless of the precise details of the reaction design, oxygen production invariably will be an energetically demanding process that must be coupled to a charge-separated state to capture, convert, and store solar energy in the form of chemical bonds. By use of a photovoltaic assembly to accomplish solar-driven charge separation, the constraints on the catalyst design are relaxed solely to provide storage. However, in bringing catalysts to a charge-separating assembly, the reaction chemistry will be performed in a heterogeneous and/or interfacial environment. Accordingly, the need to acquire a molecular-level understanding of reactions at the surfaces of solids represents another scientific challenge confronting the effective utilization of solar energy. Finally, inasmuch as the aforementioned reactions and schemes are all enacted at a metal-based platform, the role of inorganic chemistry, whether at a molecule or a surface, will be pivotal to the development of the aforementioned water-splitting cycles. Ingenious approaches to water splitting may be possible using organic catalysts and biocatalysts as well, although the ability to operate these reactions at low overpotential will represent a significant challenge.
Conclusions
The sun has a unique role in sustainable energy production, in that it is the undisputed champion of energy; the resource base presented by terrestrial insolation far exceeds that of all other renewable energy sources combined. The solar energy resource additionally far exceeds what can possibly be envisioned as a level of human consumption necessary to support even the most technologically advanced society. However, to be a material contribution to primary energy supply, solar energy must be captured, converted, and stored to overcome the diurnal cycle and the intermittency of the terrestrial solar resource. Arguably the most attractive method for this energy conversion and storage is in the form of chemical bonds, by production of cheap solar fuels. Significant advances in basic science, however, are needed for this technology to attain its full potential. Chemistry will assume a special role in this endeavor, because new materials must be created for solar capture and conversion, and because new catalysts are needed for the desired chemical bond conversions. Here we present a blueprint for a reaction chemistry, when interfaced to a charge-separation structure, that permits artificial photosynthesis to be envisioned. The progress of scientists in chemistry, biology, engineering, materials science, and physics in addressing the basic science challenges involved with realizing this artificial photosynthesis will be critical to enable humans to use the sun sustainably as their primary energy source.
Acknowledgments
We acknowledge sustained support from the U.S. Department of Energy (Office of Basic Energy Sciences) and the National Science Foundation (and in particular, Chemical Bonding Center CP-CP0533150) for basic research in renewable energy and for facilitating our ongoing perspective on global energy options.
Abbreviations
- TW
terawatt
- GDP
gross domestic product
- PV
photovoltaics
- GtC
metric tons of carbon.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
References
- 1.Energy Information Administration. Annual Energy Outlook. Washington, DC: US Dept of Energy; 2005. [Google Scholar]
- 2.Nakicenovic N, Swart R, editors. Special Report on Emissions Scenarios. Washington, DC: Intergovernmental Panel on Climate Change; 2000. pp. 48–55. [Google Scholar]
- 3.Kates R. Environment. 2000;42:10–19. [Google Scholar]
- 4.Hoffert MI, Caldeira K, Jain AK, Haites EF, Harvey LD, Potter SD, Schlesinger ME, Wigley TML, Wuebbles DJ. Nature. 1998;395:881–884. [Google Scholar]
- 5.United Nations Development Program. World Energy Assessment Report: Energy and the Challenge of Sustainability. New York: United Nations; 2003. [Google Scholar]
- 6.Wigley TML, Richels R, Edmonds JA. Nature. 1996;379:240–243. [Google Scholar]
- 7.Maier-Reimer E, Hasselmann K. Climate Dyn. 1987;2:63–90. [Google Scholar]
- 8.Petit JR, Jouzel J, Raynaud D, Barkov NI, Barnola J-M, Basile I, Bender M, Chappellaz J, Davis M, Delaygue G, et al. Nature. 1999;399:429–436. [Google Scholar]
- 9.Siegenthaler U, Stocker TF, Monnin E, Lüthi D, Schwander J, Stauffer B, Raynaud D, Barnola J-M, Fischer H, Masson-Delmotte V, et al. Science. 2005;310:1313–1317. doi: 10.1126/science.1120130. [DOI] [PubMed] [Google Scholar]
- 10.Intergovernmental Panel on Climate Change. Climate Change 2001, Synthesis Report Summary for Policymakers. Washington, DC: Intergovernmental Panel on Climate Change; 2001. Third Assessment Report. [Google Scholar]
- 11.Moniz E, Deutch J, editors. The Future of Nuclear Power. Cambridge, MA: Massachusetts Institute of Technology; 2003. [Google Scholar]
- 12.Metz B, Davidson O, de Coninck , Loos HM, Meyer L, editors. Carbon Dioxide Capture and Storage. Washington, DC: Intergovernmental Panel on Climate Change; 2005. [Google Scholar]
- 13.Solar Energy Utilization Workshop. Basic Science Needs for Solar Energy Utilization. Washington, DC: US Dept of Energy; 2005. [Google Scholar]
- 14.Shaheen SE, Ginley DS, Jabbour GE. MRS Bull. 2005;30:10–19. [Google Scholar]
- 15.Marcus RA. Angew Chem Int Ed Engl. 1993;32:1111–1121. [Google Scholar]
- 16.Taube H. Angew Chem Int Ed Engl. 1984;23:329–339. [Google Scholar]
- 17.Gray HB, Winkler JR. Proc Natl Acad Sci USA. 2005;102:3534–3539. doi: 10.1073/pnas.0408029102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cukier RI, Nocera DG. Annu Rev Phys Chem. 1998;49:337–369. doi: 10.1146/annurev.physchem.49.1.337. [DOI] [PubMed] [Google Scholar]
- 19.Chang CJ, Chang MCY, Damrauer NH, Nocera DG. Biophys Biochim Acta. 2004;1655:13–28. doi: 10.1016/j.bbabio.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 20.Darensbourg MY, Lyon EJ, Zhao X, Georgakaki IP. Proc Natl Acad Sci USA. 2003;100:3683–3688. doi: 10.1073/pnas.0536955100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peters JW, Lanzilotta WN, Lemon BJ, Seefeldt LC. Science. 1998;282:1853–1858. doi: 10.1126/science.282.5395.1853. [DOI] [PubMed] [Google Scholar]
- 22.Mejia-Rodriguez R, Chong DS, Reibenspies JH, Soriaga MP, Darensbourg MY. J Am Chem Soc. 2004;126:12004–12014. doi: 10.1021/ja039394v. [DOI] [PubMed] [Google Scholar]
- 23.Ott S, Kritikos M, Akermark B, Sun LC, Lomoth R. Angew Chem Int Ed. 2004;43:1006–1009. doi: 10.1002/anie.200353190. [DOI] [PubMed] [Google Scholar]
- 24.Borg SJ, Behrsing T, Best SP, Razavet M, Liu XM, Pickett CJ. J Am Chem Soc. 2004;126:16988–16999. doi: 10.1021/ja045281f. [DOI] [PubMed] [Google Scholar]
- 25.Borg SJ, Bondin MI, Best SP, Razavet M, Liu X, Pickett CJ. Biochem Soc Trans. 2005;33:3–6. doi: 10.1042/BST0330003. [DOI] [PubMed] [Google Scholar]
- 26.Gloaguen F, Lawrence JD, Schmidt M, Wilson SR, Rauchfuss TB. J Am Chem Soc. 2001;123:12518–12527. doi: 10.1021/ja016071v. [DOI] [PubMed] [Google Scholar]
- 27.Gloaguen F, Lawrence JD, Rauchfuss TB. J Am Chem Soc. 2001;123:9476–9477. doi: 10.1021/ja016516f. [DOI] [PubMed] [Google Scholar]
- 28.Gray HB, Maverick AW. Science. 1981;214:1201–1205. doi: 10.1126/science.214.4526.1201. [DOI] [PubMed] [Google Scholar]
- 29.Koelle U. New J Chem. 1992;16:157–169. [Google Scholar]
- 30.Koelle U, Ohst S. Inorg Chem. 1986;25:2689–2694. [Google Scholar]
- 31.Koelle U, Infelta PP, Gratzel M. Inorg Chem. 1998;27:879–883. [Google Scholar]
- 32.Connolly P, Espenson JH. Inorg Chem. 1986;25:2684–2688. [Google Scholar]
- 33.James TL, Cai LS, Muetterties MC, Holm RH. Inorg Chem. 1996;35:4148–4161. doi: 10.1021/ic960216v. [DOI] [PubMed] [Google Scholar]
- 34.Collin JP, Jouaiti A, Sauvage JP. Inorg Chem. 1988;27:1986–1990. [Google Scholar]
- 35.Hu X, Cossairt BM, Brunschwig BS, Lewis NS, Peters JC. Chem Comm. 2005:4723–4725. doi: 10.1039/b509188h. [DOI] [PubMed] [Google Scholar]
- 36.Heyduk AF, Nocera DG. Science. 2001;293:1639–1641. doi: 10.1126/science.1062965. [DOI] [PubMed] [Google Scholar]
- 37.Esswein AJ, Veige AS, Nocera DG. J Am Chem Soc. 2005;127:16641–16651. doi: 10.1021/ja054371x. [DOI] [PubMed] [Google Scholar]
- 38.Milstein D, Calabrese JC, Williams ID. J Am Chem Soc. 1986;108:6387–6389. [Google Scholar]
- 39.Dorta R, Rozenberg H, Shimon LJW, Milstein D. J Am Chem Soc. 2002;124:188–189. doi: 10.1021/ja016625u. [DOI] [PubMed] [Google Scholar]
- 40.Burn MJ, Fickes MG, Hartwig JF, Hollander FJ, Bergman RG. J Am Chem Soc. 1993;115:5875–5876. [Google Scholar]
- 41.Tani K, Iseki A, Yamagata T. Angew Chem Int Ed. 1998;37:3381–3383. doi: 10.1002/(SICI)1521-3773(19981231)37:24<3381::AID-ANIE3381>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 42.Dorta R, Togni A. Organometallics. 1998;17:3423–3428. [Google Scholar]
- 43.Morales-Morales D, Lee DW, Wang Z, Jensen CM. Organometallics. 2001;20:1144–1147. [Google Scholar]
- 44.Schrock RR, Seidel SW, Mosch-Zanetti NC, Shih K-Y, O'Donoghue MB, Davis WM, Reiff WM. J Am Chem Soc. 1997;119:11876–11893. [Google Scholar]
- 45.Ford PC. Acc Chem Res. 1981;14:31–37. [Google Scholar]
- 46.Lin W, Frei H. J Am Chem Soc. 2005;127:1610–1611. doi: 10.1021/ja040162l. [DOI] [PubMed] [Google Scholar]
- 47.Fujita E, Brunschwig BS. In: Catalysis of Electron Transfer, Heterogeneous and Gas-Phase Systems. Balzani V, editor. Vol 4. Weinheim, Germany: Wiley-VCH; 2001. pp. 88–126. [Google Scholar]
- 48.Simón-Manso E, Kubiak CP. Organometallics. 2005;24:96–102. [Google Scholar]
- 49.Hammouche M, Lexa D, Momenteau M, Savéant J-M. J Am Chem Soc. 1991;113:8455–8466. [Google Scholar]
- 50.Beley M, Collin J-P, Ruppert R, Sauvage J-P. J Am Chem Soc. 1986;108:7461–7467. doi: 10.1021/ja00284a003. [DOI] [PubMed] [Google Scholar]
- 51.Laitar DS, Müller P, Sadighi JP. J Am Chem Soc. 2005;127:17196–17197. doi: 10.1021/ja0566679. [DOI] [PubMed] [Google Scholar]
- 52.Ferreira KN, Iverson TM, Maghlaoui K, Barber J, Iwata S. Science. 2004;303:1831–1838. doi: 10.1126/science.1093087. [DOI] [PubMed] [Google Scholar]
- 53.Sauer K, Yano J, Yachandra VK. Photosynth Res. 2005;85:73–86. doi: 10.1007/s11120-005-0638-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Loll B, Kern J, Saenger W, Zouni A, Biesiadka J. Nature. 2005;438:1040–1044. doi: 10.1038/nature04224. [DOI] [PubMed] [Google Scholar]
- 55.Ruttinger W, Dismukes GC. Chem Rev. 1997;97:1–24. doi: 10.1021/cr950201z. [DOI] [PubMed] [Google Scholar]
- 56.Gersten SW, Samuels GJ, Meyer TJ. J Am Chem Soc. 1982;104:4029–4030. [Google Scholar]
- 57.Sens C, Romero I, Rodriguez M, Llobet A, Parella T, Benet-Buchholz J. J Am Chem Soc. 2004;126:7798–7799. doi: 10.1021/ja0486824. [DOI] [PubMed] [Google Scholar]
- 58.Wada T, Tsuge K, Tanaka K. Angew Chem Int Ed. 2000;39:1479–1482. doi: 10.1002/(sici)1521-3773(20000417)39:8<1479::aid-anie1479>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 59.Zong R, Thummel RP. J Am Chem Soc. 2005;127:12802–12803. doi: 10.1021/ja054791m. [DOI] [PubMed] [Google Scholar]
- 60.Jacobsen EN. Acc Chem Res. 2000;33:421–431. doi: 10.1021/ar960061v. [DOI] [PubMed] [Google Scholar]
- 61.Odom AL, Cummins CC, Protasiewicz JD. J Am Chem Soc. 1995;117:6613–6614. [Google Scholar]
- 62.Goldman AS, Roy AH, Huang Z, Ahuja R, Schinski W, Brookhart M. Science. 2006;312:257–261. doi: 10.1126/science.1123787. [DOI] [PubMed] [Google Scholar]