Abstract
Because of species selectivity, HIV research is largely restricted to in vitro or clinical studies, both limited in their ability to rapidly assess new strategies to fight the virus. To prospectively study some aspects of HIV in vivo, immunodeficient mice, transplanted with either human peripheral blood leukocytes or human fetal tissues, have been developed. Although these are susceptible to HIV infection, xenoreactivity, and short infection spans, resource and ethical constraints, as well as biased HIV coreceptor tropic strain infection, pose substantial problems in their use. Rag2−/−γc−/− mice, transplanted as newborns with human CD34+ cells, were recently shown to develop human B, T, and dendritic cells, constituting lymphoid organs in situ. Here we tested these mice as a model system for HIV-1 infection. HIV RNA levels peaked to up to 2 × 106 copies per milliliter of plasma early after infection, and viremia was observed for up to 190 days, the longest time followed. A marked relative CD4+ T cell depletion in peripheral blood occurred in CXCR4-tropic strain-infected mice, whereas this was less pronounced in CCR5-tropic strain-infected animals. Thymus infection was almost exclusively observed in CXCR4-tropic strain-infected mice, whereas spleen and lymph node HIV infection occurred irrespective of coreceptor selectivity, consistent with respective coreceptor expression on human CD4+ T cells. Thus, this straightforward to generate and cost-effective in vivo model closely resembles HIV infection in man and therefore should be valuable to study virus-induced pathology and to rapidly evaluate new approaches aiming to prevent or treat HIV infection.
Since the beginning of the HIV pandemic, research has been hampered because of the lack of assessable animal models that mirror infection in humans. HIV is a human-specific virus, and consequently laboratory rodents as mice or rats are not susceptible to infection (1). Although non-human primates such as chimpanzees can be infected, they do not develop HIV-associated immunodeficiency (2, 3), whereas sooty mangabeys, rhesus macaques, and baboons are susceptible to only HIV-related simian immunodeficiency virus (4). Therefore, HIV research in non-human primates, although of importance, remains restricted by biological as well as by ethical and financial constraints (5). Efforts to genetically engineer rodents to become HIV targets (e.g., artificial expression of human CD4, CCR5, or CXCR4) have largely failed, because, even if infection in vitro was achieved, HIV replication in vivo was limited or absent (1, 6, 7). Thus, substitute xenochimeric models have been developed by transplanting immunodeficient mice with either human peripheral blood leukocytes (hu-PBL-SCID) (8, 9) or pieces of human fetal tissues containing hematopoietic cells (SCID-hu) (10, 11). Both hu-PBL-SCID and SCID-hu mice sustain HIV infection and replication in vivo. However, in hu-PBL-SCID mice xenoreactivity and successive loss of human leukocytes limit infection to a relatively short time frame, and, possibly driven by activation-induced CCR5 expression on human cells, infection is skewed toward HIV strains with the respective coreceptor tropism (12, 13). In SCID-hu mice, because of the transfer of human hematopoietic stem cell-containing tissue, HIV infection can be observed for extended times; however, availability of transplantable human fetal organs is restricted for practical and ethical reasons, and HIV pathology in these mice is mainly limited to the tissue implants, with naïve T cells being preferentially susceptible to CXCR4-tropic strain infection (1, 14, 15). Given these limitations and the fact that no primary immune responses were generated, hu-PBL-SCID and SCID-hu mice did not fully match the demand for a small animal model that closely mirrors infection in humans.
Recently we found that injection of human cord blood CD34+ cells into newborn Rag2−/−γc−/− mice leads to development of human T, B, and dendritic cells, successive formation of primary and secondary lymphoid organs, and some in vivo immune responses (16, 17). We here evaluated these mice as a model system for both CXCR4- and CCR5-tropic HIV infection.
Results
CXCR4 and CCR5 Expression on de Novo Generated Human Thymocytes and CD4+ T Cells in Rag2−/−γc−/− Mice.
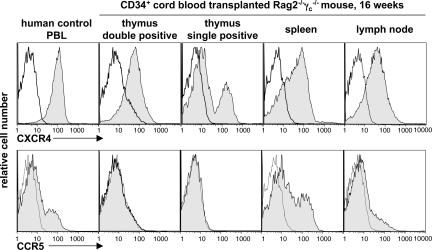
Depending on the use of chemokine receptors in combination with CD4 for cellular entry, HIV has been classified into CXCR4- or CCR5-tropic strains (18–20). In humans, CD4+ thymocytes and CD4+ T cells broadly express CXCR4, whereas CCR5 expression is restricted to a fraction of mainly CD4+ T memory cells (21). Similarly, most of thymic and peripheral CD4+ T cells in CD34+ cord blood cell-transplanted Rag2−/−γc−/− mice expressed CXCR4, whereas CCR5 was found on only a fraction of lymphoid organ CD4+ T cells that almost exclusively displayed a memory phenotype as determined by CD45RO expression (Fig. 1 and data not shown). Thus, de novo generated human CD4+ thymocytes and T cells in CD34+ cord blood cell-transplanted Rag2−/−γc−/− mice closely resemble chemokine receptor expression patterns observed in humans and therefore should be valid targets for HIV strains with respective coreceptor selectivity.
Fig. 1.
CCR5 and CXCR4 expression on human CD4+ cells in CD34+ cell-transplanted Rag2−/−γc−/− mice resembles expression patterns in humans. Histograms depict representative receptor expressions (shaded histograms) and respective isotype controls (open histograms) on CD4+ gated control human PBL and cells isolated from tissue of a mouse at 16 weeks after birth and transplantation of CD34+ cells.
Long-Term and High-Titer CCR5- and CXCR4-Tropic HIV Infection in Human CD34+ Cord Blood Cell-Transplanted Rag2−/−γc−/− Mice.
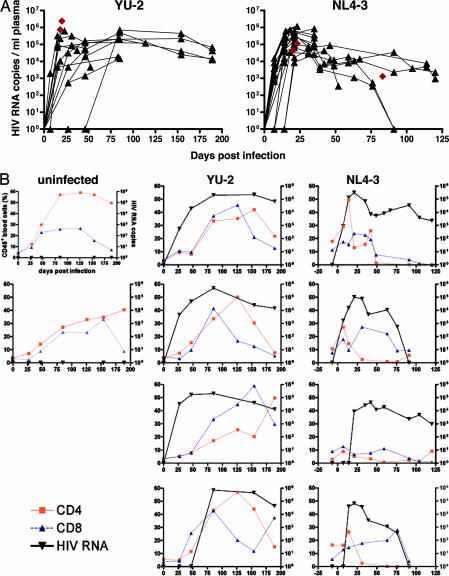
CD34+ cord blood cell-transplanted Rag2−/−γc−/− mice with a mean human peripheral blood CD45+ and CD4+ cell chimerism of 29.4 ± 18.2% and 2.7 ± 3.0%, respectively (detailed analysis of human cell engraftment in representative animals is given in Table 1, which is published as supporting information on the PNAS web site), were infected i.p. with either CCR5-tropic YU-2 (n = 15) or CXCR4-tropic NL4-3 (n = 19) HIV strains at 10–28 (mean 16.4 ± 6.7) weeks of age. Plasma levels of viral RNA were measured at successive time points. Independent of viral strains used, HIV RNA levels peaked 2–6 weeks after infection, with up to ≈2 × 106 copies per milliliter of plasma, whereas thereafter viremia mostly stabilized at lower levels and was maintained for up to 190 (YU-2) and 120 (NL4-3) days, the longest time followed (Fig. 2A). No HIV RNA was detectable at 4 weeks after injection in two YU-2- and five NL4-3-receiving animals. However, upon NL4-3 reinfection of four of the latter ones, all four became HIV RNA-positive.
Fig. 2.
Long-term and high-titer CCR5- and CXCR4-tropic HIV infection in human CD34+ cord blood cell-transplanted Rag2−/−γc−/− mice. (A) Quantitative HIV RNA plasma levels in animals successfully infected with CCR5-tropic (YU-2; n = 13) and CXCR4-tropic (NL4-3; n = 18) HIV strains. Black triangles and connector lines indicate sequential analysis of single mice, and brown diamonds indicate mice analyzed at a single time point. (B) Graphs depict quantitative HIV RNA plasma levels (copies per milliliter of plasma; right y axis) and relative CD4+ and CD8+ T cell levels (percentage of human CD45+ blood cells; left y axis) in individual mice over time (uninfected, n = 2; YU-2- and NL4-3-infected, n = 4 each), showing more pronounced CD4+ T cell depletion in CXCR4-tropic infected animals.
In a subgroup of both CCR5- and CXCR4-tropic strain-infected mice (n = 4 each), we measured HIV RNA as well as relative CD4+ and CD8+ cell counts in peripheral blood over time: partial CD4+ T cell depletion occurred in three of four mice infected with CCR5-tropic strains beyond 125 days of infection, whereas all CXCR4-tropic strain-infected animals showed a more pronounced relative peripheral blood CD4+ T cell depletion already early in infection, i.e., beyond day 25, after the initial rise of plasma HIV RNA (Fig. 2B).
HIV strains recovered from either YU-2- or NL4-3-infected chimeric Rag2−/−γc−/− mice were fully functional because cocultured mouse spleen cells propagated infection in primary human peripheral blood leukocytes (PBL) in vitro (Fig. 6, which is published as supporting information on the PNAS web site). Together these findings indicate that CD34+ cord blood cell-transplanted Rag2−/−γc−/− mice have an overall high susceptibility to both CXCR4- and CCR5-tropic HIV and develop high and sustained viral titers, comparable to titers found in HIV-infected individuals.
HIV Spreading in Lymphoid Organs of Human CD34+ Cell-Transplanted Rag2−/−γc−/− Mice Resembles HIV Infection in Humans.
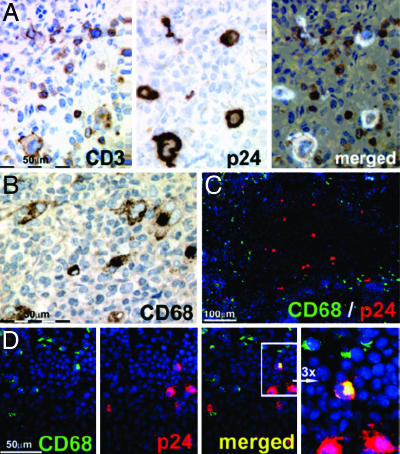
To visualize the major cell types productively infected with HIV in lymphoid organs, serial sections were taken and stained with anti-HIV p24 capsid antigen and anti-human CD3, revealing that, as expected, most infected cells were human T cells (Fig. 3A). Although CD68+ macrophages, CD11c+ dendritic cells, and CD14+ monocytic cells were generated from human CD34+ cells in mice (Fig. 3 B–D and Table 1) (17), p24-expressing, and thus productively HIV-infected non-T cells such as CD68+ macrophages, were only occasionally detected (Fig. 3 C and D), suggesting that non-T cells are a minor source of HIV in this model.
Fig. 3.
HIV p24+ cells are mostly human CD3+ cells and only occasionally non-T cells such as CD68+ macrophages. (A) Histologies show consecutive spleen sections (paraffin, 1 μm) stained with antibodies against human CD3 (Left) and p24 (Center) and a merged presentation of both (Right) in a YU-2-infected animal 18 days after infection. (B) Anti-human CD68 staining on paraffin-embedded material. (C) Merged anti-CD68 (green), HIV-p24 (red), and DAPI (blue) staining of cryoembedded spleen (6-μm sections) showing that p24+ cells mainly localize in white pulp areas (darker area; see also Fig. 4B), whereas CD68+ cells mostly localize at adjacent margins and red pulp areas. (D) Consecutive spleen cryosection staining and respective merged presentation showing a rare CD68 and p24 double-positive cell (yellow). The far-right image shows a ×3 enlargement of the area with the double-positive cell. (B–D) Representative spleen sections from a YU-2-infected animal 23 days after infection.
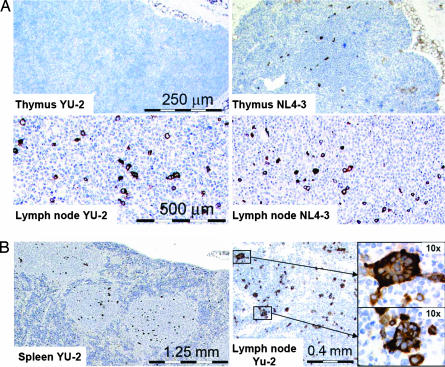
From 18 days after infection, spleens and lymph nodes of both NL4-3- and YU-2-infected animals contained p24+ cells. In thymi, however, p24 expression was detected in NL4-3-infected animals, but only infrequently or not at all in YU-2-infected animals, consistent with the expression of CXCR4 but not CCR5 on human CD4+ thymocytes (Figs. 1 and 4).
Fig. 4.
HIV spreading in lymphoid organs of human CD34+ cell-transplanted Rag2−/−γc−/− mice resembles HIV infection in humans. (A) Representative p24-stained thymus and lymph node sections of YU-2- and NL4-3-infected mice analyzed at 37 and 23 days after infection, respectively. No or very rare p24 staining is observed in thymi of CCR5-tropic YU-2-infected animals. (B) Representative tissue section of spleen (Left) and lymph node (Right) of a YU-2-infected animal at 52 days after infection showing multinucleated p24+ giant cells (enlarged Insets Right).
In both CXCR4- and CCR5-tropic HIV-infected mice, p24+ multinucleated giant cells were formed in lymph node and spleen sections (Fig. 4B), a phenomenon previously reported in brain and lymphoid tissues of HIV-infected individuals.
Thus, overall, the here-observed HIV dissemination in lymphoid organs of human CD34+ cell-transplanted Rag2−/−γc−/− mice closely resembles HIV infection in humans.
Human CD34+ Cell-Transplanted Rag2−/−γc−/− Mice Mount No or Very Limited B Cell Responses to HIV Infection.
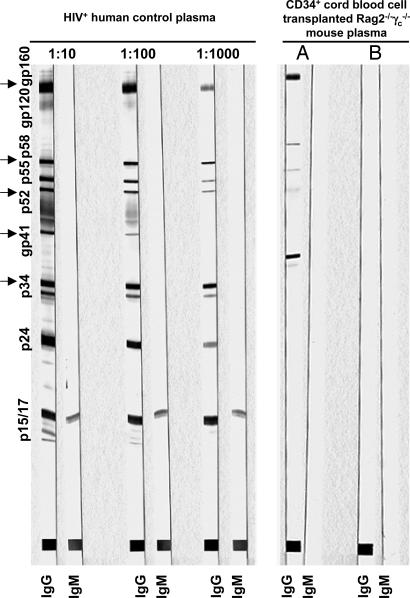
To determine human B cell responses in Rag2−/−γc−/− mice beyond 3 weeks of infection, plasma samples of n = 23 were tested for total human IgG levels, and n = 25 were analyzed for HIV-specific IgM and IgG by Western blot. As expected from our previous observations (17), total IgG levels accounted for a mean of 0.136 g/liter (range 0.0295–0.5 g/liter), an amount ≈80 times less than that found in healthy human adults. Only one animal infected with YU-2 at 23 weeks of age (11% human CD45+ cells in peripheral blood) and analyzed at 42 days after infection produced a detectable IgG response, but no measurable IgM response, against p34, gp41 (weak), p52, p58, and gp160 (Fig. 5).
Fig. 5.
Humoral immune response in HIV-infected human CD34+ cell-transplanted Rag2−/−γc−/− mice. Western blot analysis of HIV-specific IgG and IgM responses is shown. (Left) Three-step dilution of plasma from an HIV-infected patient (no antiviral therapy) showing full IgG seroconversion. (Right) Undiluted plasma of an animal showing a measurable IgG response against p34, gp41, p52, p58, and gp160 (lane A) and undiluted plasma of an animal showing no response (lane B).
We also performed a limited analysis of T cell responses: spleen cells of each two NL4-3- and YU-2-infected animals killed at 52–115 days after infection were loaded in vitro with HIV-specific peptides covering a broad range of HLA types, followed by flow cytometric measurement of intracellular IFN-γ production. However, no relevant IFN-γ production could be detected, suggesting a lack of T cell response, or a T cell response below the detection limit.
Together these results indicate that HIV-infected human CD34+ cell-transplanted Rag2−/−γc−/− mice mount no substantial or only very occasional B cell responses and, likewise, insufficient T cell responses.
Discussion
To investigate infectious agents prospectively, suitable in vivo laboratory models are needed. This poses a problem in research involving such human-specific pathogens as HIV. To this end, xenochimeric models have been developed by transplanting immunodeficient mice with cellular targets of HIV, i.e., either human PBL (8, 9) or pieces of human fetal tissues such as liver and thymus containing hematopoietic cells (SCID-hu) (10, 11); although suitable to study some aspects of HIV in vivo, both models are limited by systematic issues (1). Human PBL transplanted into SCID mice (hu-PBL-SCID) are activated within the xenogeneic environment, T cells become successively anergic, and, because of lack of both continuing hematopoiesis and appropriate hu-PBL maintenance, the xenograft is nonfunctional within several weeks (1, 9). In contrast, mice transplanted with human fetal liver and thymus (SCID-hu) de novo generate and maintain human cells, especially T cells, within the human thymus graft (10). However, SCID-hu mice are laborious and costly to generate, and availability of transplantable human fetal organs is restricted for practical and ethical reasons, limiting broader use of these mice in laboratories. Recently, major advances in generating xenogeneic mouse models that continuously produce T cells and all other major cell types of the human adaptive immune system in respective mouse organs from human hematopoietic stem and progenitor cells were achieved (reviewed in refs. 22–24). We demonstrated that newborn human cord blood CD34+ cell-transplanted Rag2−/−γc−/− mice develop de novo human T, B, and dendritic cells, form primary and secondary lymphoid organs in situ, and mount some immune responses upon tetanus toxoid vaccination or infection with EBV (17). Similar results were obtained by transplanting NOD/SCIDγc−/− mice with either human cord blood or mobilized peripheral blood CD34+ cells (25–28).
We here establish cord blood CD34+ cell-transplanted Rag2−/−γc−/− mice as a tool to study HIV infection and pathogenesis in vivo. In this model system, CXCR4 and CCR5 expression on in vivo generated human CD4+ T cells in respective lymphoid organs closely resembles HIV coreceptor expression in humans (Fig. 1) (21). Accordingly, efficient in vivo infection of human cells with both CXCR4-tropic (NL4-3) and CCR5-tropic (YU-2) HIV strains was reliably achieved (Figs. 2–4), leading to replication of fully functional HIV (Fig. 6). Although CXCR4-tropic strains infected all lymphoid organs, CCR5-tropic strain infection was largely restricted to extrathymic tissues (Fig. 4). Both viral strains led to long-term, high-titer infection with an initial viremic peak of up to 2 × 106 HIV RNA copies per milliliter of plasma, followed by a chronic phase with somewhat lower RNA levels for up to 190 days, the longest time followed (Fig. 2).
In both CXCR4- and CCR5-tropic strain-infected animals, productively HIV-infected cells, i.e., p24+ cells, were mostly CD3+ and only occasionally non-T cells such as CD68+ macrophages (Fig. 3 and data not shown). These findings are reminiscent of data acquired from ex vivo isolated lymphoid tissue of HIV-infected individuals, where productive macrophage infection by both CXCR4- and CCR5-tropic HIV strains is very infrequently observed (29–31) and likely increases only in end-stage disease with occurrence of opportunistic infections (32).
Irrespective of coreceptor selectivity, HIV-infected, multinucleated giant cells were formed (Fig. 4B), a phenomenon previously observed in brain and lymphoid tissues of HIV-infected individuals, likely associated with high viral replication, spreading infection, and CD4+ T cell loss (33, 34).
Together, these findings are clearly distinct from previous observations on HIV infection in hu-PBL SCID or SCID-hu mice: although both CXCR4- and CCR5-tropic viruses infect hu-PBL-SCID mice, CCR5-tropic viruses are more aggressive, likely because of xenogeneic activation and CCR5 up-regulation of transferred mature T cells, and, with consecutive graft failure, infection is limited to few weeks; in contrast, SCID-hu mice develop few human CCR5-carrying cells, and CXCR4-tropic HIV replication is limited rather exclusively to the fetal tissue grafts; furthermore, in both SCID models, by virtue of their nature, no HIV dissemination to respective lymphoid organs occurs.
In humans, CCR5-tropic HIV strains are primarily transmitted and dominate infection over extended times. In approximately half of late-stage HIV patients, CXCR4-tropic strains emerge, either as cause or consequence of accelerated immunodeficiency, a matter still under debate (35). Previously, it was demonstrated that CXCR4-tropic HIV strains led to pronounced cell depletion in human fetal thymus grafts in SCID-hu mice (14) and replicated more efficiently in primary human thymocytes in vitro (36). Interestingly, CXCR4-tropic HIV infection in CD34+ cell-transplanted Rag2−/−γc−/− mice, led to more rapid blood CD4+ cell loss than CCR5-tropic infection (Fig. 2B). Thus, these findings suggest a causative rather than secondary role of CXCR4 virus in accelerated immunodeficiency, possibly in part by destruction of emerging thymocytes (35, 37).
An ultimate goal in the use of substitute xenogeneic small animal models is the generation of robust human primary adaptive immune responses to rapidly test potential new vaccine candidates, a matter unfortunately not met so far in human hematopoietic stem and progenitor cell-transplanted animals (1, 23, 24). However, some limited evidence for primary immune responses in the xenogeneic setting were reported: (i) SCID-hu mice were resistant to opportunistic infections, suggesting some direct or indirect immune function of the human grafts (10); (ii) we have demonstrated that human CD34+ cell-transplanted Rag2−/−γc−/− mice generate some low-level specific IgG responses to tetanus toxoid upon repeated vaccination beyond 12 weeks of age, and, after EBV infection, some mice showed inverted CD4:CD8 ratios, and CD8 T cells proliferated in vitro when stimulated with autologous EBV-transformed target cells (17); (iii) ovalbumin-specific human IgM and IgG responses were observed in NOD/SCIDγc−/− mice (27). Although in the data presented here all HIV-infected Rag2−/−γc−/− mice tested produced human IgG at levels on average 80-fold lower than healthy human adults, only 1 of 25 mounted a detectable HIV-specific IgG response (Fig. 5). Similarly, although we had limited data, we did not detect HIV-specific T cell responses, as determined by IFN-γ detection upon in vitro restimulation. Low or absent immune responses to HIV might in part be due to preferential destruction of virus reactive cells by HIV (38); however, in this setting the more likely explanation might be inefficient MHC selection of human T cells in the mouse thymus and possible lack of some cross-reactive cytokines and chemokines in the xenogeneic environment (16, 17, 23, 24). Thus, generating primary HIV-specific immune responses remains a challenge that might be solved by adding human MHC, cytokines, chemokines, or stromal cell compounds to the recipient mouse background.
In summary, the data presented here establish newborn human CD34+ cell-transplanted Rag2−/−γc−/− mice (17) as a tool to study HIV infection and pathogenesis in vivo. Upon CCR5-tropic or CXCR4-tropic HIV challenge these mice develop long-term, high-titer, and lymphoid organ disseminated infection closely resembling HIV infection in humans. This straightforward to generate, cost-effective, ethically unproblematic, and easy to monitor in vivo model should thus be valuable to study virus-induced pathology, as well as pharmacologic or genetic approaches aiming to prevent or treat HIV infection.
Materials and Methods
Cord Blood Samples.
Human cord blood was obtained with written parental informed consent from healthy full-term newborns with approval of the local ethical board. CD34+ cells were enriched by using immunomagnetic beads (Miltenyi Biotec, Bergisch Gladbach, Germany) as described (17). Cells were either frozen or transplanted immediately. Animals used in this study received 50,000–600,000 (mean 227,500 ± 140,000) CD34+ selected cells.
Mice.
Human CD34+ cell-reconstituted mice were generated as described in accordance with the guidelines of the Institute for Research in Biomedicine (Bellinzona, Switzerland) animal facility (17). Rag2−/−γc−/− mice were originally kindly provided by M. Ito (Central Institute for Experimental Animals, Kawasaki, Japan).
HIV-1 Infection.
Viral stocks were obtained by calcium phosphate transfection (Promega, Madison, WI) of 293T cells with pNL4-3 or pYU-2. pYU-2 and pNL4-3 were obtained from B. H. Hahn (University of Alabama at Birmingham, Birmingham, AL) and M. A. Martin (Laboratory of Molecular Microbiology, National Institute of Allergy and Infectious Diseases, Bethesda, MD), respectively, through the National Institutes of Health AIDS Research and Reference Reagent Program. Forty-eight hours after transfection, virus was harvested, filtered (0.22 μm), and frozen at −80°C until use. Mice were injected i.p. with 0.2 ml of PBS containing either YU-2 or NL4-3 at a tissue culture-infecting dose 50 of 2 × 106. Infection was performed in a Biosafety Level 3 laboratory in accordance with the Institute for Research in Biomedicine and the Institute of Veterinary Bacteriology (University of Berne) animal facility guidelines.
Analysis.
Plasma HIV RNA concentrations were determined by Cobas Amplicor RT-PCR assay (Roche Diagnostics, Basel, Switzerland). p24 antigen levels in cell culture supernatants were quantified by ELISA as described (39). Human cell engraftment in mice was measured by flow cytometry as described (17). Anti-CXCR4 (12G5) and anti-CCR5 (2D7) antibodies were from BD Pharmingen (San Diego, CA). Immunohistochemical stainings were performed in an automated Ventana Discovery Module (Ventana, Strasbourg, France). Paraffin sections were incubated in a 1:5 dilution of mouse monoclonal antibody to HIV-1 p24 (clone Kal-1; DAKO Diagnostics, Zug, Switzerland). Staining for CD3 (SP7; Lab Vision, Fremont, CA) was performed according to Ventana protocols. Immunofluorescent stainings and microscopy were done as described (40). Briefly, slides were sequentially incubated with (i) mouse anti-HIV-1 p24 diluted 1:10 (DAKO Diagnostics); (ii) 5 μg/ml Cy3-conjugated goat anti-mouse Ig (Amersham, Little Chalfont, U.K.); (iii) 5 μg/ml mouse IgG (Sigma, Buchs, Switzerland); (iv) FITC-conjugated mouse anti-human CD68 (1:20; DAKO Diagnostics); (v) rabbit anti-FITC (1:2,000; DAKO Diagnostics); and (vi) 5 μg/ml Alexa Fluor 488-conjugated goat anti-rabbit Ig (Molecular Probes, Leiden, The Netherlands) and 0.5 μmol/liter DAPI (Sigma). To assess nonspecific binding, tissue from untransplanted and/or uninfected transplanted mice was stained as controls.
Immune Responses.
Human IgG levels in serum were measured on LC-Partigen plates (Behring, Marburg, Germany). HIV-specific Western blot analysis was done by using a commercially available kit (New Lav BlotI; Bio-Rad, Hercules, CA). HIV-specific T cell responses were evaluated as previously described (41): briefly, 106 splenocytes from HIV-infected and uninfected mice were pulsed with pools of overlapping peptides covering the HIV gag [National Institutes of Health HIV-1 Consensus B Gag (15-mer) Peptides Set: pool I, catalog nos. 7872–7933; pool II, catalog nos. 7934–7994] or HIV nef [National Institutes of Health HIV-1 Consensus B Nef (15-mer) Peptides Complete Set, catalog no. 5189] or with a 17-peptide pool [National Institutes of Health catalog nos. 7891, 7920, 7926, 7945, 7976, 7980, 7983 (gag), 5507, 5527, 5544, 5576 (pol), 6287, 6412, 6420 (env), 5172, 5183 (nef), and 6078 (vpr)] at a concentration of 2 × 10−6 M per peptide. One hour later, brefeldin was added for 5 h. Splenocytes were stained with mAb against CD4, CD8, and intracellularly against IFN-γ by using the Cytofix/Cytoperm permeabilization/fixation kit (BD Pharmingen).
Supplementary Material
Acknowledgments
We thank the staff of Ospedale San Giovanni (Bellinzona, Switzerland), University Hospital Zurich, and Maternité Triemli (Zurich, Switzerland) for cord blood collection. We thank Friederike Burgener and Helen Steinmann for technical assistance in processing histopathology tissues. HIV peptides were generously provided by the National Institutes of Health AIDS Research and Reference Reagent Program. This work was supported in part by the EMDO Foundation (R.F.S.), the Baugartenstiftung (R.F.S.), Swiss National Science Foundation Grants 3100A0-108352/1 (to R.F.S. and M.G.M.) and 3100A0-102221 (to M.G.M.), and the Bill and Melinda Gates Foundation (M.G.M.).
Abbreviations
- PBL
peripheral blood leukocyte
- hu-PBL-SCID mice
human PBL SCID mice
- SCID-hu mice
SCID human thymus/liver mice
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
References
- 1.Jamieson BD, Zack JA. AIDS. 1999;13(Suppl A):S5–S11. [PubMed] [Google Scholar]
- 2.Levy JA. J Med Primatol. 1996;25:163–174. doi: 10.1111/j.1600-0684.1996.tb00013.x. [DOI] [PubMed] [Google Scholar]
- 3.Nath BM, Schumann KE, Boyer JD. Trends Microbiol. 2000;8:426–431. doi: 10.1016/s0966-842x(00)01816-3. [DOI] [PubMed] [Google Scholar]
- 4.Joag SV. Microbes Infect. 2000;2:223–229. doi: 10.1016/s1286-4579(00)00266-5. [DOI] [PubMed] [Google Scholar]
- 5.VandeBerg JL, Zola SM. Nature. 2005;437:30–32. doi: 10.1038/437030a. [DOI] [PubMed] [Google Scholar]
- 6.Browning J, Horner JW, Pettoello-Mantovani M, Raker C, Yurasov S, DePinho RA, Goldstein H. Proc Natl Acad Sci USA. 1997;94:14637–14641. doi: 10.1073/pnas.94.26.14637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lores P, Boucher V, Mackay C, Pla M, Von Boehmer H, Jami J, Barre-Sinoussi F, Weill JC. AIDS Res Hum Retroviruses. 1992;8:2063–2071. doi: 10.1089/aid.1992.8.2063. [DOI] [PubMed] [Google Scholar]
- 8.Mosier DE. Semin Immunol. 1996;8:255–262. doi: 10.1006/smim.1996.0032. [DOI] [PubMed] [Google Scholar]
- 9.Mosier DE, Gulizia RJ, Baird SM, Wilson DB, Spector DH, Spector SA. Science. 1991;251:791–794. doi: 10.1126/science.1990441. [DOI] [PubMed] [Google Scholar]
- 10.McCune J, Kaneshima H, Krowka J, Namikawa R, Outzen H, Peault B, Rabin L, Shih CC, Yee E, Lieberman M, et al. Annu Rev Immunol. 1991;9:399–429. doi: 10.1146/annurev.iy.09.040191.002151. [DOI] [PubMed] [Google Scholar]
- 11.Namikawa R, Kaneshima H, Lieberman M, Weissman IL, McCune JM. Science. 1988;242:1684–1686. doi: 10.1126/science.3201256. [DOI] [PubMed] [Google Scholar]
- 12.Nakata H, Maeda K, Miyakawa T, Shibayama S, Matsuo M, Takaoka Y, Ito M, Koyanagi Y, Mitsuya H. J Virol. 2005;79:2087–2096. doi: 10.1128/JVI.79.4.2087-2096.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rizza P, Santini SM, Logozzi MA, Lapenta C, Sestili P, Gherardi G, Lande R, Spada M, Parlato S, Belardelli F, Fais S. J Virol. 1996;70:7958–7964. doi: 10.1128/jvi.70.11.7958-7964.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berkowitz RD, Alexander S, Bare C, Linquist-Stepps V, Bogan M, Moreno ME, Gibson L, Wieder ED, Kosek J, Stoddart CA, McCune JM. J Virol. 1998;72:10108–10117. doi: 10.1128/jvi.72.12.10108-10117.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krowka JF, Sarin S, Namikawa R, McCune JM, Kaneshima H. J Immunol. 1991;146:3751–3756. [PubMed] [Google Scholar]
- 16.Chicha L, Tussiwand R, Traggiai E, Mazzucchelli L, Bronz L, Piffaretti JC, Lanzavecchia A, Manz MG. Ann NY Acad Sci. 2005;1044:236–243. doi: 10.1196/annals.1349.029. [DOI] [PubMed] [Google Scholar]
- 17.Traggiai E, Chicha L, Mazzucchelli L, Bronz L, Piffaretti JC, Lanzavecchia A, Manz MG. Science. 2004;304:104–107. doi: 10.1126/science.1093933. [DOI] [PubMed] [Google Scholar]
- 18.Berger EA, Doms RW, Fenyo EM, Korber BT, Littman DR, Moore JP, Sattentau QJ, Schuitemaker H, Sodroski J, Weiss RA. Nature. 1998;391:240. doi: 10.1038/34571. [DOI] [PubMed] [Google Scholar]
- 19.Berger EA, Murphy PM, Farber JM. Annu Rev Immunol. 1999;17:657–700. doi: 10.1146/annurev.immunol.17.1.657. [DOI] [PubMed] [Google Scholar]
- 20.Speck RF, Wehrly K, Platt EJ, Atchison RE, Charo IF, Kabat D, Chesebro B, Goldsmith MA. J Virol. 1997;71:7136–7139. doi: 10.1128/jvi.71.9.7136-7139.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bleul CC, Wu L, Hoxie JA, Springer TA, Mackay CR. Proc Natl Acad Sci USA. 1997;94:1925–1930. doi: 10.1073/pnas.94.5.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kosco-Vilbois MH. Nat Biotechnol. 2004;22:684–685. doi: 10.1038/nbt0604-684. [DOI] [PubMed] [Google Scholar]
- 23.Legrand N, Weijer K, Spits H. J Immunol. 2006;176:2053–2058. doi: 10.4049/jimmunol.176.4.2053. [DOI] [PubMed] [Google Scholar]
- 24.Macchiarini F, Manz MG, Palucka AK, Shultz LD. J Exp Med. 2005;202:1307–1311. doi: 10.1084/jem.20051547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hiramatsu H, Nishikomori R, Heike T, Ito M, Kobayashi K, Katamura K, Nakahata T. Blood. 2003;102:873–880. doi: 10.1182/blood-2002-09-2755. [DOI] [PubMed] [Google Scholar]
- 26.Yahata T, Ando K, Nakamura Y, Ueyama Y, Shimamura K, Tamaoki N, Kato S, Hotta T. J Immunol. 2002;169:204–209. doi: 10.4049/jimmunol.169.1.204. [DOI] [PubMed] [Google Scholar]
- 27.Ishikawa F, Yasukawa M, Lyons B, Yoshida S, Miyamoto T, Yoshimoto G, Watanabe T, Akashi K, Shultz LD, Harada M. Blood. 2005;106:1565–1573. doi: 10.1182/blood-2005-02-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shultz LD, Lyons BL, Burzenski LM, Gott B, Chen X, Chaleff S, Kotb M, Gillies SD, King M, Mangada J, et al. J Immunol. 2005;174:6477–6489. doi: 10.4049/jimmunol.174.10.6477. [DOI] [PubMed] [Google Scholar]
- 29.Embretson J, Zupancic M, Ribas JL, Burke A, Racz P, Tenner-Racz K, Haase AT. Nature. 1993;362:359–362. doi: 10.1038/362359a0. [DOI] [PubMed] [Google Scholar]
- 30.Haase AT. Annu Rev Immunol. 1999;17:625–656. doi: 10.1146/annurev.immunol.17.1.625. [DOI] [PubMed] [Google Scholar]
- 31.Jayakumar P, Berger I, Autschbach F, Weinstein M, Funke B, Verdin E, Goldsmith MA, Keppler OT. J Virol. 2005;79:5220–5226. doi: 10.1128/JVI.79.8.5220-5226.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Orenstein JM, Fox C, Wahl SM. Science. 1997;276:1857–1861. doi: 10.1126/science.276.5320.1857. [DOI] [PubMed] [Google Scholar]
- 33.Soontornniyomkij V, Nieto-Rodriguez JA, Martinez AJ, Kingsley LA, Achim CL, Wiley CA. Clin Neuropathol. 1998;17:95–99. [PubMed] [Google Scholar]
- 34.Wenig BM, Thompson LD, Frankel SS, Burke AP, Abbondanzo SL, Sesterhenn I, Heffner DK. Am J Surg Pathol. 1996;20:572–587. doi: 10.1097/00000478-199605000-00004. [DOI] [PubMed] [Google Scholar]
- 35.Schuitemaker H, Koot M, Kootstra NA, Dercksen MW, de Goede RE, van Steenwijk RP, Lange JM, Schattenkerk JK, Miedema F, Tersmette M. J Virol. 1992;66:1354–1360. doi: 10.1128/jvi.66.3.1354-1360.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmitt N, Nugeyre MT, Scott-Algara D, Cumont MC, Barre-Sinoussi F, Pancino G, Israel N. AIDS. 2006;20:533–542. doi: 10.1097/01.aids.0000210607.63138.bc. [DOI] [PubMed] [Google Scholar]
- 37.Meissner EG, Duus KM, Loomis R, D'Agostin R, Su L. Curr HIV Res. 2003;1:275–285. doi: 10.2174/1570162033485258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Douek DC, Brenchley JM, Betts MR, Ambrozak DR, Hill BJ, Okamoto Y, Casazza JP, Kuruppu J, Kunstman K, Wolinsky S, et al. Nature. 2002;417:95–98. doi: 10.1038/417095a. [DOI] [PubMed] [Google Scholar]
- 39.Moore JP, McKeating JA, Weiss RA, Sattentau QJ. Science. 1990;250:1139–1142. doi: 10.1126/science.2251501. [DOI] [PubMed] [Google Scholar]
- 40.Kuster H, Opravil M, Ott P, Schlaepfer E, Fischer M, Gunthard HF, Luthy R, Weber R, Cone RW. Am J Pathol. 2000;156:1973–1986. doi: 10.1016/S0002-9440(10)65070-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oxenius A, Price DA, Hersberger M, Schlaepfer E, Weber R, Weber M, Kundig TM, Boni J, Joller H, Phillips RE, et al. J Infect Dis. 2004;189:1199–1208. doi: 10.1086/382028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.