Abstract
Complex biological systems are increasingly understood in terms of the algorithms that guide the behavior of system components and the information pathways that link them. Much attention has been given to robust algorithms, or those that allow a system to maintain its functions in the face of internal or external perturbations. At the same time, environmental variation imposes a complementary need for algorithm versatility, or the ability to alter system function adaptively as external circumstances change. An important goal of systems biology is thus the identification of biological algorithms that can meet multiple challenges rather than being narrowly specified to particular problems. Here we show that emigrating colonies of the ant Temnothorax curvispinosus tune the parameters of a single decision algorithm to respond adaptively to two distinct problems: rapid abandonment of their old nest in a crisis and deliberative selection of the best available new home when their old nest is still intact. The algorithm uses a stepwise commitment scheme and a quorum rule to integrate information gathered by numerous individual ants visiting several candidate homes. By varying the rates at which they search for and accept these candidates, the ants yield a colony-level response that adaptively emphasizes either speed or accuracy. We propose such general but tunable algorithms as a design feature of complex systems, each algorithm providing elegant solutions to a wide range of problems.
Keywords: biological complexity, plasticity, robustness
Complex systems arise when large numbers of functionally diverse units interact selectively and nonlinearly to produce coherent patterns (1, 2). Understanding complexity, which characterizes every level of biological hierarchy from genes to ecosystems, poses major empirical and theoretical challenges (2–5). A growing computational approach attempts to understand complex systems by identifying the communication pathways among units and the algorithms governing their interactions (6). This line of attack has elucidated the function of complex biochemical systems, including bacterial chemotaxis (7), intracellular signaling cascades (8), and the specificity and sensitivity of T cell recognition (9). It also has been applied to the organization of insect societies, where complex interactions among workers yield adaptive colony-level behaviors, such as efficient division of labor and the construction of sophisticated nests (4, 10). Expressing the behavior of complex systems in terms of underlying algorithms allows hypotheses to be “dry-tested” by using computer simulations (2). It also helps to reveal fundamental design features, such as feedback control, redundancy, and modularity (11, 12).
An important design feature of biological algorithms is the ability to perform robustly under variable environmental conditions (7, 11, 13, 14). For example, the bacteria Escherichia coli can detect and climb chemical gradients over a wide range of absolute concentrations. This ability emerges directly from the connectivity of its receptor complexes, each of which follows a simple activation/deactivation algorithm, rather than “fine-tuning” of parameters to particular chemical concentrations (7). Similar robustness to external conditions is observed in T cells (9), gene regulatory networks (15), and foraging honey bee colonies (16). These examples emphasize homeostasis, or the maintenance of a constant state or function in the midst of a variable environment. In a wide array of situations, however, biological systems must instead alter their function adaptively as conditions change. For example, a forager's sensitivity to risk can rise markedly with its energy budget (17), and a decision-maker's relative valuation of speed and accuracy of choice varies with decision urgency (18, 19). In these cases, an algorithm can perform robustly only by remaining sensitive to changes in conditions. In this study, we determine whether emigrating ant colonies achieve this kind of sensitivity by tuning a decision algorithm to emphasize either speed or accuracy.
Ants of the genus Temnothorax use a well understood algorithm to solve the problem of choosing a new home after destruction of their fragile nest cavity (typically a rock crevice or hollow nut) (20–25). The colony can decide among multiple options of different quality without the necessity for direct comparison among sites by well informed individuals. A decision instead emerges from a competition among recruitment efforts launched by independent discoverers at each site. The competition is mediated by an active minority of ants who scout for sites and employ a distinctive process of graded commitment to any that they find (Fig. 1). This algorithm enhances the probability of the colony ending up in the best available nest, but the ants' behavior is dominated by the need to quickly end their dangerous exposure. As a result, colonies frequently split among multiple sites or even fail to select the best one available.
Fig. 1.
Decision algorithm used by each active ant during colony emigration. Search consists of exploratory journeys from the old nest and gives way to assessment once the ant finds a site. Assessment ceases at a rate that depends on site quality, and the next behavior is chosen on the basis of a quorum rule. At low site population, the ant canvasses her fellow active ants by leading them to the site in slow tandem runs (43). Once the population has surpassed a threshold, the ant fully commits to the site and uses speedy transports to bring the passive adults and brood that constitute the majority of the colony.
Colonies can also organize emigrations and choose among nest sites in a very different circumstance: when their old nest remains intact but better quality nests become available nearby. In these “unforced” emigrations, the ants do not face a critical need for immediate shelter but instead can deliberate among alternatives from relative safety. Colonies take longer to complete such emigrations than they do when faced with “forced” emigrations occasioned by the destruction of their old nest (26), possibly improving their likelihood of moving directly to the best site. Thus, these two situations potentially represent extremes of a speed/accuracy tradeoff: forced emigrations emphasizing speed and unforced emigrations emphasizing accuracy.
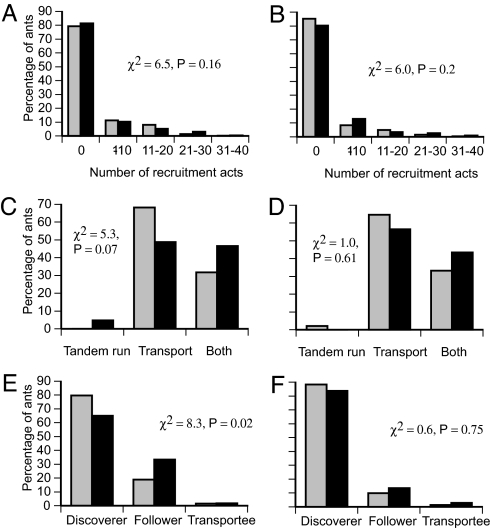
To determine whether ants use the same decision algorithm regardless of urgency, we first simulated unforced emigrations by using a fuller version of the algorithm shown in Fig. 1 (see Fig. 6, which is published as supporting information on the PNAS web site). In previous work, we derived this algorithm and accurately measured its parameters by using detailed observation of individual ants during forced migrations (24). The algorithm's 19 behavioral states and 44 parameters provide a thorough description of the decision rules and information pathways used by active ants. Simulations based on this algorithm reliably predict the behavior of colonies in forced emigrations (24). In this study, we set the algorithm's parameters to values measured in unforced emigrations to determine whether it can also account for behavior in those conditions. Parameters were measured from six unforced emigrations by three colonies moving into either good or mediocre nests. The resulting simulations effectively reproduced the colonies' observed behavior. Recruitment acts per ant, methods of arrival at the new site, and numbers of ants performing each recruitment type were largely indistinguishable between observed and simulated data (Fig. 2). Emigration dynamics were also well reproduced, with both observed and simulated emigrations reaching most key milestones at similar times (see Table 1, which is published as supporting information on the PNAS web site).
Fig. 2.
Predicted (gray bars) and observed (black bars) values for the distribution of recruitment activity, recruitment type, and arrival type in unforced emigrations. (A and B) Histogram of number of recruitment acts by each active ant. (C and D) Histogram of recruitment methods used by each active ant. (E and F) Histogram of method of arrival by active ants at the new nest. (A, C, and E) Emigrations to good nests. (B, D, and F) Emigrations to mediocre nests.
Another series of simulations examined how the ants might trade off decision speed and accuracy by tuning key parameters of their decision algorithm as urgency changes. In these simulations, colonies simultaneously had available two new nest sites, one good and one mediocre. Parameters were set to baseline values estimated from six forced emigrations by three colonies. Three parameters were then systematically varied to test their influence on colony performance: (i) Search, the probability that an ant leaves the old nest to search for new sites; (ii) Accept, the rate at which an ant begins recruitment to a site she has found; and (iii) Quorum, the threshold population of the new nest at which recruiters switch from tandem running to transporting. The switch to transport accelerates recruitment and marks a scout's highest level of commitment to choosing a site as the colony's new home. One hundred simulations were run for each combination of values. In each simulation, we measured the speed of emigration as the time at which the old site was completely abandoned and the accuracy of decision-making as the proportion of the colony's members inside the good site at the time of old nest abandonment.
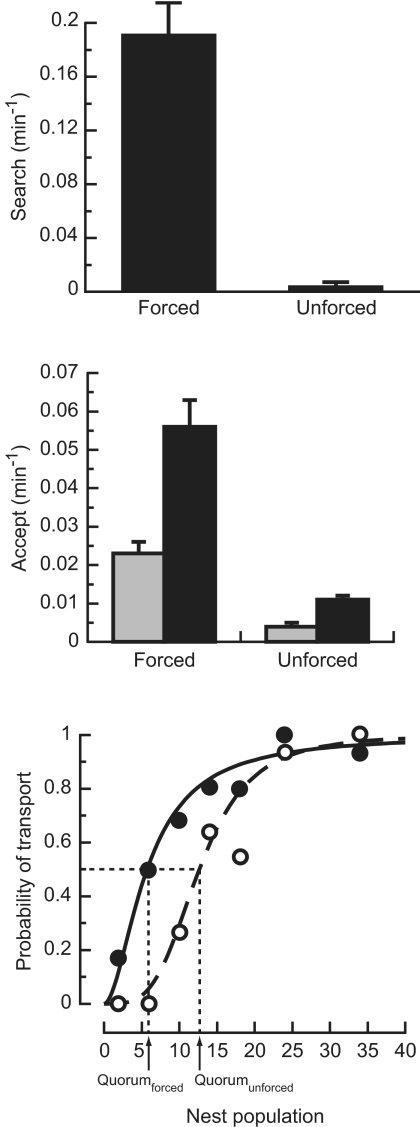
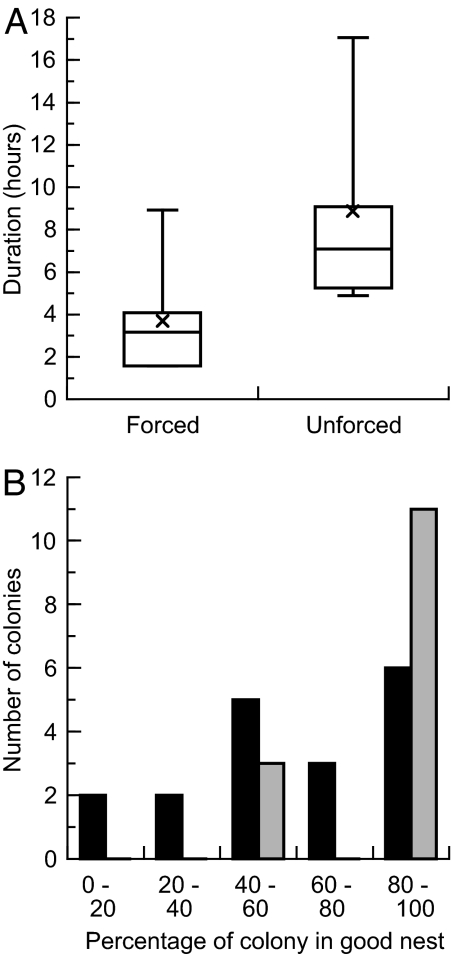
The results showed significant effects of all three parameters on speed and accuracy (Fig. 3). Higher values of Accept and Search led to shorter emigrations with more splitting between the good and mediocre nests. Raising Quorum had more subtle effects in the opposite direction, with reduced splitting frequency and a relatively small impact on speed. Data from actual colonies showed that all three of these parameters differed significantly between unforced and forced emigrations. Both Search and Accept were higher in forced moves, and Quorum was lower (Fig. 4). By using these observed values, we ran additional simulations to predict the relative speed and accuracy of decision-making expected in each type of emigration. The results predicted markedly faster and less accurate decision-making in forced emigrations, with colonies abandoning the old nest in 87 ± 77 min and bringing 84 ± 11% of their population to the good nest. The corresponding values for the unforced case were 565 ± 314 min and 97 ± 7% (n = 1,000 simulations in each condition). These predictions were confirmed by the actual performance of 18 colonies choosing between good and mediocre nests under high or low urgency (Fig. 5). Forced emigrations finished significantly earlier than unforced ones (Wilcoxon test: W = 252, P < 0.0001), and they were significantly less accurate, with only 65 ± 32% of each colony's population ending up in the good nest, compared with 90 ± 18% for the unforced treatment (Wilcoxon test: W = 67, P < 0.05).
Fig. 3.
Predicted effects of Accept, Search, and Quorum on the duration and decision-making accuracy of emigrations. In each image, two of the three parameters are varied independently; all other parameters are set to values estimated from forced emigrations. Plots show mean duration and accuracy over 100 simulations at each parameter combination. Quorum is the threshold population at which the probability of switching to transport reaches 50%. Accept gives the recruitment initiation rate at good nests; the rate for mediocre nests was obtained by multiplying by the factor 0.52 (the ratio of observed values of Accept for mediocre and good nests).
Fig. 4.
Effect of emigration urgency on key parameters of the ants' decision algorithm. (Top) Ants in forced conditions have significantly higher Search, meaning that scouts are more likely to leave the old nest to look for a new one (X12 = 151, P < 0.001, n = 119). (Middle) Greater urgency also leads to higher Accept, so that scouts who find a nest begin recruiting to it more quickly (X12 = 166, P < 0.001, n = 401). Regardless of urgency, Accept is higher to good nests (black bars) than to mediocre nests (gray bars), enhancing the likelihood of the colony choosing the better nest (unforced: X12 = 28, P < 0.001, n = 193; forced: X12 = 30, P < 0.001, n = 208). Brackets show standard errors. (Bottom) Quorum is significantly lower in forced emigrations, meaning that a recruiter's probability of switching from slow tandem runs to fast transports rises to 50% at lower site populations and thus earlier in the emigration (ANOVA: F4142 = 37, P < 0.0001). In forced emigrations (filled circles and solid line), Quorum is only 5.7 ± 0.5 ants, compared with 12.7 ± 0.6 ants for the unforced case (open circles and dotted line).
Fig. 5.
Speed and accuracy of decision-making in forced and unforced emigrations. (A) Time until all colony members have been moved out of the old nest for each treatment, showing that higher urgency induces faster emigrations. The ends of each box mark the upper and lower quartiles, and the horizontal line inside the box gives the median. The cross marks the mean, and brackets show the data range. (B) Histograms of the degree to which colonies split between the good and mediocre new nest sites, for forced (black bars) and unforced (gray bars) emigrations. Under higher urgency, colonies are more likely to divide their population between the sites, or even to move entirely into the worse nest, rather than achieving consensus on the single best site.
These results show that an algorithm originally described in emergency moves can account in detail for the behavior of colonies in deliberative moves. Moreover, we found that the ants tune the algorithm's parameters to match the distinct demands of each situation, emphasizing either decision speed or accuracy. The parameters exerting the greatest influence on this tradeoff are the rates for initiating search and recruitment. These rates determine how rapidly an ant ascends a scale of increasingly strong commitment to a site that she has found (Fig. 1). Other complex decision-making systems, both social (27) and intracellular (9, 28, 29), incorporate analogous multistep schemes. The central importance of speed/accuracy tradeoffs to decision-making may explain this ubiquity. Indeed, these other systems may also tune the rates at which one step gives way to the next according to decision urgency.
Quorums are another widespread property of decision-making systems (30–32). Earlier work on T. albipennis emphasized how quorum size changed with environmental conditions under forced emigrations (26, 33). Our study suggests, however, that quorum size has only a small effect on the speed/accuracy tradeoff. Instead, computer simulations predict that reliance on a quorum consistently improves decision accuracy without imposing a large cost in speed. For example, the quorum of 11.3 seen in unforced emigrations is predicted to improve accuracy by 40.7% compared with a quorum of zero. The corresponding effect on emigration speed is only 5.7%. This insensitivity of speed probably reflects a tradeoff between time devoted to recruiting transporters and time taken by those transporters to move the colony (22). At the same time, the quorum enhances decision accuracy by reducing the effect of errors made by individual units and by ensuring that information spreads through the system before a decision is made.
Other group-living organisms face decision-making problems similar to those of emigrating Temnothoraxcolonies. Although they use different behavioral tools, their collective decision mechanisms share fundamental features with the algorithm described here. Both Messor ants (34) and honey bees (27) rely on a recruitment system that amplifies local population densities through positive feedback: mass recruitment by pheromone trails and the dance language, respectively. Both species also make key behavioral changes on the basis of local nest mate numbers. Social spiders carry out coordinated emigrations if their nest is damaged or as part of a partially nomadic life history (35). For them, reinforcement of routes by the addition of silk draglines may play a role analogous to pheromone trails (36, 37). Cockroaches decrease their probability of leaving a temporary shelter as a function of its population, leading to collective selection of only one out of a number of locally available shelters (38). Bacteria have intricate information-sharing mechanisms, including quorum-sensing, which they deploy to make collective decisions affecting sporulation, virulence, gene exchange, and other traits (39). As long as individuals can respond to changes in urgency by adjusting key features of their behavior, then these societies may also be able to tune their decision algorithms to different environmental contingencies.
All collective decision-makers must balance speed and accuracy, but the optimal balance is likely to vary widely with species and context. For example, the relative value of accuracy may rise with the cost of nest moving, determined by factors such as colony size or distance to the new nest. Another important cost is that of secondary moves needed to correct an inferior initial choice, when speed is favored at the cost of accuracy. Honey bees, whose decision mechanism bears many resemblances to that of Temnothorax (27), invest heavily in comb construction, brood-rearing, and food storage at their nest. Because most of these costs cannot be recouped should the colony later have to abandon an inferior site, bees might be expected to place an unusually high premium on making an accurate choice the first time. The relative importance of speed and accuracy should also vary for foraging decisions. For example, colonies relying on rapid exploitation of ephemeral food sources may place more value on speed than those that invest heavily in a long-term food source.
A tunable algorithm represents a general means for complex biological systems to solve multiple problems without needing specific solutions for each one. It is reminiscent of many self-organized algorithms that show high sensitivity to environmental conditions, allowing them to adapt their behavior to prevailing circumstances (4, 40). For example, as the local density of pheromone-depositing ants increases, they collectively switch their foraging strategy from random search to pheromone trails (41). In this case, individual ants do not change their behavior, but instead rely on the appropriate collective structure to self-organize as a result of changes in the environment. The tunable algorithm described here, in contrast, works on quantitative alteration in the behavior of the group members themselves. Because this tunability offers greater control to the collective, these algorithms may be a common design feature of complex systems, each providing efficient solutions to a variety of problems. In this sense, they underscore the close links between a system's adaptive flexibility and the kind of functional complexity that results from interplay among hierarchical levels (42). A colony's complex organization and behavior emerge from the cooperative interactions of its members, but this group-level complexity in turn shapes and expands the capabilities of individual workers, granting them the capacity to adaptively alter group behavior as the changing environment requires.
Methods
Colonies, Nests, and Emigrations.
Parameter estimates were based on emigrations by three queenright colonies of Temnothorax curvispinosus with 120–190 workers and ample brood. All workers were uniquely paint-marked to allow quantification of individual behavior. Each colony emigrated four times: in forced and unforced conditions to either a good or a mediocre nest. Nests consisted of a wood slat (2.4 mm thick) with a cavity cut through its center, sandwiched between two glass microscope slides (50 × 76 mm). An entrance hole was drilled through the center of the upper slide. Good and mediocre sites had rectangular cavities of the same size (25 × 33 mm), but good sites had a smaller entrance (1.6 vs. 4.8 mm diameter). In the forced treatment, the colony's original good-quality nest was destroyed to induce emigration. In the unforced treatment, the original nest remained intact but was of poor quality, so that the ants could improve their situation by moving. Poor nests had both a small, round cavity (17 mm diameter) and a very large entrance (9.5 mm diameter). Colonies strongly prefer the good design to the mediocre one and either of these to the poor one (25).
Emigrations were observed in a fiberglass tray (75 × 60 × 7 cm). The old and new nests were placed near opposite walls, 65 cm apart. Observations began when the new nest was made available to the ants; in the forced emigrations, this coincided with the destruction of the old nest by removal of its roof. Video cameras recorded all activity at both the old and new nests for up to 8 hours. If the emigration was still underway when videotaping ceased, the colony was checked daily until it had completed its move. Further information on these emigrations is presented elsewhere (25).
To test for tradeoffs between speed and accuracy of decision-making, each of 18 colonies was twice induced to choose between a good and a mediocre nest site, once in forced and once in unforced conditions. In each emigration, the colony in its old nest was placed against the center of one wall of a square polystyrene dish (20 × 20 cm). The two new nests were placed in the opposite corners of the dish. Precautions were taken against confounding effects of testing order or side bias. Colonies were checked periodically to determine the time at which the old site was completely abandoned and the proportion of the colony's members inside the good site at that time.
Simulations.
We used a described algorithm (24) and estimated its parameters from data pooled for all three individually marked colonies. Methods followed those published earlier (24), except for minor departures detailed in Supporting Text, which is published as supporting information on the PNAS web site. The algorithm was encoded in Objective C to produce a discrete-time agent-based simulation. For each colony, we simulated 500 emigrations to both mediocre and good nests. Each run used a colony-specific population, but other parameters were identical across all simulations.
We compared simulated and observed unforced emigrations by using four performance statistics: (i) the distribution of recruitment effort across individual ants; (ii) the proportion of active ants arriving at the new nest by each possible method (independent discovery, after a tandem run, and being transported); (iii) the proportion of ants performing each recruitment type (tandem runs and transports); and (iv) the duration between successive emigration milestones (emigration start, first tandem run, first transport, and completion of 50% of transports). The first three statistics were evaluated with a χ2 test, using the simulation results as expected frequencies. For the durations, we tested the null hypothesis that the data and the simulation had the same mean, using the statistic
where Y and S are, respectively, the mean and standard deviation of the data, and μ is the mean of the simulation. Assuming a normal distribution for the durations, t is Student-t distributed with two degrees of freedom.
Effect of Urgency on Parameter Values.
Accept was estimated by survival analysis of the duration between each active ant's first entry into the new site and her first recruitment to it. The effect of emigration urgency on Accept was tested by applying analysis of deviance to this survival model and to another that included urgency as a covariate. An analogous approach tested for an effect of new nest quality. The parameter Search was similarly tested by survival analysis of the duration of each stay in the old nest before an exploratory journey. To estimate Quorum, we determined the proportion Y of transports in groups of recruitment acts binned by site population P. We then fit these data to the Hill function
where Quorum gives the population at which Y = 0.5 and k determines the nonlinearity of the relationship. To detect an effect of emigration urgency, we fit another model with separate Quorum and k for forced and unforced emigrations. An ANOVA then determined whether the more complex model significantly improved the fit.
Supplementary Material
Acknowledgments
S.C.P. was supported by Pew Charitable Trusts Award 2000-002558. D.J.T.S. was supported by a Royal Society University Research Fellowship.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
References
- 1.Hasty J, McMillen D, Collins JJ. Nature. 2002;420:224–230. doi: 10.1038/nature01257. [DOI] [PubMed] [Google Scholar]
- 2.Kitano H. Foundations of Systems Biology. Cambridge, MA: MIT Press; 2001. [Google Scholar]
- 3.Bar-Yam Y. Dynamics of Complex Systems. Reading, MA: Addison–Wesley; 1997. [Google Scholar]
- 4.Camazine S, Deneubourg JL, Franks NR, Sneyd J, Theraulaz G, Bonabeau E. Self-Organization in Biological Systems. Princeton: Princeton Univ Press; 2001. [Google Scholar]
- 5.Levin SA. Ecosystems. 1998;1:431–436. [Google Scholar]
- 6.Kitano H. Nature. 2002;420:206–210. doi: 10.1038/nature01254. [DOI] [PubMed] [Google Scholar]
- 7.Alon U, Surette MG, Barkai N, Leibler S. Nature. 1999;397:168–171. doi: 10.1038/16483. [DOI] [PubMed] [Google Scholar]
- 8.Schoeberl B, Eichler-Jonsson C, Gilles ED, Muller G. Nat Biotechnol. 2002;20:370–375. doi: 10.1038/nbt0402-370. [DOI] [PubMed] [Google Scholar]
- 9.George AJT, Stark J, Chan C. Trends Immunol. 2005;26:653–659. doi: 10.1016/j.it.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 10.Fewell JH. Science. 2003;301:1867–1870. doi: 10.1126/science.1088945. [DOI] [PubMed] [Google Scholar]
- 11.Stelling J, Sauer U, Szallasi Z, Doyle FJ, Doyle J. Cell. 2004;118:675–685. doi: 10.1016/j.cell.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 12.Sumpter DJT. Philos Trans R Soc London B. 2006;361:5–22. doi: 10.1098/rstb.2005.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carlson JM, Doyle J. Proc Natl Acad Sci USA. 2002;99:2538–2545. doi: 10.1073/pnas.012582499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kitano H. Nat Rev Genet. 2004;5:826–837. doi: 10.1038/nrg1471. [DOI] [PubMed] [Google Scholar]
- 15.von Dassow G, Meir E, Munro EM, Odell GM. Nature. 2000;406:188–192. doi: 10.1038/35018085. [DOI] [PubMed] [Google Scholar]
- 16.Seeley TD. The Wisdom of the Hive. Cambridge, MA: Belknap, Harvard Univ Press; 1995. [Google Scholar]
- 17.Bateson M, Kacelnik A. In: Cognitive Ecology: The Evolutionary Ecology of Information Processing and Decision Making. Dukas R, editor. Chicago: Univ of Chicago Press; 1998. pp. 297–341. [Google Scholar]
- 18.Chittka L, Dyer AG, Bock F, Dornhaus A. Nature. 2003;424:388. doi: 10.1038/424388a. [DOI] [PubMed] [Google Scholar]
- 19.Smith PL, Ratcliff R. Trends Neurosci. 2004;27:161–168. doi: 10.1016/j.tins.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 20.Franks NR, Pratt SC, Mallon EB, Britton NF, Sumpter DJT. Philos Trans R Soc London B. 2002;357:1567–1583. doi: 10.1098/rstb.2002.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pratt SC, Pierce NE. Anim Behav. 2001;62:281–287. [Google Scholar]
- 22.Pratt SC, Mallon EB, Sumpter DJT, Franks NR. Behav Ecol Sociobiol. 2002;52:117–127. [Google Scholar]
- 23.Pratt SC. Behav Ecol. 2005;16:488–496. [Google Scholar]
- 24.Pratt SC, Sumpter DJT, Mallon EB, Franks NR. Anim Behav. 2005;70:1023–1036. [Google Scholar]
- 25.Pratt SC. Insectes Sociaux. 2005;52:383–392. [Google Scholar]
- 26.Dornhaus A, Franks NR, Hawkins RM, Shere HNS. Anim Behav. 2004;67:959–963. [Google Scholar]
- 27.Seeley TD, Visscher PK, Passino KM. Am Sci. 2006;94:220–229. [Google Scholar]
- 28.Furdui CM, Lew ED, Schlessinger J, Anderson KS. Mol Cell. 2006;21:711–717. doi: 10.1016/j.molcel.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 29.Xie HF, Ye M, Feng R, Graf T. Cell. 2004;117:663–676. doi: 10.1016/s0092-8674(04)00419-2. [DOI] [PubMed] [Google Scholar]
- 30.Conradt L, Roper TJ. Trends Ecol Evol. 2005;20:449–456. doi: 10.1016/j.tree.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 31.Seeley TD, Visscher PK. Behav Ecol Sociobiol. 2004;56:594–601. [Google Scholar]
- 32.Waters CM, Bassler BL. Annu Rev Cell Dev Biol. 2005;21:319–346. doi: 10.1146/annurev.cellbio.21.012704.131001. [DOI] [PubMed] [Google Scholar]
- 33.Franks NR, Dornhaus A, Fitzsimmons JP, Stevens M. Proc R Soc London Ser B; 2003. pp. 2457–2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jeanson R, Deneubourg JL, Grimal A, Theraulaz G. Behav Ecol Sociobiol. 2004;55:388–394. [Google Scholar]
- 35.Aviles L. Biol J Linn Soc. 2000;70:325–339. [Google Scholar]
- 36.Jeanson R, Deneubourg JL, Theraulaz G. Anim Behav. 2004;67:531–537. [Google Scholar]
- 37.Saffre F, Mailleux AC, Deneubourg JL. J Insect Behav. 1999;12:277–282. [Google Scholar]
- 38.Ame JM, Rivault C, Deneubourg JL. Anim Behav. 2004;68:793–801. [Google Scholar]
- 39.Ben Jacob E, Becker I, Shapira Y, Levine H. Trends Microbiol. 2004;12:366–372. doi: 10.1016/j.tim.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 40.Ben-Jacob E, Cohen I, Gutnick DL. Annu Rev Microbiol. 1998;52:779–806. doi: 10.1146/annurev.micro.52.1.779. [DOI] [PubMed] [Google Scholar]
- 41.Beekman M, Sumpter DJT, Ratnieks FLW. Proc Natl Acad Sci USA. 2001;98:9703–9706. doi: 10.1073/pnas.161285298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ben-Jacob E. Philos Trans R Soc London A. 2003;361:1283–1312. doi: 10.1098/rsta.2003.1199. [DOI] [PubMed] [Google Scholar]
- 43.Franks NR, Richardson T. Nature. 2006;439:153. doi: 10.1038/439153a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.