Abstract
The subcellular localization of the cluster of differentiation 63 (CD63) tetraspanin and its interaction with the class II MHC antigen presentation pathway were examined in the context of phagocytosis by live cell imaging, by using monomeric red fluorescent protein-tagged mouse CD63 expressed in primary bone marrow-derived cell cultures. Upon phagocytosis of Cryptococcus neoformans and polystyrene beads, CD63 was recruited selectively to C. neoformans-containing phagosomes in a MyD88-independent acidification-dependent manner. Bead-containing phagosomes, within a C. neoformans-containing cell, acidified to a lesser extent and failed to recruit CD63 to a level detectable by microscopy. CD63 recruitment to yeast phagosomes occurred independently of class II MHC and LAMP-1. These observations indicate that the composition of distinct phagosomal compartments within the same cell is determined by phagosomal cargo and may affect the outcome of antigen processing and presentation.
Keywords: dendritic cells, live cell imaging, lysosome, phagocytosis, yeast
Professional antigen-presenting cells (APCs) acquire microbial pathogens by phagocytosis and process them for presentation by class II MHC molecules. Internalization of particulatematter can be mediated through a number of receptors, including Fc receptors, complement receptors, integrins, and scavenger receptors. Each mode of entry is associated with specific morphological changes, all of which ultimately result in formation of the phagosome (reviewed in ref. 1). Phagosome maturation is complex and not completely understood. Once formed, the phagosome undergoes a series of fusion and fission events that sequentially incorporate elements of early endosomes, late endosomes, and lysosomes; these events result in dynamic delivery and removal of material (reviewed in ref. 2).
Several pathogens successfully evade immune attack by exploiting the phagosomal environment to render it favorable for replication and survival. Examples include Leishmania donovani (3), Salmonella typhimurium (4), Mycobacterium tuberculosis (5), and Cryptococcus neoformans (6). Furthermore, internalization of different pathogenic organisms may yield functionally distinct phagosomes. Whatever the routes involved in pathogen entry and subsequent phagosome maturation, current models would benefit from a more accurate description of phagocytosis in living cells through use of suitably tagged endosomal proteins, such as the class II MHC products that ultimately present pathogen-derived peptides (7).
Here we investigate the cluster of differentiation 63 (CD63) tetraspanin within the endosomal pathway by tagging it with monomeric red fluorescent protein (mRFP1) and using live cell imaging to observe its behavior in primary mouse APCs. CD63, also known as LAMP-3, was characterized originally as a platelet activation marker (8) and has been used as a marker of late endosomes and lysosomes (9). More recently, the focus has shifted to its interaction with class II MHC and its role in antigen presentation. In professional APCs such as dendritic cells (DCs), subcellular fractionation and biochemical analysis show that human CD63 segregates into class II MHC-rich domains within the endocytic pathway and cell surface (10–12). Furthermore, this tetraspanin protein preferentially resides in membrane patches enriched for particular class II MHC–peptide complexes (13). Disruption of these microdomains in immortalized B cells significantly reduces their ability to activate T cells. The lysosomal sorting sequence in its C terminus (14) has led to the hypothesis that CD63 may act as part of a tetraspanin web that chaperones class II MHC through the endosomal pathway and recycles between the cell surface and late endosomes (13). How tetraspanins and class II MHC are integrated into membrane domains conducive to antigen presentation is not known.
Because APCs largely acquire source materials for antigen presentation by phagocytosis, we investigated the behavior of CD63 in relation to class II MHC in primary cells that actively phagocytose particulate matter. APCs derived from the bone marrow of either C57BL/6 (B6) or class II MHC-eGFP (enhanced GFP) knockin mice were transduced with a lentivirus encoding mRFP1-tagged CD63. We used Cryptococcus neoformans (CN) to induce phagosome formation. These phagosomes recruited CD63 only when the phagosomes successfully acidified, presumably by fusion with vesicles containing vacuolar ATPase. Phagocytosis of polystyrene beads by the same cells that acquired CN failed to show CD63 localization on the phagosomal membrane and acidified to a lesser extent. Within one and the same cell, phagosomal content determines phagosomal membrane composition and may ultimately influence antigen processing and presentation.
Results
CD63-mRFP1 and HA-CD63 Fusion Proteins Mature and Show an Intracellular Distribution Comparable to Their Untagged Counterparts.
Although antibodies exist for many human tetraspanins, anti-mouse CD63 antibodies that effectively recognize native fully glycosylated CD63 are not available. To characterize the behavior of CD63 and validate the use of tagged CD63 derivatives for live cell imaging, we used two epitope-tagged fusion constructs. For biochemical analysis, including the identification of associated proteins, we used an N-terminal affinity tag comprising a HA epitope. For visualization of the subcellular localization and trafficking patterns of CD63 by optical microscopy, we tagged mouse CD63 at its C terminus with mRFP1, a fluorophore derived from dsRED but engineered to prevent the pronounced tetramerization of its precursor (15).
CD63 contains three predicted N-linked glycosylation sites within its large extracellular loop. Maturation of CD63 in human DCs involves the acquisition of complex-type N-linked glycans; this modification manifests by a gradual transition over a 1-h period from a discrete 37-kDa band to a heterodisperse smear characteristic of terminal glycosylation (16). Given the lack of proper antibodies, we were unable to test the efficacy of our fusion proteins by comparing them to endogenous CD63. By making use of epitope tags grafted onto CD63, however, we were able to compare the characteristics of the fusion proteins to those of the closely related human CD63. Using retroviral vectors, we transduced each CD63-encoding construct into the mouse macrophage-like cell line RAW 264.7. Pulse–chase analysis and immunoprecipitation with either anti-HA or -mRFP1 antibody (Fig. 1A) showed that after 90 min of chase, both fusion proteins acquired endoglycosidase H (EndoH) resistance, which is indicative of proper folding and transport from the endoplasmic reticulum to the Golgi. Treatment of immunoprecipitates containing CD63-mRFP1 (obtained from cells labeled for 3 h) with PNGase F caused collapse to a single band identical in size to that of the EndoH digestion products (Fig. 1B). To determine the extent of trafficking beyond the Golgi and subcellular localization at steady state, we reasoned that the highly homologous mouse and human forms of CD63 should behave similarly. We introduced the mouse CD63-mRFP1 construct into U373 human astrocytoma cells through lentiviral infection. Using an anti-human CD63 monoclonal antibody, we observed nearly perfect colocalization with the tagged mouse CD63 (Fig. 1C); small amounts of mouse CD63-mRFP1 were detectable at the cell surface as well. Addition of the mRFP1 tag thus preserves the subcellular localization of CD63. Taken together, these data justify the use of both fusion proteins to derive meaningful data on the role of mouse CD63 within the endocytic pathway by live cell imaging.
Fig. 1.
CD63 fusion proteins mature normally. (A and B) RAW 264.7 cells were transduced to stably express mouse CD63-mRFP1 or HA-CD63. Cells were pulse-labeled for 15 min with [35S]cysteine and methionine and chased for 0, 90, or 180 min (A) or labeled for 3 h (B). Cells were lysed in 1% Brij58 and proteins immunoprecipitated with anti-mRFP1 or anti-HA antibody. At each time point, half of the sample was treated with endoglycosidase H (A) or F (B) for 1 h at 37°C. Samples were run on a 12% SDS-polyacrylamide gel and polypeptides visualized by autoradiography. The “CHO” labels indicate the number of N-linked glycans and complex-type sugars (C.T.) present. (C) Human astrocytoma U373 cells expressing mouse CD63-mRFP1 were fixed, permeabilized, and stained with anti-human CD63 antibody. Localization of mouse CD63-mRFP1 (Left) and human CD63 (Center) and the merged image (Right).
CD63 Recruitment to the Phagosome Is Rapid and Depends on Content.
DC maturation can be determined by the level of class II MHC on the cell surface. Upon LPS stimulation, these cells rearrange their endosomal compartments and form tubular structures that deliver class II MHC to the cell surface (17–19). In class II MHC-eGFP bone marrow-derived DCs lentivirally transduced to express CD63-mRFP1, we observed partial overlap between CD63 and class II MHC in immature DCs, whereas in fully mature cells, the majority of CD63 was present in intracellular compartments, clearly segregated from surface class II MHC (Fig. 6, which is published as supporting information on the PNAS web site). These data suggest that if CD63 reaches the cell surface, then it must separate from the associated class II MHC and recycle back to an intracellular location. Because we demonstrate by immunoprecipitation–Western blot that these molecules do interact in mouse cells (Fig. 7, which is published as supporting information on the PNAS web site), these data collectively raise the question of where these molecules first interact.
Phagocytosis represents an important means by which most microbial pathogens gain intracellular access and enter the antigen-processing pathway. To study CD63 and class II MHC interactions within the context of this pathway, we chose the phagosome as a likely environment where these proteins might initially interact. The process of phagocytosis has been studied by using inert polystyrene beads, and we therefore sought to study the behavior of these molecules within the context of this widely used model. In CD63-mRFP1-expressing bone marrow-derived cells, time-lapse experiments examining both early (within minutes of ingestion) and late (24 h after ingestion) time points failed to reveal detectable changes in CD63 distribution on bead phagosomes (Fig. 2A). Class II MHC also seemed unresponsive to the presence of the bead, with only trace amounts being recruited to the phagosomal membrane after 24-h incubation. Opsonizing beads or coating them with ovalbumin also failed to produce any detectable change in the phagosomes (data not shown). Complete ingestion of beads was confirmed by phase-contrast microscopy as well as z-stack images of cells. In all cases, only cells with clear internalization of beads were used for analysis.
Fig. 2.
CD63 and class II MHC are recruited selectively to yeast phagosomes. (A) B6-derived bone marrow cultures expressing CD63-mRFP1 were exposed to “dragon green” polystyrene beads. Still images show CD63-mRFP1 distribution in the cell. (B) Bone marrow cells from B6 mice expressing CD63-mRFP1 (Left) or from class II MHC-eGFP-expressing mice (Right) were incubated with CN. The images display the phagosomal distribution of CD63-mRFP1 and class II MHC-eGFP. (C) B6-derived bone marrow cultures were transduced with CD63-mRFP1 and incubated with both CN and dragon green polystyrene beads. An example of CD63-mRFP1 distribution in a cell having taken up two yeast, and one bead is shown. The far-right bright-field image demonstrates the presence of CN within the cell. (Scale bars, 10 μm.)
Given the ability of certain intracellular pathogens to modify the architecture of the phagosome (3–5), we hypothesized that, by exposing the bone marrow DCs to a pathogenic organism, we might observe differences in CD63 and class II MHC distribution. We selected CN, a pathogenic encapsulated yeast (20), as our model organism (H99, Serotype A), for its clinical importance and ease of detection. Upon phagocytosis of CN, a bright fluorescent ring appeared on the phagosome, indicating robust recruitment of CD63-mRFP1 or class II MHC-eGFP (Fig. 2B). This observation was not limited only to CN but was also seen when cells phagocytosed Saccharomyces cerevisiae (Fig. 8, which is published as supporting information on the PNAS web site; ref. 21).
Within an individual cell that had ingested both beads and CN, the acquisition of CD63 (Fig. 2C) and of class II MHC (data not shown) by the phagosomes remained dependent on their contents. Phagosomes containing CN developed a bright fluorescent ring of CD63-mRFP1, whereas those containing beads remained dark. Acquisition of CD63, therefore, does not seem to be readily triggered by the act of phagocytosis alone but requires additional stimuli absent from inert beads but present on yeast. Furthermore, this observation demonstrates the ability of different phagosomal contents to elicit differential recruitment of CD63, class II MHC, and almost certainly other molecules to the phagosome. Entry of beads and CN into membrane-delimited compartments was verified by observation of actin flashing in cells expressing actin-GFP (22) and by electron microscopy (Fig. 9, which is published as supporting information on the PNAS web site).
Class II MHC Is Recruited to the Phagosome Before CD63.
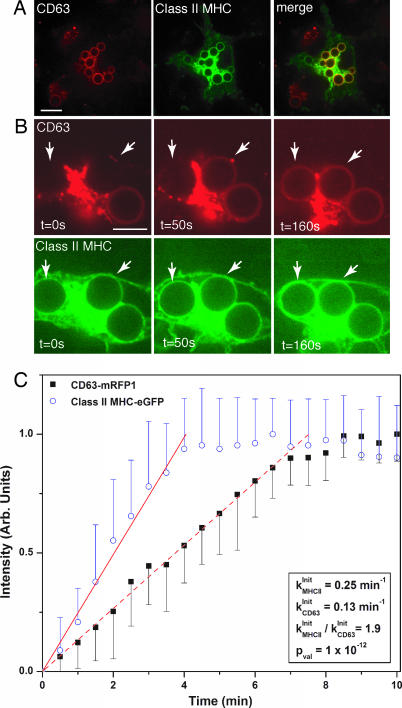
By transducing bone marrow-derived cells from a class II MHC-eGFP mouse with CD63-mRFP1 lentivirus, we confirmed that both molecules are recruited to the same CN phagosome (Fig. 3A). In addition, through time-lapse microscopy, we were able to examine whether these routes of vesicular transport emanate from sources of different membrane composition or from a single homogeneous source. Time-lapse analysis revealed that class II MHC arrives at the phagosomal membrane separately and slightly earlier than CD63 (Fig. 3B). We examined the kinetics of recruitment more closely by doing a quantitative analysis of increasing fluorescence intensity of multiple independent phagosomal events. Analysis was done in single-labeled cells to avoid bleedthrough of red fluorescence into the green channel. We also used Cap59−/− CN, an acapsular mutant (23) showing enhanced uptake by phagocytes, to facilitate collection of a large number of phagocytic events. Cap59−/− yeast induced the same endosomal rearrangements as wild-type CN, the only difference being that terminal levels of CD63 and class II MHC were slightly lower (Fig. 10, which is published as supporting information on the PNAS web site), a point that did not interfere with resolution of recruitment kinetics. By identifying nascent phagosomes in cells expressing either class II MHC-eGFP or CD63-mRFP1, we could monitor the recruitment of each molecule over time (Movies 1 and 2, which are published as supporting information on the PNAS web site).
Fig. 3.
Class II MHC recruitment to the phagosome precedes that of CD63. (A) Within 10 min after addition of CN to bone marrow cultures derived from class II MHC-eGFP mice and transduced to express CD63-mRFP1, phagosomes acquire both CD63-mRFP1 and class II MHC (Left, CD63-mRFP1; Center, class II MHC; and Right, merge). (Scale bar, 10 μm.) (B) Time-lapse images in both red and green channels show that class II MHC-eGFP appears around the phagosomes (indicated by the arrows) slightly before CD63-mRFP1. (Scale bar, 5 μm.) (C) Kinetic analysis of fluorescence acquisition around phagosomes. Each data point represents the average intensity measured at each time point for at least nine independent Cap59−/− CN phagocytic events for CD63-mRFP1 (■) and class II MHC-eGFP (○) recruitment. The linear fits indicate the initial rates of recruitment for each protein to the phagosome.
We treated the data using a first-order kinetics model and determined initial rates of recruitment using linear regression. The ratio of the recruitment rates of class II MHC-eGFP to CD63-mRFP1 was 1.8 (P = 2 × 10−12), demonstrating, in a statistically significant manner, that recruitment into phagosomes of class II MHC proceeds at a consistently different and more rapid pace than that of CD63-mRFP1 (Fig. 3C). The increase in fluorescence observed for CD63-mRFP1 and class II MHC-eGFP is gradual, not saltatory, and thus is consistent with the involvement of transport vesicles far smaller than the target organelles. The near-constant size of the phagosome in the course of maturation further implies a balance of fusion and fission events. These observations suggest that these reporters must have their origin in membrane vesicles or distinct membrane microdomains, and that multiple discrete input pathways exist for gradual modification of the composition of the phagosomal membrane.
Phagosome Acidification Coincides with CD63 Recruitment.
We attempted to change the CD63 recruitment phenotype of either beads or CN by manipulation of cellular surface molecules known to be involved in yeast phagocytosis. Adsorption of mannosylated BSA or IgG onto beads to force engagement of the mannose and FcγII receptors did not result in CD63 recruitment. Furthermore, uptake of CN by bone marrow DCs derived from a MyD88−/− mouse failed to abrogate recruitment of this tetraspanin, excluding involvement of MyD88-dependent Toll-like receptors (TLR; see Fig. 11, which is published as supporting information on the PNAS web site). Conversely, polystyrene beads with adsorbed LPS failed to recruit CD63 (data not shown), suggesting that if TLR4 is involved in this process, its ligation alone is insufficient to induce the endosomal rearrangement observed with yeast. We next focused on identifying physical changes to the phagosome itself in an effort to understand the mechanism whereby this tetraspanin traffics. Because CD63 is widely accepted as a lysosomal marker, it seemed logical that it may be delivered to the phagosome through fusion with lysosomal compartments, thereby leading to a change in pH. By incubating CD63-mRFP1-transduced cells with Lysosensor, a pH indicator that accumulates in acidic vesicles and fluoresces when protonated, in conjunction with CN and/or beads, we assessed the kinetics of phagosome acidification as it relates to CD63 recruitment. Consistently, the appearance of CD63 in the phagosome coincided with that of Lysosensor, which reached maximal fluorescence by 10 min after internalization (Fig. 4A). Beads generated a significantly lower level of acidification (Fig. 4B), suggesting that perhaps trace amounts of CD63 are recruited to these phagosomes at levels not detectable by live cell imaging.
Fig. 4.
CD63 recruitment and phagosome acidification coincide. Bone marrow cultures derived from B6 mice and transduced to express CD63-mRFP1 were incubated with CN and the pH indicator, Lysosensor. (A) Time-lapse imaging of phagosome acidification as determined by Lysosensor recruitment (Upper) and of CD63-mRFP1 recruitment (Lower) are shown. Representative images have been selected at 0, 200, and 500 sec. (Scale bar, 5 μm.) (B) Colocalization of Lysosensor (Left) and of CD63-mRFP1 (Center) in the CN phagosome after at least 10-min incubation is apparent in the merged image (Right). (Scale bar, 10 μm.)
Acidification of the Phagosome Is Required for CD63 Recruitment but Not for Other Lysosomal Markers.
Because phagosome acidification and CD63 recruitment largely coincided, we asked whether these events were interdependent. Bafilomycin is a selective and potent inhibitor of vacuolar ATPase (24), the enzyme complex responsible for pumping protons across the membrane, and hence vacuolar acidification. We added bafilomycin to cell cultures (1 μM) and effectively blocked vacuolar acidification. In each experiment, we verified lack of acidification by absence of Lysosensor dye from phagosomes (data not shown). In cells transduced to express CD63-mRFP1, bafilomycin successfully blocked CD63 recruitment to CN-containing phagosomes (Fig. 5A). We repeated this experiment using cells derived from a class II MHC-eGFP knockin mouse as well as with B6 bone marrow-derived cells transduced to express LAMP-1-GFP. We observed unabated recruitment of both class II MHC and LAMP-1 to the yeast phagosomes despite the presence of bafilomycin. These results further support our finding that CD63 and class II MHC traffic to the phagosome independently as a result of different subcellular signals. Furthermore, that acidification is required for the recruitment of CD63, but not for LAMP-1, distinguishes these molecules as residents of different endosomal subcompartments, an argument bolstered by their nonidentical subcellular distribution when probed by immunofluorescence (Fig. 5B). To date, CD63 and LAMP-1 have been largely used interchangeably as lysosomes markers.
Fig. 5.
Phagosomal recruitment of CD63, but not class II MHC or LAMP-1, is blocked by inhibition of vacuolar ATPase. Bone marrow-derived cell cultures from B6 mice were lentivirally transduced with CD63-mRFP1 or LAMP1-GFP. Alternatively, bone marrow cultures were derived from class II MHC-eGFP mice. (A) Cells were incubated with CN in the absence or presence of 1 μM bafilomycin and followed for CD63-mRFP1 (Left), class II MHC-eGFP (Center), or LAMP1-GFP (Right) recruitment. Images shown were taken after 30-min incubation. (Scale bars, 5 μm.) (B) B6 bone marrow cells were transduced with CD63-mRFP1 and paraformaldehyde-fixed. Analysis by immunofluorescence with anti-mRFP1 (Left) and LAMP-1 (Center) antibodies shows colocalization of these two molecules (Right). (Scale bars, 10 μm.)
Discussion
Specialized membrane microdomains stabilized by proteins of the tetraspanin family are functionally important, but their origin and organization in living cells have not been well studied. The importance of “tetraspanin webs” in a wide array of cellular processes is becoming increasingly clear (reviewed in ref. 25), including their role in antigen presentation (26). Here we have addressed the behavior of CD63 in the maturation of phagosomes using live cell imaging. To date, characterization of CD63 has been accomplished in human cell lines, largely through biochemical analysis (12, 16, 27). The data presented here focus on the behavior of CD63 and class II MHC in live primary cultures of mouse APCs in the context of antigen capture by phagocytosis.
Our observation that phagosomal composition depends on the identity of the ingested particle, even when two different particles are within a single cell, may have important implications for antigen processing and presentation. This specificity may be due, in part, to the initial interaction of a phagocyte with a target pathogen. The combination of surface molecules involved depends on the pathogen itself. For example, Leishmania relies on complement and scavenger receptors to gain entry (28), whereas yeast engage the mannose receptor to trigger cellular uptake (29). Because phagosomes are, at least in part, plasma membrane-derived (30), the initial composition of the phagosomal environment must differ depending on the selective engagement of surface molecules. These differences become more pronounced as phagosomes mature and pathogens manipulate their immediate surroundings to their advantage (28, 31, 32).
Pathogens that are able to interfere specifically with the vacuolar ATPase proton pump may be capable of manipulating antigen presentation through a direct effect not only on endosomal pH and thus lysosomal protease activity but also on phagosomal compartment composition. CN can thrive in an acidic milieu and shows reduced viability in the absence of phagosome acidification (33). Histoplasma capsulatum, another fungal pathogen, actively recruits vacuolar ATPase and thereby controls the pH of its immediate environment (34). Likewise, Helicobacter pylori blocks proper acidification by inhibiting fusion of its compartment with late lysosomal compartments. This obstruction interferes with antigen presentation, presumably because efficient antigen processing cannot take place in the absence of functional proteases (35). We propose that each pathogen, by virtue of its unique surface architecture, may be delivered to a cellular compartment with distinct processing proteases and with different routes of access to the class II MHC molecules themselves.
Blocking acidification inhibited recruitment of CD63 to the CN-containing phagosome but did not affect that of class II MHC or LAMP-1. This result suggests that the acquisition of lysosomal proteins by the phagosome is a multistep process consisting first of class II MHC and LAMP-1 recruitment and acidification and then CD63 recruitment. This tetraspanin must be directly responsive to signals triggered upon acidification, serving either as part of a delivery complex or as a structural purpose once within the confines of the phagosome. Signaling by the vacuolar ATPase (vATPase) itself has been shown to influence the recruitment of AOP ribosylation factor 6 (Arf6) and ADP ribosylation factor nucleotide-binding site opener (ARNO) (36).
The observed robust recruitment of CD63 may indicate optimal access to antigen-loading machinery and subsequently efficient processing and presentation. Immunoprecipitations with the CDw78 antibody from cells lysed in weak detergents identified a complex of associated proteins, including CD63, CD82, HLA-DR, and HLA-DM, which catalyzes exchange of class II-associated invariant chain peptide (CLIP) peptide with antigenic peptide (13). Selective recruitment of CD63 to CN-containing phagosomes may represent a physiological phenomenon whereby antigens destined for processing and presentation gain more efficient access to components of the antigen-presentation pathway. CD63, as part of a family of molecules that characteristically play a structural role within the cell, could be recruited to phagosomes to tether the antigen-loading machinery together. The notion that phagosomal contents influence their processing and subsequent presentation has been proposed in other systems as well (37, 38).
CD63 and other tetraspanins can be coimmunoprecipitated with type II phosophoinositide 4-kinase (39, 40) and γ-glutamyl transpeptidase (41). The presence of these enzymes suggests that their recruitment to specific tetraspanin-rich areas of membranes may mediate regulated signaling events. In the context of the phagosome, recruitment of CD63 may, in turn, attract signaling molecules responsible for regulating downstream antigen-processing events. Signal initiation could result in up-regulated transcription as well as recruitment of preexisting molecules to the phagosome.
That polystyrene beads ultimately do recruit modest amounts of class II MHC molecules, albeit with considerable delay (≥24 h), and possibly of CD63 below the level of detection, suggests that both bead and CN may gain access to the class II MHC compartment. In support of this argument, ratiometric imaging showed marked differences in the nature of macrophage phagosomes containing either opsonized erythrocytes or beads (42). Specifically, the endoplasmic reticulum (ER) membrane was not detected around phagocytosed erythrocytes, whereas it was abundant around beads. Although we did not probe for phagosomal ER markers in this study, the discrepancy observed in CD63 and class II MHC recruitment between beads and CN underscores the obvious difference between synthetic beads and real microorganisms. The identity of markers to assess maturation of phagosomes to phagolysosomes is probably best determined using microorganisms. Although electron microscopy and immunofluorescence demonstrate the presence of molecules expected to participate in antigen processing and presentation within bead phagosomes, the modest quantities of CD63 and class II MHC recruited to these phagosomes make polystyrene beads a less effective tool to study phagosome maturation events in living cells.
Functional antigen-presentation assays using tetraspanin knockout models are needed to determine the relative importance of tetraspanins to the class II MHC pathway. How the phagosomal environment is defined, either by surface interactions that immediately direct entry into a defined pathway or by the nature of the phagocytosed particle inducing selective recruitment of molecules, remains to be determined.
Materials and Methods
Plasmids and Constructs.
Mouse CD63 and mRFP1 cDNA were kind gifts from H. Hotta (Department of Microbiology, Kobe University School of Medicine, Hyogo, Japan) and R. Tsien (Department of Pharmacology, University of California at San Diego, La Jolla, CA), respectively. The HA-TEV-SBP affinity purification tag has been described (43). HATEVSBP-CD63 and CD63-mRFP1 were cloned in the pGEM-T vector (Promega, Madison, WI) for sequence verification, and HATEVSBP-CD63 was transferred into pMIG-W, a mouse stem-cell retroviral vector with a downstream IRES-GFP sequence (44). CD63-mRFP1 and actin-GFP were subcloned into the pHAGE vector, a third-generation lentiviral self-inactivating nonreplicative vector used for transduction of bone marrow stem cells (45). LAMP1-GFP (fusion constructs were kind gifts from Ira Mellman (Department of Cell Biology, Yale University, New Haven, CT) and Maggie So (Department of Molecular Microbiology and Immunology, Oregon Health and Science University, Portland, OR) was also subcloned into pHAGE.
Cell Lines and Tissue Culture.
The mouse macrophage cell line RAW 264.7, human U373 astrocytoma cells, and viral packaging cell line HEK293T cells were all grown in DMEM (Gibco, Grand Island, NY) supplemented with 10% FCS (HyClone, Logan, UT) and penicillin/streptomycin (Gibco). A20 mouse B cells were grown in RPMI medium 1640 (Gibco) supplemented with 10% FCS/50 μM 2-mercaptoethanol/penicillin/streptomycin. RAW 264.7 and A20 cells stably expressing HATEVSBP-CD63 or CD63-mRFP1 were generated through retroviral and lentiviral infection, respectively (see below).
Mouse Strains and Bone Marrow Cultures.
Class II MHC-eGFP knockin mice and wild-type mice on a mixed 129SVJ-B6 background were killed according to approved protocols, and bone marrow cells cultures were prepared as described (7) and seeded in equal aliquots of 6 × 105 cells/200 μl on eight-well Labtek II chambered coverglass wells (Nalge Nunc, Naperville, IL). Media were replaced every 48 h.
Retroviral and Lentiviral Transduction of Cell Lines and Bone Marrow Cultures.
Lentivirus were made as described (45). Cellular transduction was done in 96-well U-bottom (A20 cells) or 96-well flat-bottom (RAW 264.7 cells) plates whereby 100 μl of viral supernatant supplemented with 8 μg/ml Polybrene (Sigma, St. Louis, MO) was placed onto plated cells, spun at 500 × g for 2 h at room temperature, and placed at 37°C overnight. Supernatants were replaced with normal media the next day, and cells were cultured as usual. Successful infection was determined by examination under an epifluorescence microscope for the presence of green or red fluorescence.
Antibodies and Reagents.
For immunoprecipitation, anti-HA mouse monoclonal 3F10 antibody was used (Roche, Basel, Switzerland). We raised polyclonal antisera against mRFP1 by bacterial expression of mRFP1 and subsequent immunization of rabbits.
Immunofluorescence was done with monoclonal rat-anti-mouse LAMP-1 (PharMingen, San Diego, CA). Secondary antibodies used were anti-rat or -rabbit Alexafluor 647 (Molecular Probes, Eugene, OR). Additional details regarding immunofluoresence, pulse–chase, and immunoprecipations are found in Supporting Text, which is published as supporting information on the PNAS web site.
Image Acquisition and Analysis.
Images were acquired by using a spinning disk confocal microscope (Perkin–Elmer Ultraview RS; Perkin–Elmer, Boston, MA), and either the Ultraview ERS system or a Prairie 3-W laser with Acousto-optical tunabe filter (Prairie Technologies, Middleton, WI) was used. Details are found in Supporting Text.
Supplementary Material
Acknowledgments
We thank Gustavo Mostoslavsky (Department of Genetics, Harvard Medical School, Boston, MA) for the pHAGE lentiviral vector, Eleftherios Mylonakis (Division of Infectious Diseases, Massachusetts General Hospital) for the H99 CN isolate and the Cap59−/− CN mutant, and George Bell for helpful discussions. K.A.-T. is a National Institutes of Health National Research Service Award fellow (1F32CA105862-01). J.C.L. is a Gilead Fellow of the Life Sciences Research Foundation (Baltimore, MD). J.M.V. is supported by the Ellison Medical Foundation and the National Institutes of Health (5K08AI57999), and a portion of this research was conducted while J.M.V. was a Pfizer postdoctoral fellow in Infectious Diseases. H.L.P. is supported by the National Institutes of Health (5R01AI034893-13).
Abbreviations
- APC
antigen-presenting cell
- CN
Cryptococcus neoformans
- CD63
cluster of differentiation 63
- mRFP1
monomeric red fluorescent protein
- DC
dendritic cell
- eGFP
enhanced GFP
Footnotes
The authors declare no conflict of interest.
References
- 1.Underhill DM, Ozinsky A, Hajjar AM, Stevens A, Wilson CB, Bassetti M, Aderem A. Nature. 1999;401:811–815. doi: 10.1038/44605. [DOI] [PubMed] [Google Scholar]
- 2.Vieira OV, Botelho RJ, Grinstein S. Biochem J. 2002;366:689–704. doi: 10.1042/BJ20020691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lodge R, Descoteaux A. J Leukocyte Biol. 2004:39–40. [Google Scholar]
- 4.Hashim S, Mukherjee K, Raje M, Basu SK, Mukhopadhyay A. J Biol Chem. 2000;275:16281–16288. doi: 10.1074/jbc.275.21.16281. [DOI] [PubMed] [Google Scholar]
- 5.Vergne I, Chua J, Lee H-H, Lucas M, Belisle J, Deretic V. Proc Natl Acad Sci USA. 2005;102:4033–4038. doi: 10.1073/pnas.0409716102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tucker SC, Casadevall A. Proc Natl Acad Sci USA. 2002;99:3165–3170. doi: 10.1073/pnas.052702799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boes M, Cerny J, Massol R, Op den Brouw M, Kirchhausen T, Chen JZ, Ploegh HL. Nature. 2002;418:983–988. doi: 10.1038/nature01004. [DOI] [PubMed] [Google Scholar]
- 8.Nieuwenhuis HK, van Oosterhout JJ, Rozemuller E, van Iwaarden F, Sixma JJ. Blood. 1987;70:838–845. [PubMed] [Google Scholar]
- 9.Escola J-M, Kleijmeer MJ, Stoorvogel W, Griffith JM, Yoshie O, Geuze HJ. J Biol Chem. 1998;273:20121–20127. doi: 10.1074/jbc.273.32.20121. [DOI] [PubMed] [Google Scholar]
- 10.Rubinstein E, LeNaour F, LagaudriereGesbert C, Billard M, Conjeaud H, Boucheix C. Eur J Immunol. 1996;26:2657–2665. doi: 10.1002/eji.1830261117. [DOI] [PubMed] [Google Scholar]
- 11.Hammond C, Denzin LK, Pan M, Griffith JM, Geuze HJ, Cresswell P. J Immunol. 1998;161:3282–3291. [PubMed] [Google Scholar]
- 12.Mantegazza AR, Barrio MM, Moutel S, Bover L, Weck M, Brossart P, Teillaud J-L, Mordoh J. Blood. 2004;104:1183–1190. doi: 10.1182/blood-2004-01-0104. [DOI] [PubMed] [Google Scholar]
- 13.Vogt AB, Spindeldreher S, Kropshofer H. Immunol Rev. 2002;189:136–151. doi: 10.1034/j.1600-065x.2002.18912.x. [DOI] [PubMed] [Google Scholar]
- 14.Rous BA, Reaves BJ, Ihrke G, Briggs JAG, Gray SR, Stephens DJ, Banting G, Luzio JP. Mol Biol Cell. 2002;13:1071–1082. doi: 10.1091/mbc.01-08-0409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Campbell RE, Tour O, Palmer AE, Steinbach PA, Baird GS, Zacharias DA, Tsien RY. Proc Natl Acad Sci USA. 2002;99:7877–7882. doi: 10.1073/pnas.082243699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Engering A, Kuhn L, Fluitsma D, Hoefsmit E, Pieters J. Eur J Biochem. 2003;270:2412–2420. doi: 10.1046/j.1432-1033.2003.03609.x. [DOI] [PubMed] [Google Scholar]
- 17.Turley SJ, Inaba K, Garrett WS, Ebersold M, Unternaehrer J, Steinman RM, Mellman I. Science. 2000;288:522–527. doi: 10.1126/science.288.5465.522. [DOI] [PubMed] [Google Scholar]
- 18.Kleijmeer M, Ramm G, Schuurhuis D, Griffith J, Rescigno M, Ricciardi-Castagnoli P, Rudensky AY, Ossendorp F, Melief CJM, Stoorvogel W, et al. J Cell Biol. 2001;155:53–64. doi: 10.1083/jcb.200103071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chow A, Toomre D, Garrett W, Mellman I. Nature. 2002;418:988–994. doi: 10.1038/nature01006. [DOI] [PubMed] [Google Scholar]
- 20.Del Poeta M. Eukaryot Cell. 2004;3:1067–1075. doi: 10.1128/EC.3.5.1067-1075.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Munoz P, Bouza E, Cuenca-Estrella M, Maria Eiros J, Jesus Perez M, Sanchez-Somolinos M, Rincon C, Hortal J, Pelaez T. Clin Infect Dis. 2005;40:1625–1634. doi: 10.1086/429916. [DOI] [PubMed] [Google Scholar]
- 22.Aderem A, Underhill DM. Annu Rev Immunol. 1999;17:593–623. doi: 10.1146/annurev.immunol.17.1.593. [DOI] [PubMed] [Google Scholar]
- 23.Chang YC, Kwonchung KJ. Mol Cell Biol. 1994;14:4912–4919. doi: 10.1128/mcb.14.7.4912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bowman EJ, Siebers A, Altendorf K. Proc Natl Acad Sci USA. 1988;85:7972–7976. doi: 10.1073/pnas.85.21.7972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hemler ME. Nat Rev Mol Cell Biol. 2005;6:801–811. doi: 10.1038/nrm1736. [DOI] [PubMed] [Google Scholar]
- 26.Kropshofer H, Spindeldreher S, Rohn TA, Platania N, Grygar C, Daniel N, Wolpl A, Langen H, Horejsi V, Vogt AB. Nat Immunol. 2002;3:61–68. doi: 10.1038/ni750. [DOI] [PubMed] [Google Scholar]
- 27.Engering A, Pieters J. Int Immunol. 2001;13:127–134. doi: 10.1093/intimm/13.2.127. [DOI] [PubMed] [Google Scholar]
- 28.Sibley LD. Science. 2004;304:248–253. doi: 10.1126/science.1094717. [DOI] [PubMed] [Google Scholar]
- 29.Ezekowitz R, Sastry K, Bailly P, Warner A. J Exp Med. 1990;172:1785–1794. doi: 10.1084/jem.172.6.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Touret N, Paroutis P, Terebiznik M, Harrison RE, Trombetta S, Pypaert M, Chow A, Jiang A, Shaw J, Yip C. Cell. 2005;123:157–170. doi: 10.1016/j.cell.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 31.Cossart P, Sansonetti PJ. Science. 2004;304:242–248. doi: 10.1126/science.1090124. [DOI] [PubMed] [Google Scholar]
- 32.Clemens D, Horwitz M. J Exp Med. 1995;181:257–270. doi: 10.1084/jem.181.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Levitz SM, Nong S-H, Seetoo KF, Harrison TS, Speizer RA, Simons ER. Infect Immunol. 1999;67:885–890. doi: 10.1128/iai.67.2.885-890.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Newman SL. Trends Microbiol. 1999;7:67–71. doi: 10.1016/s0966-842x(98)01431-0. [DOI] [PubMed] [Google Scholar]
- 35.Molinari M, Salio M, Galli C, Norais N, Rappuoli R, Lanzavecchia A, Montecucco C. J Exp Med. 1998;187:135–140. doi: 10.1084/jem.187.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hurtado-Lorenzo A, Skinner M, El Annan J, Futai M, Sun-Wada GH, Bourgoin S, Casanova J, Wildeman A, Bechoua S, Ausiello DA, et al. Nat Cell Biol. 2006;8:124–136. doi: 10.1038/ncb1348. [DOI] [PubMed] [Google Scholar]
- 37.Oh Y, Swanson J. J Cell Biol. 1996;132:585–593. doi: 10.1083/jcb.132.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blander JM, Medzhitov R. Science. 2004;304:1014–1018. doi: 10.1126/science.1096158. [DOI] [PubMed] [Google Scholar]
- 39.Yauch RL, Hemler ME. Biochem J. 2000;351:629–637. [PMC free article] [PubMed] [Google Scholar]
- 40.Israels SJ, McMillan-Ward EM. Thromb Haemostasis. 2005;93:311–318. doi: 10.1160/TH04-08-0503. [DOI] [PubMed] [Google Scholar]
- 41.Nichols TC, Guthridge JM, Karp DR, Molina H, Fletcher DR, Holers VM. Eur J Immunol. 1998;28:4123–4129. doi: 10.1002/(SICI)1521-4141(199812)28:12<4123::AID-IMMU4123>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 42.Henry RM, Hoppe AD, Joshi N, Swanson JA. J Cell Biol. 2004;164:185–194. doi: 10.1083/jcb.200307080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lilley BN, Ploegh HL. Nature. 2004;429:834–840. doi: 10.1038/nature02592. [DOI] [PubMed] [Google Scholar]
- 44.Cherry SR, Biniszkiewicz D, van Parijs L, Baltimore D, Jaenisch R. Mol Cell Biol. 2000;20:7419–7426. doi: 10.1128/mcb.20.20.7419-7426.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mostoslavsky G, Kotton DN, Fabian AJ, Gray JT, Lee JS, Mulligan RC. Mol Ther. 2005;11:932–940. doi: 10.1016/j.ymthe.2005.01.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.