Abstract
We report the 13C content of preserved organic carbon for a 150 million-year section of late Archean shallow and deepwater sediments of the Hamersley Province in Western Australia. We find a 13C enrichment of ≈10‰ in organic carbon of post-2.7-billion-year-old shallow-water carbonate rocks relative to deepwater sediments. The shallow-water organic-carbon 13C content has a 29‰ range in values (−57 to −28‰), and it contrasts with the less variable but strongly 13C-depleted (−40 to −45‰) organic carbon in deepwater sediments. The 13C enrichment likely represents microbial habitats not as strongly influenced by assimilation of methane or other 13C-depleted substrates. We propose that continued oxidation of shallow settings favored the expansion of aerobic ecosystems and respiring organisms, and, as a result, isotopic signatures of preserved organic carbon in shallow settings approached that of photosynthetic biomass. Facies analysis of published carbon-isotopic records indicates that the Hamersley shallow-water signal may be representative of a late Archean global signature and that it preceded a similar, but delayed, 13C enrichment of deepwater deposits. The data suggest that a global-scale expansion of oxygenated habitats accompanied the progression away from anaerobic ecosystems toward respiring microbial communities fueled by oxygenic photosynthesis before the oxygenation of the atmosphere after 2.45 billion years ago.
Keywords: organic carbon, isotopes, methanotrophy, oxygen
Resolving changes in early ecosystems and their geochemical context is critical to understanding both the means and pace of events that culminated in atmospheric oxygenation beginning possibly as early as 2.45 gigaannum (Ga) before the present, based on the sulfur isotopic record (1) but certainly by 2.3 Ga, based on other geochemical observations (2). Oxygenic photosynthesis must have evolved before this event, but conjectures for its onset range from pre-3.5 Ga (3, 4) to 2.7 Ga (5, 6) to 2.4 Ga (7). Whenever it occurred, its inevitable impact on ecosystems must have been profound.
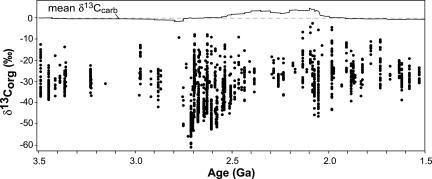
The 13C content of sedimentary organic matter represents mixed contributions from many biological materials. Isotopic signatures within modern and Phanerozoic marine sediments generally fall in a narrow range, and they reflect those of phytoplankton (8) because heterotrophic respiration imparts small, if any, isotopic shifts in the preserved organic matter. In contrast, late Archean organic carbon displays a wide range in 13C content (expressed as δ13C, ‰ = 103 (13Rsample/13Rstandard −1), and 13R = 13C/12C; Fig. 1), with values generally below −35 to −40‰ in shales (9, 10) and even lower in some shallow-water deposits at ≈2.7 Ga (Fig. 1). As hypothesized by Hayes (5, 6), strong 13C depletion in organic matter, either in bulk or as the insoluble fraction (kerogen; δker), indicates that late Archean ecosystems incorporated 13C-depleted substrates, particularly methane.
Fig. 1.
Compilation of published kerogen and total organic carbon δ13C values (δorg) for all sedimentary rock types. Data from this work are included, and the geochronology is updated (see also Supporting References, which are published as supporting information on the PNAS web site). The top curve is the mean inorganic carbon δ13C composition for marine carbonate rocks published by Shields and Veizer (16). Values are not corrected for postdepositional alteration.
If kerogen δ13C patterns in different sedimentary environments reflect differences in the dominant pathways for carbon assimilation and cycling, then the isotopic record may also reflect secular changes in microbial communities within different environmental settings. Unlike carbonate isotopic records, which are highly sensitive to marine versus lacustrine distinctions, organic-carbon isotopic signatures record the influence of biological productivity and recycling within and below the photic zone. Thus, we focus this work on relative water-column depth, and we present kerogen δ13C data for a well preserved, 150 million-year stratigraphic section from the late Archean, in which we distinguish isotopic signatures of shallow versus deepwater sedimentary environments. We specifically evaluate this isotopic record after the pronounced depletion in organic 13C signatures at 2.72 Ga, and we compare it with published isotopic records from other regions to assess global changes in microbial ecosystems during this important time.
Results
Kerogen δ13C values for 175 samples from a 2.72- to 2.57-Ga section in the Hamersley Province, Western Australia, vary between −57‰ and −28‰ (Fig. 2 A–D), spanning the published range for late Archean organic carbon. Large variations (5–16‰) occurred at 1- to 10-m scales, and they are correlated with lithology (Fig. 2 A–D). For example, in the WRL1 core, increases and decreases in dolomitic siltstone relative to shale are accompanied by enrichment and depletion of δ13C in kerogens, respectively. Similar δ13C–lithologic relationships have been observed for the 2.52- to 2.46-Ga Transvaal rocks on the Kaapvaal Craton (9, 10), although they have a narrower range in carbon isotopic compositions for bulk organic matter (δorg) range.
Fig. 2.
Depositional facies and δker profiles. (A–D) Shown are shallow-water carbonates (circles) deposited on a marine platform or other shallow setting (yellow) or turbidite (orange), mixed shale/carbonate lithologies (triangles), including shallow-water shale facies (blue) or deepwater slope facies (green), massive black shales of deepwater basin facies (black, squares), basalts (pink), carbonate breccia (white), and fluvial sandstones (brown). Symbols are larger than analytical reproducibility. Arrows denote impact-event beds. (A) WRL1 core. (B) RHDH2a core. (C) SV1 core. (D) Tumbiana Formation (Fm.) outcrop. (E) Histograms of δker with respect to depositional facies.
Facies were defined by sedimentological characteristics, and they were grouped into shallow-water deposits and deeper slope and distal basin deposits. Briefly, the upper Carawine Dolomite with abundant stromatolites is well recognized as a shelf deposit having platform architecture (11). The Tumbiana very shallow carbonate environment flanked a large, southerly subsiding rift basin (12), and it received a high flux of terrestrial detritus. The Tumbiana Formation has been interpreted as a fluvial–lacustrine deposit (13) (but others suggest that it may also contain shallow marine deposits; see ref. 14 and Supporting Discussion, which is published as supporting information on the PNAS web site). The Warrie Member overlies a transgressive shoreline deposit in northern exposures, and it contains sedimentary structures consistent with a shallow marine shelf environment (see Supporting Discussion). Carbonates of the Wittenoom Dolomite and lower Carawine Dolomite largely consist of platform (shelf) detritus within turbidite deposits (11). Finely laminated, dolomite-rich black shales (10–60 wt % dolomite), interbedded with platform carbonate deposits in the Carawine Dolomite, reflect lagoonal or other localized, restricted-circulation settings on the platform. Other finely laminated black shales and dolomitic and tuffaceous siltstones characterize deepwater slope deposits, whereas massive, pyritic black shales were deposited in basin settings more distal to the shelf.
The isotopic record shown in Fig. 2 A–D reveals both facies- and time-related signals. Over the entire period, the shallow facies show a wide range of δker values, with dolomitic shales of the restricted platform facies 2–14‰ depleted in 13C relative to the platform carbonates immediately above and below. Kerogens in deepwater shales are strongly depleted in 13C, whereas δker values for the slope facies are intermediate to those for basin and shallow-water carbonate shelf facies (Fig. 2E). Sedimentology suggests that the slope facies may have received organic matter transported and deposited from shallower environments, which was mixed with autochthonous inputs.
To assess temporal changes in δker, we merged the records in Fig. 2 by using correlations based on available U-Pb geochronology, impact spherule beds, and the overall framework of stratigraphic sections (Fig. 3 and Fig. 6, which is published as supporting information on the PNAS web site). The range of δker values broadens significantly in younger strata over the course of 100 million years. This broadening is largely the result of a ≈23‰ enrichment in carbonate-hosted δker values, which approach −28‰ near the top of the section, and the relative stability of deepwater-basin δker values from 2.7 Ga to 2.6 Ga. Regardless of its environmental setting, the lowermost unit (i.e., the Tumbiana carbonate unit) is characterized by very shallow deposition, in which restricted conditions may have existed locally. The extreme δker values recorded in this unit define the first of two prominent 10–12‰ steps in carbonate-hosted kerogen (Fig. 3) at 2.72 and 2.6 Ga. Both enrichments are exceptional, especially compared with the notable Cretaceous-to-modern rise in the δ13C of marine organic carbon, which was only 5–7‰ (15). In between, the δker signatures for the Warrie Member and Carawine Dolomite are largely consistent (−38 ± 3‰) for ≈50 million years, despite a major marine transgression and bolide impact. Shallow marine carbonate δ13C (δcarb) values remain near 0‰ throughout the study interval, reflecting stability of global average δcarb at this time (16) (Figs. 1 and 3).
Fig. 3.
Composite graph of δker data versus time. The curve represents the average in carbonate-hosted δker, but it is not quantitative. For symbol and color key, see Fig. 2. U-Pb geochronology and impact-event beds constrain correlations between cores and outcrops (OC). Inorganic-carbon isotope values from carbonates (δ13Ccarb) are near 0‰ for nonevaporitic units of the Tumbiana Formation (0.0 ± 1.2‰, n = 36) and Carawine (0.0 ± 0.8‰, n = 32) and Wittenoom Dolomites (−0.5 ± 2.0‰, n = 66), and they are consistent with δ13Ccarb for well preserved marine carbonates from five other Archean provinces (−0.3 ± 0.7‰, n = 324) (see Supporting Discussion).
All samples experienced relatively low thermal stress (metamorphism no greater than prehnite–pumpellyite grade; subgreenschist), consistent with nongraphitic kerogen H/C ratios of 0.15–0.30). Losses of carbon associated with dehydrogenation could have resulted in an isotopic enrichment of 2–2.5‰ (10) for all samples. We see no systematic relationship between total organic carbon abundance and δker values (see Supporting Discussion); thus, there is no obvious evidence for thermally related patterns in δker. There is no physical or chemical evidence for residues from nonindigenous oil migration.
Discussion
Observational data and computational models suggest that the Archean atmosphere was composed of N2, CO2, CH4, and H2 (17, 18), consistent with the suggestion that the carbon cycle was dominated by autotrophy, fermentation, acetogenesis, and methanogenesis (19, 20). Moderately high CO2 levels (up to 10× the present atmospheric level) (18, 20, 21) provided ample substrate for autotrophs, such that isotope fractionation likely approached maximum values. Assuming an 8‰ difference between carbonate and dissolved CO2, carbon fixation by ribulose-bisphosphate carboxylase/oxygenase (Rubisco) and the Calvin–Benson cycle could account for δker values as low as −37‰, as in most modern eukaryotes (form IB; maximum fractionation, ≈29‰; refs. 22 and 23). Most modern cyanobacteria have Rubisco form IB, whereas some marine species have form IA (maximum fractionation, ≈24‰; ref. 24). Still, cyanobacteria biomass typically yields less fractionation (e.g., 17‰ for Synechococcus sp.; ref. 25) because of other factors. If modern cyanobacterial biology and physiology are representative of their ancestors, then cyanobacteria most likely would have contributed δker values of 32–25‰. Photoautotrophic purple bacteria, which have Rubisco form II (maximum fractionation, 19.5‰; ref. 26), would yield similar values. Fractionations are even lower (≤14‰; ref. 27) for anoxygenic photoautotrophic bacteria that employ either the 3-hydroxypropionate pathway or the reductive tricarboxylic acid cycle. Iron abundances (<2 wt %; refs. 28 and 29) reported for Hamersley carbonates do not suggest a profuse supply of Fe(II) necessary for an ecosystem dominated by anoxygenic Fe(II)-oxidizing photoautotrophs, like that envisioned for banded iron formation deposits (7, 30). Sulfur in primary minerals is nearly absent in the Wittenoom Dolomite (most pyrite occurs in isolated roll-up laminae or as coatings of clasts), suggesting that sulfur was not the dominant redox partner for carbon in this system. Considering the limited availability of electron donors in shallow environments, the highest δker values probably represent inputs from ancestral cyanobacteria, which is consistent with the finding of molecular fossils (2α-methyl hopanes) most likely from cyanobacteria in late Archean rocks (31, 32), although difficulties interpreting the molecular record have been noted (7).
In all of the above scenarios, photoautotrophy under a high CO2 atmosphere cannot explain 13C-depleted values below −37‰. The Archean anaerobic communities envisioned by Kasting, Siefert, and coworkers (17–20) included microorganisms that degraded and recycled carbon substrates. In modern settings with anaerobic habitats, such processes yield a 13C-depleted biomass. Specifically, recycling of fermentation-derived CO2 or acetate can yield a 13C-depleted biomass, especially for anaerobes using the acetyl-CoA pathway (33). Nonfermenting acetogens, which compete for H2 with other organisms that possess greater energy-yielding metabolisms (e.g., methanogens), can produce acetate that is extremely 13C-depleted relative to CO2 (≈59‰) (34). Incorporation of this product into biomass can also yield low δorg values.
Similarly, methane oxidation and assimilation, either aerobically or anaerobically, can produce a biomass with extremely low δ13C values (5, 6, 35, 36), such as those recorded in our oldest samples. Methane oxidation requires electron acceptors such as O2 or another suitable oxidant, such as SO42− (37, 38) or NO32− (39), as observed for anaerobic methane-oxidizing consortia. The dramatic drop in δker values at ≈2.7 Ga (Fig. 1) may represent a sharp increase in the availability of oxidized electron acceptors that was directly (6) or indirectly (40) a consequence of oxygen production. If so, the ≈2.7-Ga δorg event is evidence that oxygenic photosynthesis originated by that time.
If the extremely 13C-depleted δker values of late Archean shallow-water facies represent the onset of methane assimilation triggered by oxygenic processes (6), we anticipate a subsequent trend, at least in shallow settings, from an ecosystem that recycled methane toward an ecosystem based on photoautotrophic carbon assimilation and heterotrophic carbon cycling employing electron acceptors that are thermodynamically favored over fermentative pathways. This expectation is consistent with the ≈23‰ enrichment that we observe in carbonate-hosted kerogens (Fig. 3).
The enrichment of 13C in shallow-water carbonate products is best explained by a decrease in the importance of anaerobic processes most likely involving methane recycling and an increase in heterotrophic respiration (Fig. 4). A decline in the fermentative supply of hydrogen to methanogenic and acetogenic communities must have limited carbon recycling by these anaerobic organisms in shallow waters. In deeper settings, δker values remained highly 13C-depleted, indicating that these environments continued to be dominated by anaerobic communities and were not (yet) influenced by the major changes in carbon cycling taking place in shallow waters.
Fig. 4.
Schematic illustration of interpreted ecological changes in carbon cycling and their imprint on the late Archean Hamersley δ13Cker record. Anaerobic microbial processes in strongly reducing conditions (arrows 1–3) led to 13C depletion relative to photosynthate in preserved organic carbon like that observed for deepwater facies throughout the study interval. By 2.72 Ga, incipient oxygenation of very shallow habitats enabled the incorporation of methane into biomass by organisms using O2, SO42−, or other suitable electron acceptors (arrows 4–5), producing extremely 13C-depleted biomass. Continued oxidation of shallow-water environments after 2.72 Ga favored microbial respiration (arrows 6–7), which limited the supply of acetate and hydrogen (arrow 1) to methane production and acetatogenesis. Because organic-matter degradation by respiration is accompanied by little isotopic discrimination, the isotopic signature of organic carbon preserved in shallow environments approached values of photosynthetic biomass by ≈2.60 Ga.
Atmospheric oxygenation by ≈2.3 Ga (1, 2) must have been preceded by oxidation of ocean surface waters, which likely began with phototrophic havens in isolated shallow-water environments (6). These so-called “oxygen oases,” characterized by local production of O2 and oxidized substrates, contrasted with the reducing atmosphere and deeper ocean (6, 40). Our data provide additional evidence for oxygen oases in the late Archean, and they place the development of oxygen oases in the context of specific depositional environments. The presence of environmental O2 is consistent with δ33S and δ34S records from the ≈2.63-Ga Hamersley strata that have been interpreted as indicating sulfate-limited sulfate respiration in shallow (but not deep) settings under an anoxic atmosphere (41). An ecological shift toward greater importance of heterotrophic respiration associated with oxygen oases is also consistent with rapid diversification among such organisms some time after the onset of oxygenic photosynthesis, as suggested in RNA-based phylogenetic patterns (42). Our δ13C data from the Hamersley succession document profound changes in shallow-water microbial ecosystems at the end of the Archean, and they suggest the beginnings of global carbon-cycle reorganization instigated by the sustained release of molecular oxygen into an otherwise anaerobic world.
Available data for other localities indicate that the facies–δker relationship is an Archean feature and that the shallow-water enrichment documented by our data may not have been unique to the Hamersley Province. When δorg values from Fig. 1 are sorted by environment based on published lithofacies descriptions (Fig. 5) and data from sections known to be highly altered are removed, a consistent pattern emerges. For shallow facies, the lowest δorg values (less than −45‰) are exclusively from the Hamersley (Fig. 5A), likely reflecting preservational bias and possibly limited occurrence of similar facies and ecosystems. Even so, Kaapvaal shallow-water facies δorg values show a less distinct and more protracted temporal 13C enrichment. Other shallow-water δorg values come from the Hamersley, the Wabigoon Belt (Superior Province), and the Belingwe Greenstone Belt (Zimbabwe Craton), and they have δorg values similar to those of the mid-Archean. These values account for the upward spread in δorg data of the 2.7- to 2.6-Ga global record (Fig. 1). Although some 13C enrichment in these data can be attributed to alteration during Greenschist metamorphism, the isotopic record suggests that shallow-water ecosystems dominated by methane assimilation may have been highly sensitive to environmental conditions and possibly favored in restricted, stromatolitic settings. Notably, the Hamersley 13C enrichment in shallow-water units over time coincides with the regional expansion of carbonate shelf environments. Thus, the Hamersley record probably reflects localized-to-regional effects driven by regional tectonic evolution that nonetheless also reflect the global rise in oxygenation. Additional, facies-specific high-resolution δker records will help to provide a better understanding of environmental influences on late Archean and early Proterozoic δker patterns.
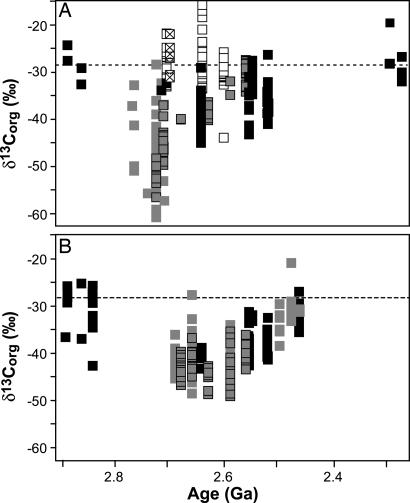
Fig. 5.
Temporal distribution of δorg values of the global record separated into shallow (A) and deepwater (B) facies. Data were reported previously in the literature for the Hamersley Province (Pilbara Craton; gray), Kaapvaal Craton (black), Zimbabwe (white), and Superior Province (white with X) and in this work (Hamersley Province; gray with black outline; restricted platform facies not included in A). Data from known highly metamorphosed units, units of uncertain facies, and oxide-BIF units were excluded. Dashed lines approximate the expected δorg composition if organic carbon were derived solely from photoautotrophs.
Temporally resolved δorg records for deepwater facies are mostly limited to the Kaapvaal and Hamersley regions, although 2.69-Ga sulfidic shales with δorg values less than or equal to −45‰ have been cited for the Keewatin Group (Superior Province) (35, 43). The 2.72- to 2.59-Ga Hamersley δorg record (see Fig. 3) shows little variability in deepwater facies; however, after 2.59 Ga, other Hamersley and Kaapvaal δorg data indicate a deepwater temporal 13C enrichment of ≈13‰ (Fig. 5B). Initiation of this shift lags behind the Hamersley shallow-water δorg enrichment by >100 million years, yet both approached −28‰, approximating the expected photosynthate value, between ≈2.45 and 2.3 Ga. These enrichment patterns suggest that by ≈2.45 Ga, the deepwater environments experienced changes in cycling of photosynthate carbon in the upper water column much like the shift that occurred in shallow environments. We suggest that the 13C enrichment reflects decreased dominance of anaerobic processes associated with the expansion of photoautotrophic oxygenation into the photic zones of offshore environments over this time. Our interpretation is supported by extensive iron deposition and diminishing mass-independent isotope signature in pyrite sulfur from deepwater shales (1) beginning at ≈2.45 Ga. Perhaps the lag between shallow and deepwater isotopic enrichment reflects a shift in cyanobacterial habitats from sediments in the photic zone of shallow environments to photic-zone water columns inclusive of deepwater environments.
In summary, our facies-specific dissection of the organic-carbon isotopic record reveals temporal and spatial patterns that document profound late Archean and early Proterozoic biogeochemical changes preceding atmospheric oxygenation after 2.45 Ga. Specifically, facies-resolved isotopic records indicate that microbial communities in shallow environments led a global-scale transition away from purely anaerobic communities. The isotopic depletion characteristic of anaerobic ecosystems and methane-assimilating communities was gradually replaced by 13C-enriched signatures, consistent with a growing importance of cyanobacterial inputs accompanied by heterotrophic respiration. Ultimately, this change was fueled by increased availability of electron acceptors derived from oxygenic photosynthesis in photic zones. These data support the conclusion that oxygenic photosynthesis, originating some time before 2.72 Ga, eventually triggered the rise of aerobic ecosystems and fueled their expansion from shallow settings into the photic zones of deepwater environments between 2.72 and ≈2.45 Ga.
Materials and Methods
We used conventional methods to isolate kerogen fractions from rocks, measure δ13C values of kerogen (δker), and determine organic and inorganic carbon and sulfur elemental abundances (Supporting Materials and Methods; Figs. 7 and 8 and Table 1, which are published as supporting information on the PNAS web site). Stratigraphy and sedimentology were derived from our field and core descriptions, and they were supplemented by observations published in the geologic literature. Drill core and outcrop rock samples were collected from a 2.72- to 2.57-Ga section in the Hamersley Province, Western Australia.
Supplementary Material
Acknowledgments
We thank John M. Hayes for sharing his insight, providing advice on revisions, and handling the review of this manuscript. We also thank Marilyn Fogel, Roger Summons, David DesMarais, Yanan Shen, George Cody, Jim Kasting, Jenn Macalady, Shuhei Ono, and others for constructive comments on early manuscript drafts. We are grateful to Rio Tinto Exploration for providing access to core samples, Matt Hurtgen for field assistance, and Mark Barley and Brian Krapez for field guidance. This work was supported by National Science Foundation Grants EAR-00-73831 and EAR-00-80267 (both to K.H.F.), an American Association for Petroleum Geologists Grant-in-Aid (to J.L.E.), a Pennsylvania Space Grant Consortium fellowship [National Aeronautics and Space Administration (NASA) Grant NGT5-40090] (to J.L.E.), NASA Astrobiology Institute Cooperative Agreement NNA04CC06A (Penn State Astrobiology Research Center) (to K.H.F. and J.L.E.), and NASA Astrobiology Institute Cooperative Agreements NNA04CC09A (Carnegie Institution of Washington) and the Geophysical Laboratory, Carnegie Institution of Washington (to J.L.E.).
Abbreviations
- δcarb
carbon isotopic composition of inorganic carbon of carbonates
- δker
carbon isotopic composition of kerogen
- δorg
carbon isotopic composition of bulk organic matter
- Ga
gigaannum before present
- Rubisco
ribulose-bisphosphate carboxylase/oxygenase.
Footnotes
The authors declare no conflict of interest.
References
- 1.Farquhar J, Wing B. Earth Planet Sci Lett. 2003;213:1–13. [Google Scholar]
- 2.Holland H. The Chemical Evolution of the Atmosphere and Oceans. Princeton: Princeton Univ Press; 1984. p. 598. [Google Scholar]
- 3.Cloud P. In: Earth's Earliest Biosphere: Its Origin and Evolution. Schopf J, editor. Princeton: Princeton Univ Press; 1983. pp. 14–31. [Google Scholar]
- 4.Rosing M, Frei R. Earth Planet Sci Lett. 2004;217:237–244. [Google Scholar]
- 5.Hayes J. In: Early Life on Earth, Nobel Symposium No. 84. Bengston S, editor. New York: Columbia Univ Press; 1994. pp. 220–236. [Google Scholar]
- 6.Hayes J. In: Earth's Earliest Biosphere: Its Origin and Evolution. Schopf J, editor. Princeton: Princeton Univ Press; 1983. pp. 291–301. [Google Scholar]
- 7.Kopp R, Kirshvink J, Hilburn I, Nash C. Proc Natl Acad Sci USA. 2005;102:131–136. doi: 10.1073/pnas.0504878102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freeman K. In: Stable Isotope Geochemistry. Valley JW, Cole DR, editors. Vol 43. Washington, DC: Min Soc Am; 2001. pp. 579–605. [Google Scholar]
- 9.Strauss H, Beukes N. Precambrian Res. 1996;79:57–71. [Google Scholar]
- 10.Des Marais D. In: Stable Isotope Geochemistry. Valley JW, Cole DR, editors. Vol 43. Washington, DC: Min Soc Am; 2001. pp. 555–578. [Google Scholar]
- 11.Simonson B, Schubel K, Hassler S. Precambrian Res. 1993;60:287–335. [Google Scholar]
- 12.Blake T, Buick R, Brown S, Barley M. Precambrian Res. 2004;133:143–173. [Google Scholar]
- 13.Buick R. Science. 1992;255:74–77. doi: 10.1126/science.11536492. [DOI] [PubMed] [Google Scholar]
- 14.Sakurai R, Ito M, Ueno Y, Kitajima K, Maruyama S. Precambrian Res. 2005;138:255–273. [Google Scholar]
- 15.Arthur M, Dean W, Claypool G. Nature. 1985;315:216–218. [Google Scholar]
- 16.Shields G, Veizer J. Geochem Geophys Geosyst. 2002;3:1031. [Google Scholar]
- 17.Kasting J. Precambrian Res. 2005;137:119–129. [Google Scholar]
- 18.Kharecha P, Kasting J, Siefert J. Geobiology. 2005;3:53–76. [Google Scholar]
- 19.Kasting J, Siefert J. Science. 2002;296:1066–1068. doi: 10.1126/science.1071184. [DOI] [PubMed] [Google Scholar]
- 20.Pavlov A, Kasting J, Brown L, Rages K, Freedman R. J Geophys Res. 2000;105:981–990. doi: 10.1029/1999je001134. [DOI] [PubMed] [Google Scholar]
- 21.Nakamura K, Kato Y. Geochim Cosmochim Acta. 2004;68:4595–4618. [Google Scholar]
- 22.Roeske C, O'Leary M. Biochemistry. 1984;23:6275–6284. [Google Scholar]
- 23.Tabita F. Photosynth Res. 1999;60:1–28. [Google Scholar]
- 24.Scott K, Schwedock J, Schrag DP, Cavanaugh CM. Environ Microbiol. 2004;6:1210–1219. doi: 10.1111/j.1462-2920.2004.00642.x. [DOI] [PubMed] [Google Scholar]
- 25.Popp B, Laws E, Bidigare R, Dore J, Hanson K, Wakeham S. Geochim Cosmochim Acta. 1998;62:66–77. [Google Scholar]
- 26.Robinson J, Scott K, Swanson S, O'Leary M, Horken K, Tabita F, Cavanaugh C. Limnol Oceanogr. 2003;48:48–54. [Google Scholar]
- 27.Sirevåg R. In: Anoxygenic Photosynthetic Bacteria: Advances in Photosynthesis. Blankenship RE, Madigan MT, Bauer CE, editors. Vol 2. Dordrecht, The Netherlands: Kluwer; 1995. pp. 871–883. [Google Scholar]
- 28.Lindsay J, Brasier M. Precambrian Res. 2002;114:1–34. [Google Scholar]
- 29.Veizer J, Clayton R, Hinton RW, Brunn V, Mason T, Buck S, Hoefs J. Geochim Cosmochim Acta. 1990;54:2217–2729. [Google Scholar]
- 30.Konhauser K, Hamade T, Raiswell R, Morris R, Ferris F, Southam G, Canfield D. Geology. 2002;30:1079–1082. [Google Scholar]
- 31.Summons R, Jahnke L, Hope J, Logan G. Nature. 1999;400:554–557. doi: 10.1038/23005. [DOI] [PubMed] [Google Scholar]
- 32.Brocks J, Logan G, Buick R, Summons R. Science. 1999;285:1033–1036. doi: 10.1126/science.285.5430.1033. [DOI] [PubMed] [Google Scholar]
- 33.House C, Schopf J, Stetter K. Org Geochem. 2003;34:345–356. [Google Scholar]
- 34.Gelwicks J, Risatti J, Hayes J. Org Geochem. 1989;14:441–446. doi: 10.1016/0146-6380(89)90009-0. [DOI] [PubMed] [Google Scholar]
- 35.Schoell M, Wellmer F. Nature. 1981;290:696–699. [Google Scholar]
- 36.Hinrichs K-U. Geochem Geophys Geosyst. 2002;3:1042. [Google Scholar]
- 37.Orphan V, House C, Hinrichs K-U, McKeegan K, DeLong E. Science. 2001;293:484–487. doi: 10.1126/science.1061338. [DOI] [PubMed] [Google Scholar]
- 38.Hinrichs K-U, Hayes J, Sylva S, Brewer P, DeLong E. Nature. 1999;398:802–805. doi: 10.1038/19751. [DOI] [PubMed] [Google Scholar]
- 39.Raghoebarsing A, Pol A, van de Pas-Schoonen K, Smolders A, Ettwig K, Rijpstra W, Schouten S, Sinninghe J. Nature. 2006;440:918–921. doi: 10.1038/nature04617. [DOI] [PubMed] [Google Scholar]
- 40.Kasting J, Holland H, Kump L. In: The Proterozoic Biosphere: A Multidisciplinary Study. Schopf J, Klein C, editors. Cambridge: Cambridge Univ Press; 1992. pp. 159–164. [Google Scholar]
- 41.Ono S, Eigenbrode J, Pavlov A, Kharecha P, Rumble D, Kasting J, Freeman K. Earth Planet Sci Lett. 2003;213:15–30. [Google Scholar]
- 42.Sheridan P, Freeman K, Brenchley J. Geomicrobiol J. 2003;20:1–14. [Google Scholar]
- 43.Strauss H. Geochim Cosmochim Acta. 1986;50:2653–2662. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.