Abstract
Androgen receptors (ARs) are phosphorylated at multiple sites in response to ligand binding, but the kinases mediating AR phosphorylation and the importance of these kinases in AR function have not been established. Here we show that cyclin-dependent kinase 1 (Cdk1) mediates AR phosphorylation at Ser-81 and increases AR protein expression, and that Cdk1 inhibitors decrease AR Ser-81 phosphorylation, protein expression, and transcriptional activity in prostate cancer (PCa) cells. The decline in AR protein expression mediated by the Cdk inhibitor roscovitine was prevented by proteosome inhibitors, indicating that Cdk1 stabilizes AR protein, although roscovitine also decreased AR message levels. Analysis of an S81A AR mutant demonstrated that this site is not required for transcriptional activity or Cdk1-mediated AR stabilization in transfected cells. The AR is active and seems to be stabilized by low levels of androgen in “androgen-independent” PCas that relapse subsequent to androgen-deprivation therapy. Significantly, the expression of cyclin B and Cdk1 was increased in these tumors, and treatment with roscovitine abrogated responses to low levels of androgen in the androgen-independent C4-2 PCa cell line. Taken together, these findings identify Cdk1 as a Ser-81 kinase and indicate that Cdk1 stabilizes AR protein by phosphorylation at a site(s) distinct from Ser-81. Moreover, these results indicate that increased Cdk1 activity is a mechanism for increasing AR expression and stability in response to low androgen levels in androgen-independent PCas, and that Cdk1 antagonists may enhance responses to androgen-deprivation therapy.
The androgen receptor (AR) has a central role in prostate cancer (PCa), and androgen-deprivation therapy (ADT) is the standard treatment for metastatic PCa. However, patients invariably recur with more aggressive tumors, which have been termed “hormone-refractory” or “androgen-independent” PCas (1). The mechanisms responsible for the progression to androgen-independent PCa are not clear, but high levels of AR expression and renewed expression of androgen-regulated genes indicate that AR transcriptional activity is reactivated (2–9). Like other steroid receptors, ARs undergo posttranslational modifications including acetylation, ubiquitination, sumoylation, and phosphorylation. The AR N terminus, which harbors the strong ligand-independent activation function 1 (AF1) that interacts with the C-terminal ligand-binding domain and regulatory proteins, is constitutively phosphorylated at Ser-94 and becomes phosphorylated at multiple additional sites in response to ligand binding (10–13). AR reactivation in androgen-independent PCa models is associated with AR stabilization and increased transcriptional activity in response to low levels of androgen (14–17). Multiple kinase pathways, including protein kinase A, phosphatidylinositol 3-kinase (PI3-kinase), and Ras/Raf/MAP kinases, have been implicated in hypersensitive androgen responses, but the identity of kinases that directly phosphorylate ARs and the functional importance of AR phosphorylation have not been established (15, 18–23).
Previous studies have shown that AR Ser-81 is phosphorylated in response to androgens (11), but transient transfection studies in AR-negative cell lines indicate that this site is not required for AR transcriptional activity (13). Here we identify cyclin-dependent kinase 1 (Cdk1, also called Cdc2) as an AR Ser-81 kinase. Cdk1 transfection increased Ser-81 phosphorylation and AR expression, whereas Cdk1 inhibitors markedly decreased AR Ser-81 phosphorylation, protein levels, and transcriptional activity in LNCaP PCa cells. The decline in ARs mediated by roscovitine, a Cdk inhibitor, was prevented by proteosome inhibitors, indicating that Cdk1 can enhance AR protein stability. Significantly, roscovitine also abrogated Ser-81 phosphorylation and AR stabilization in response to low levels of androgen in the androgen-independent C4-2 PCa cell line. These findings indicate that Cdk1 can stabilize ARs and that increased Cdk1 activity may enhance AR responses to low levels of androgen in androgen-independent PCa.
Results
Cdk1 Mediates AR Ser-81 Phosphorylation and Protein Stabilization.
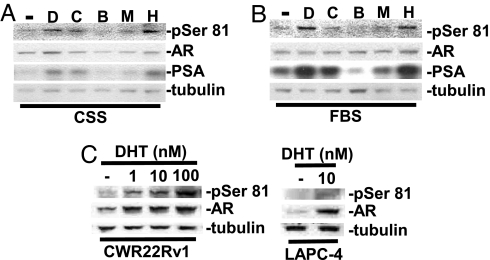
Recent studies using an antibody against AR phospho-Ser-81 (pSer-81) have shown that phosphorylation at this site correlates with androgen-stimulated transcriptional activation and that this site is hypophosphorylated in mutants defective in DNA binding (15, 24). In agreement with these results, AR Ser-81 in LNCaP PCa cells was not substantially phosphorylated in steroid hormone-depleted medium [RPMI medium 1640 with charcoal/dextran-stripped serum (CSS)] (Fig. 1A). Dihydrotestosterone (DHT), cyproterone acetate, and hydroxyflutamide stimulated Ser-81 phosphorylation, which is consistent with their known agonist activities for the T877A mutant AR in LNCaP cells (25, 26). In contrast, the AR antagonists bicalutamide and mifepristone did not stimulate Ser-81 phosphorylation, which supports a link between agonist activity and Ser-81 phosphorylation. Fig. 1A further shows that Ser-81 phosphorylation parallels the expression of prostate-specific antigen (PSA), a strongly androgen-regulated protein.
Fig. 1.
Agonist-dependent AR Ser-81 phosphorylation. (A and B) LNCaP cells were split in normal medium (10% FBS) for 2 days, and the medium was then changed to RPMI medium 1640 plus 5% CSS (A) or was unchanged (B) for 2 days. AR agonists and antagonists were as follows: 10 nM DHT (D) or mifepristone (M) or 10 μM cyproterone acetate (C), bicalutamide (B), or hydroxyflutamide (H) was then added for 24 hr. (C) CWR22Rv1 and LAPC-4 cells were grown in normal medium for 2 days, and the culture was refreshed with 5% CSS medium for 2 days. DHT was then added for 24 hr as indicated. The cells were harvested in 2% SDS, and equal amounts of total protein were immunoblotted.
Similar results were obtained when the cells were cultured in medium that was not steroid hormone-depleted. In this case, higher basal Ser-81 phosphorylation could be detected without the addition of ligand, and this was increased by treatment with AR agonists (Fig. 1B). Moreover, the AR antagonist bicalutamide markedly repressed the basal Ser-81 phosphorylation. Finally, the effects of each drug on Ser-81 phosphorylation correlated with their effects on PSA protein expression. AR Ser-81 phosphorylation was also induced by DHT in other PCa cell lines, CWR22Rv1 and LAPC-4 (Fig. 1C). Significantly, DHT and other AR agonists also increased total AR protein levels, consistent with previous data showing that unliganded ARs are rapidly degraded and that agonist binding stabilizes AR protein (27).
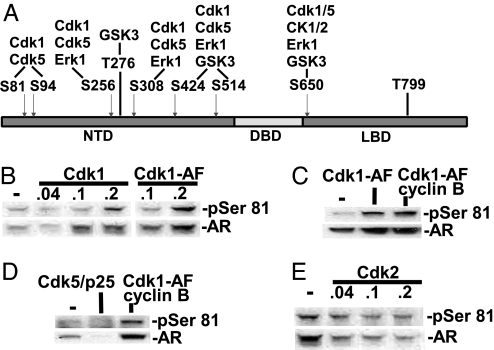
To identify potential Ser-81 kinases that may regulate AR functions, we used the Scansite program (http://scansite.mit.edu), which indicated that Cdk1 (also called Cdc2) and Cdk5 were the strongest candidates (Fig. 2A). Significantly, Ser-81 is one of six Ser/Pro sites in the AR N-terminal domain that are identified as strong potential Cdk1 and Cdk5 sites. Cdk1 is activated by cyclin B and by Cdc25-mediated removal of inhibitory phosphates at the end of the G2 phase, and Cdk1 activity is critical for mitosis (28, 29). Consistent with the Scansite prediction, cotransfection of AR and Cdk1 into 293T cells resulted in increased Ser-81 phosphorylation (Fig. 2B). Significantly, there was also an increase in total AR protein. Ser-81 phosphorylation and total AR expression were similarly increased by cotransfection of an activated Cdk1, Cdk1-AF, which has double mutations on the Cdc25-targeted inhibitory Thr-14/Tyr-15 residues, and could be further enhanced by cotransfection of Cdk1-AF and cyclin B (Fig. 2 B and C). In contrast, transfection of Cdk5 and its activator p25 did not increase Ser-81 phosphorylation, instead causing a marked decrease in total AR expression (Fig. 2D). Transfection of another proline-directed Cdk, Cdk2, similarly resulted in decreased Ser-81 phosphorylation and lower AR levels (Fig. 2E). Taken together, these results support the conclusion that Cdk1 is an AR Ser-81 kinase and further indicate that Cdk1 can enhance AR protein expression.
Fig. 2.
Cdk1 can phosphorylate AR Ser-81 and enhance AR protein expression. (A) Predictions for kinases targeting Ser/Thr/Pro sites. NTD, N-terminal domain; DBD, DNA-binding domain; LBD, ligand-binding domain. (B–E) 293T cells were transfected with 100 ng of AR plasmid, together with other plasmids (100 ng each or 0.04–0.2 μg as indicated), with empty pCDNA3.1 vector to equalize total plasmids. After overnight transfection, the cells were incubated in 5% CSS medium with 10 nM DHT for 24 hr, and equal amounts of protein were immunoblotted.
Cdk1 Inhibition Decreases Ser-81 Phosphorylation and AR Stabilization.
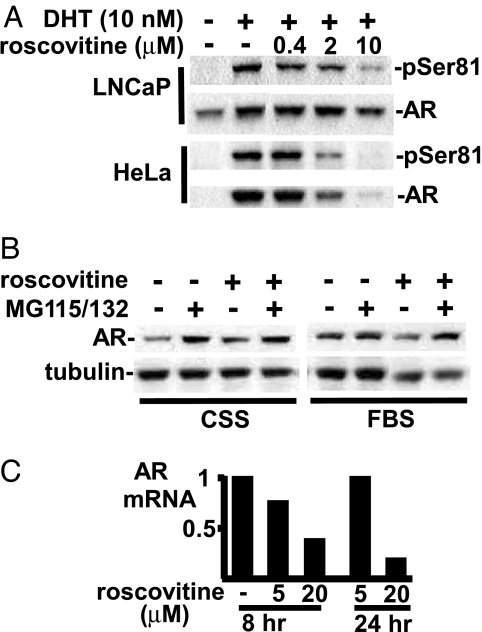
We next carried out inhibitor studies to determine whether endogenous Cdk1 mediates AR Ser-81 phosphorylation in PCa cells. LNCaP cells were treated with DHT (10 nM) in the absence or presence of roscovitine, a selective inhibitor of cellular Cdks (Cdk1, Cdk2, Cdk5, Cdk7, and Cdk9) in the 1 to 10 μM range (28, 30, 31). As shown in Fig. 3A, roscovitine at 0.4 and 2 μM partially blocked DHT-stimulated phosphorylation of Ser-81 on the endogenous AR. Increasing the concentration to 10 μM roscovitine resulted in an almost complete block of Ser-81 phosphorylation and decreased AR protein levels. Roscovitine similarly suppressed Ser-81 phosphorylation and AR protein expression in AR-transfected HeLa cells, with 10 μM yielding nearly complete suppression.
Fig. 3.
Cdk inhibition decreases Ser-81 phosphorylation and AR protein levels. (A) LNCaP- and AR-transfected (50 ng) HeLa cells were incubated in 5% CSS medium with DHT (10 nM) and different doses of roscovitine for 24 hr. (B) LNCaP cells were grown in either normal medium or 5% CSS medium with 10 nM DHT. Roscovitine (10 μM) and proteosome inhibitors MG115 (5 μg/ml) and MG132 (5 μg/ml) were added for 8 hr as indicated. (C) LNCaP cells grown in normal medium were treated with 5 or 20 μM roscovitine for 8 or 24 hr, as indicated. Total RNA was isolated for real-time RT-PCR analysis of AR expression versus 18S rRNA, and levels were normalized to the untreated cells.
It should be noted that DHT markedly stabilizes the expression of transfected AR in HeLa cells, with AR protein being almost undetectable because of degradation in the absence of DHT (Fig. 3A, HeLa, lanes 1 and 2). The marked decrease in AR protein expression at 2 and 10 μM roscovitine suggested that the drug was increasing AR degradation, which was assessed by using proteosome inhibitors. Consistent with the rapid proteosome-mediated degradation of unliganded AR, proteosome inhibitor treatment increased the level of AR protein in LNCaP cells cultured in steroid hormone-depleted medium, without or with roscovitine (20 μM; Fig. 3B). In contrast, proteosome inhibition did not substantially increase AR levels when the cells were cultured in steroid hormone containing medium (10% FBS), consistent with the decreased turnover of AR under these conditions. However, the decrease in AR protein in 10% FBS mediated by roscovitine (20 μM) was abrogated by proteosome inhibition, indicating that AR degradation was increased by roscovitine.
Previous studies in LNCaP cells have shown that the decline in AR protein levels in response to androgen withdrawal results in an increase in AR message levels, reflecting feedback inhibition of the AR gene by the androgen-liganded AR (32). In contrast, quantitative real-time RT-PCR experiments showed that the roscovitine-mediated decline in AR protein levels in LNCaP cells did not cause an increase in AR message levels, with a substantial decrease in message at 20 μM roscovitine (Fig. 3C). Taken together, these results demonstrate that roscovitine at lower concentrations inhibits androgen-stimulated Ser-81 phosphorylation, whereas higher concentrations (≈10 μM) cause a decrease in AR protein that is associated with both decreased AR message levels and increased protein degradation.
Cdk1 Inhibitors Suppress AR Transcriptional Activity.
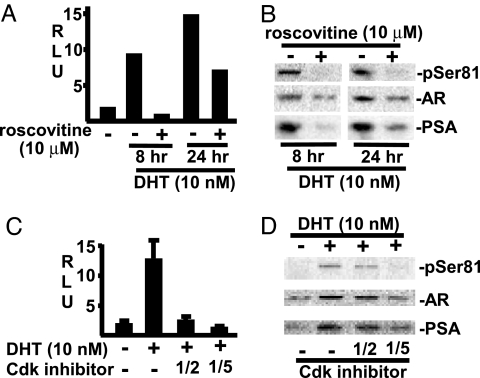
To assess the effects of Cdk1 inhibition on AR transcriptional activity, we cotransfected LNCaP cells with a firefly luciferase reporter regulated by the androgen-responsive PSA promoter/enhancer (pPSA–luciferase) and a control Renilla luciferase reporter regulated by the CMV enhancer (pRL–CMV). Roscovitine markedly repressed the pPSA–luciferase activity stimulated by 8 hr of DHT exposure and repressed the pPSA–luciferase activity to a lesser extent after 24 hr (Fig. 4A). Immunoblotting further showed that the roscovitine treatment completely blocked Ser-81 phosphorylation at 8 hr and markedly blocked it at 24 hr and that roscovitine treatment also decreased total AR protein levels (Fig. 4B). Moreover, consistent with the pPSA–luciferase results, DHT-stimulated expression of endogenous PSA protein was markedly repressed. Because roscovitine is not specific for Cdk1 and can also inhibit Cdk2, Cdk5, Cdk7, and Cdk9, we examined two additional Cdk inhibitors with overlapping specificities. LNCaP cells were transfected as described above and stimulated for 8 hr with DHT in the absence or presence of NU6102 (Cdk1/Cdk2 inhibitor with IC50 of 8 μM for cell growth) (33) or 3-amino-1H-pyrazolo[3,4-β]quinoxaline (Cdk1/Cdk5 inhibitor) (34). Significantly, both drugs repressed DHT-stimulated transcriptional activity and Ser-81 phosphorylation, with less marked decreases in AR and endogenous PSA protein, further indicating that Cdk1 was mediating these effects (Fig. 4D).
Fig. 4.
Cdk1 inhibitors repress Ser-81 phosphorylation and AR and PSA protein expression. RLU, relative light unit. (A and B) LNCaP cells were transfected overnight with pRL–CMV (2.5 ng) and pPSA–luciferase (50 ng) reporters and then incubated in 5% CSS medium with 10 nM DHT and 10 μM roscovitine for 8 or 24 hr, as indicated. The cells were harvested and divided for luciferase assays (A) or immunoblotting (B). (C and D) LNCaP cells were transfected and analyzed as in A and B but were treated with the Cdk1/Cdk2 inhibitor NU6102 (10 μM) or the Cdk1/Cdk5 inhibitor 3-amino-1H-pyrazolo[3,4-β]quinoxaline (0.1 mM) as indicated.
AR Transcriptional Activity Is Not Ser-81-Dependent.
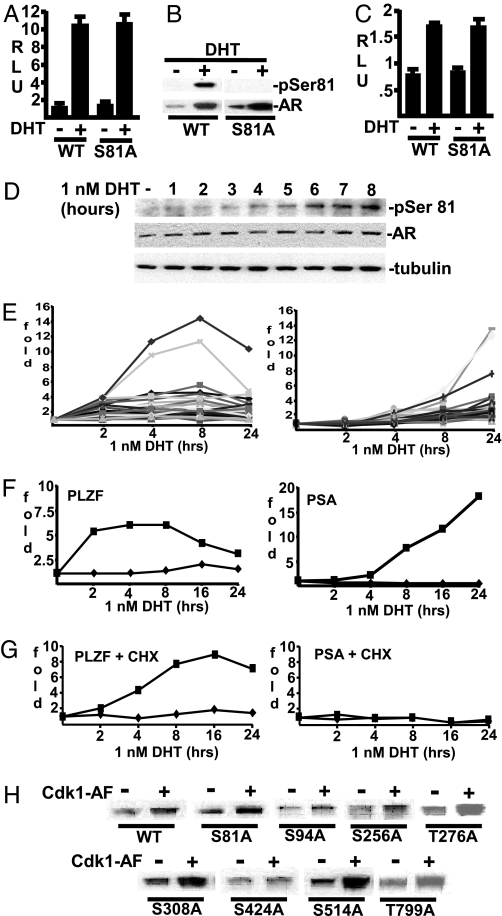
Although Cdk1 inhibitors could suppress both Ser-81 phosphorylation and AR transcriptional activity, previous transfection studies in AR-negative cell lines have shown that Ser-81 is not required for AR transcriptional activity (13, 35). We similarly found that transfected ARs and an S81A mutant had comparable levels of androgen-stimulated transcriptional activity in HeLa cells, as assessed on an ARE4–luciferase reporter gene (Fig. 5A). Significantly, WT and S81A mutant ARs were expressed at comparable levels and were both strongly stabilized by DHT (Fig. 5B). Consistent with the specificity of the pSer-81 antibody, there was no reactivity against the S81A mutant (Fig. 5B). Similar results were obtained in transfected 293T cells (Fig. 5C).
Fig. 5.
Ser-81 phosphorylation is not required for AR transcriptional activity. RLU, relative light unit. (A and B) HeLa cells were transfected overnight with pRL–CMV (2.5 ng) and ARE4–luciferase (50 ng) reporters, together with AR WT (50 ng) or AR S81A mutant (50 ng) expression vectors, and then incubated in 5% CSS medium with DHT (10 nM) for 24 hr, as indicated. The cells were harvested and divided for luciferase assay (A) or immunoblotting (B). (C) An experiment similar to that shown in A was performed with 293T cells. (D) LNCaP cells were split in RPMI medium 1640 plus 5% CSS for 2 days and then treated with 1 nM DHT for different time points, and equal amounts of proteins were immunoblotted as indicated. (E) LNCaP cells were split in RPMI medium 1640 with 5% CSS for 2 days and then treated with 1 nM DHT for 2–24 hr, and total RNA from duplicate plates was analyzed on Affymetrix U133A GeneChip array. Androgen-stimulated genes were clustered into early responsive (Left) and late responsive (Right) groups. (F and G) LNCaP cells were split in RPMI medium 1640 with 5% CSS for 2 days and then treated with DHT (1 nM) (■) or vehicle (♦) for 2–24 hr, without (F) or with (G) cycloheximide (CHX) (10 μg/ml). Total RNA was isolated for real-time RT-PCR analysis of PSA and PLZF gene expression versus 18S rRNA, and values were normalized to levels before DHT addition. (H) 293T cells were transfected with 100 ng of WT AR or single-site (Ser/Thr) mutant expression vectors, together with 100 ng of activated Cdk1 (Cdk1-AF) expression vector or empty pCDNA3.1 vector. After overnight transfection, the cells were incubated in 5% CSS medium with 10 nM DHT for 24 hr and harvested in 2% SDS, and equal amounts of total protein were immunoblotted for AR.
Chromatin immunoprecipitation studies in LNCaP cells have shown that DHT stimulates the very rapid (within 15 min) recruitment of AR and coactivator proteins to endogenous androgen-regulated genes, including the PSA gene (36, 37). Therefore, time course studies were done to assess whether Ser-81 phosphorylation was linked to chromatin recruitment of the endogenous AR in PCa cells. In contrast to the rapid recruitment of AR to androgen-regulated genes, a clear increase in Ser-81 phosphorylation was first detected at ≈4 hr and was not maximal until 8 hr of DHT stimulation, indicating that Ser-81 phosphorylation was not required for AR recruitment to androgen-regulated genes (Fig. 5D).
We next used Affymetrix oligonucleotide microarrays to determine how the time course of Ser-81 phosphorylation compared with the induction of androgen-regulated genes. Significantly, this analysis identified a large number of genes that were rapidly induced within 2–4 hr, whereas strong induction of many other genes, including PSA, was not observed until 4 or 8 hr (Fig. 5E). Real-time RT-PCR confirmed the rapid induction of PLZF, a previously identified androgen-regulated gene, relative to PSA (Fig. 5F) (38). Interestingly, whereas cycloheximide treatment did not block the induction of PLZF (although maximal induction took ≈8, instead of 2, hr), it abrogated the DHT-stimulated expression of PSA, indicating that PSA transcription depended on new protein synthesis (Fig. 5G). Taken together, these results indicate that the expression of rapidly induced androgen-regulated genes is independent of Ser-81, although Ser-81 phosphorylation may contribute to the subsequently delayed induction of other androgen-regulated genes.
Cdk1-Mediated AR Stabilization Is Not Ser-81-Dependent.
We next determined whether the Cdk1-mediated increase in AR protein depended on Ser-81 phosphorylation. For these experiments, 293T cells were cotransfected with ARs and mutants, without or with activated Cdk1, and AR levels were assessed by immunoblotting. Significantly, Cdk1 enhanced the expression of both the WT and S81A mutant, indicating that Cdk1 can stabilize AR protein expression by a mechanism that is independent of Ser-81 phosphorylation (Fig. 5H). Similar to the S81A mutant, other Ser/Thr/Pro site mutants were also stabilized by activated Cdk1 (Fig. 5H). However, the degree to which this stabilization occurred varied, indicating that one or more of these other sites on the AR may contribute to Cdk1-mediated AR stabilization.
Roscovitine Abrogates AR Stabilization at Low Androgen Levels in Androgen-Independent PCa Cells.
The progression to androgen-independent PCa after ADT is associated with high levels of AR expression and renewed expression of multiple androgen-regulated genes, indicating that AR transcriptional activity is reactivated despite castrate levels of androgen (2–9). Studies in xenograft and cell line models indicate that the AR in androgen-independent tumors can be stabilized and transcriptionally activated by low levels of androgens, but the mechanisms mediating this hypersensitivity to androgens are not yet clear (14–17). Interestingly, we have found that the most highly overexpressed cell-cycle-regulatory genes in androgen-independent PCa are Cdk1, cyclin B1, and cyclin B2 (3.3, 2.7, and 2.6-fold increases in median expression, respectively), with the cyclin B1 increase being statistically significant (6). Previous studies have also found increased expression of cyclin B1 and Cdc25 in PCa, and this has been associated with more aggressive PCa and progression to androgen-independent PCa (39–41). Based on these observations, we examined androgen-independent PCa cells to determine whether Cdk1 may mediate enhanced responses to low levels of androgen.
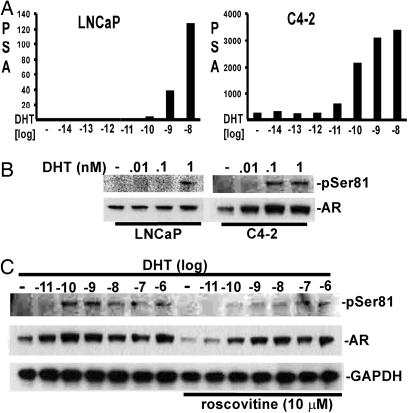
C4-2 cells were derived from an LNCaP xenograft that relapsed after castration and have been used as a model of androgen-independent PCa (14, 17, 42). Consistent with previous results, C4-2 cells expressed substantial basal levels of PSA message in steroid hormone-depleted medium and could be further stimulated by as little as 10 pM DHT (Fig. 6A). In contrast, 100 pM to 1 nM DHT was required to stimulate PSA expression by LNCaP cells. Similarly, AR phosphorylation at Ser-81 was not stimulated until the DHT concentration reached ≈1 nM in LNCaP cells, whereas in C4-2 cells, Ser-81 phosphorylation and AR protein expression were increased at 10–100 pM DHT (Fig. 6B). Significantly, roscovitine abrogated the hypersensitive response to low levels of DHT, with 10- to 100-fold higher DHT concentrations being required for maximal Ser-81 phosphorylation and AR protein expression in the roscovitine-treated C4-2 cells (Fig. 6C).
Fig. 6.
Roscovitine inhibits hypersensitive responses to DHT in androgen-independent C4-2 cells. (A and B) LNCaP cells or C4-2 cells were split in RPMI medium 1640 plus 5% CSS for 2 days, and different doses of DHT were then added for 24 hr as indicated. (A) Total RNA was isolated for real-time RT-PCR analysis of PSA expression versus 18S rRNA and levels were normalized to untreated cells. (B) Total proteins were harvested in 2% SDS and equal amounts were immunoblotted. (C) C4-2 cells were split in RPMI medium 1640 plus 5% CSS for 2 days, and different doses of DHT were added for 24 hr, with or without 10 μM roscovitine, as indicated.
Discussion
The unliganded AR undergoes rapid degradation, whereas androgen binding stabilizes AR and triggers conformational changes that result in DNA binding and transcriptional activation. Ligand binding induced AR phosphorylation at multiple sites (primarily N-terminal Ser/Pro sites), but the kinases mediating AR phosphorylation and their importance for AR function have not been established (11, 13, 43, 44). Transfected Cdk1 stimulated AR phosphorylation at Ser-81 and increased AR protein expression, whereas Cdk1 inhibitors decreased Ser-81 phosphorylation of the endogenous AR in LNCaP PCa cells and similarly decreased AR protein expression and transcriptional activity. The decrease in AR protein expression in response to the Cdk inhibitor roscovitine was prevented by treatment with proteosome inhibitors, indicating that Cdk1 enhances AR protein stability. However, roscovitine also decreased AR message levels, indicating that Cdk1 or possibly another Cdk may increase AR gene transcription or stability of the AR message. Analyses of an S81A AR mutant in transfected cells demonstrated that this site was not required for AR transcriptional activity or for AR stabilization mediated by androgen or Cdk1. Significantly, the expression of cyclin B and Cdk1 is increased in androgen-independent tumors that relapse subsequent to ADT (6), and treatment with roscovitine abrogated hypersensitive responses to low levels of androgen in the androgen-independent C4-2 PCa cell line. Taken together, these findings identify Cdk1 as a Ser-81 kinase and indicate that Cdk1 stabilizes AR protein by phosphorylation at a site(s) distinct from Ser-81. Moreover, these results indicate that increased Cdk1 activity is a mechanism for increasing AR expression and stability in response to low androgen levels in androgen-independent PCa and that Cdk1 antagonists may enhance or prolong responses to ADT.
Consistent with previous data, Ser-81 phosphorylation was induced by AR agonists but was not required for AR transcriptional activity or stabilization in response to androgens or activated Cdk1 in transfected cells. Moreover, we found that phosphorylation of the endogenous AR at Ser-81 in LNCaP cells was not increased until ≈4 hr after androgen stimulation, indicating that it was not required for the expression of rapidly induced genes such as PLZF. This delay is consistent with data showing that Ser-81 is not phosphorylated until after DNA binding and transcription initiation and is progressively phosphorylated beyond 6 hr (12, 24). Interestingly, the DHT-stimulated expression of many other genes (including PSA) was delayed for several hours, but this delay with respect to PSA seems to reflect a requirement for new protein synthesis and is not clearly related to Ser-81 phosphorylation. Although these data show that Ser-81 is not required for AR transcriptional activity or stabilization in transfected cells, the unique location of Ser-81 within the N-terminal polyglutamine stretch, the length of which affects AR stability and transcriptional activity (45), suggests that there may be some new functions for phosphorylation at this site.
Cdk1 is a candidate kinase for multiple Ser/Pro sites in addition to Ser-81 that are phosphorylated in the AR N terminus (11, 12, 43, 44), but mutagenesis results indicated that none of these sites were essential for Cdk1-mediated AR stabilization. One interpretation of these data is that AR can be stabilized by Cdk1-mediated phosphorylation at any one of multiple Ser/Pro sites in the AR. Alternatively, AR may be stabilized by a mechanism that is independent of AR phosphorylation, including the indirect effects of Cdk1 on other pathways due to altered cell-cycle kinetics. It should also be noted that, although Cdk2 and Cdk5 did not enhance AR Ser-81 phosphorylation or expression, other Cdks may contribute to the effects of Cdk antagonists in PCa cells, including possible roles for Cdk7 and Cdk9 in regulating AR message levels. Significantly, previous studies have identified AR interactions with Cdk6 and Cdk7, whereas Cdk2 has been reported to interact with the progesterone receptor (46–49). We have used Cdk1 siRNA and short hairpin RNA (shRNA) in efforts to further assess the role of Cdk1, but Cdk1 is required for mitosis, and it has not yet been possible to generate Cdk1-depleted PCa cells.
The standard treatment for PCa that has spread outside the prostate is to suppress or ablate testicular androgen production (i.e., ADT), but patients eventually develop recurrent tumors. Importantly, although there is a decline in the expression of AR protein and androgen-regulated genes in response to ADT, both AR protein and androgen-regulated genes are highly expressed in the recurrent tumors. This AR reactivation is associated with increased AR message levels and with AR stabilization and increased transcriptional activity in response to low levels of androgens in PCa cell lines and xenograft models (6, 8, 14, 50). Significantly, previous studies have indicated that Cdk1 activity is increased in more aggressive and androgen-independent PCa (6, 39–41). Consistent with Cdk1 enhancing AR activity in androgen-independent PCa, we found that Cdk inhibition with roscovitine abrogated the hypersensitive response to low concentrations of DHT in androgen-independent C4-2 cells. Taken together, these observations indicate that increased Cdk1 activity may play a role in androgen-independent PCa by enhancing AR stability and activity in response to low androgen concentrations.
We suggest that a physiological function of Cdk1 is to phosphorylate AR, possibly at multiple sites, and to prevent its degradation. Increased Cdk1 activity would then provide a mechanism for enhancing AR stability and expression in androgen-independent tumors that recur subsequent to ADT, although additional mechanisms including phosphorylation by other kinases, acetylation, and increased expression of chaperone proteins may also enhance AR expression. Further studies are clearly necessary to determine whether Cdk1 stabilizes AR directly by phosphorylation (and to precisely define the relevant sites) or by other mechanisms and to determine whether Cdk1 directly or indirectly enhances AR message levels. In any case, these data indicate that Cdk antagonists may enhance responses to ADT and have efficacy in androgen-independent PCa treatment. Moreover, although the efficacy of pan-Cdk antagonists currently in clinical trials remains to be established, these data suggest that selective Cdk1 antagonists that are now under development may be particularly effective in a subset of cancers including androgen-independent PCa (28, 51).
Materials and Methods
Reagents and Plasmids.
Cdk inhibitors were from Calbiochem (Darmstadt, Germany), and all other drugs were from Sigma (St. Louis, MO). Sera (FBS and CSS) were from HyClone (Logan, UT). Reporter genes have been described (52), and the pCIneo-hAR plasmid was from Lirim Shemshedini (University of Toledo, Toledo, OH). Cdk1, Cdk1-AF (T14A/Y15F mutant), Cdk2, and cyclin B plasmids were from Azad Bonni (Department of Pathology, Harvard Medical School, Boston, MA). Cdk5 and p25 plasmids were from Li-Huei Tsai (Department of Pathology, Harvard Medical School) and Bradley M. Denker (Brigham and Women's Hospital, Harvard Medical School). AR and AR pSer-81 antibodies were from Upstate Biotechnology (Lake Placid, NY), anti-PSA was from BioDesign (Kennebunk, ME), and anti-tubulin was from Chemicon (Temecula, CA).
Transient Transfections and Reporter Gene Assays.
LNCaP and C4-2 cells were grown in RPMI medium 1640 with 10% FBS, LAPC-4 cells were grown in Iscove's modified Dulbecco's medium with 10% FBS, and CWR22Rv1, HeLa, and 293T cells were grown in DMEM with 5% FBS. Cells were transfected overnight at ≈80% confluence with Lipofectamine 2000 and then switched to medium containing 5% CSS, with or without treatments as indicated. The ratios between firefly and Renilla luciferase activities (in relative light units) were measured with Promega's (Madison, WI) Dual-Luc reporter assay kit, and the results reflect the mean and standard deviation from triplicate samples.
Real-Time RT-PCR.
Total RNA was isolated with Trizol (Invitrogen, Carlsbad, CA), and real-time RT-PCR was performed with TaqMan kits (PE Biosystems, Foster City, CA) and an ABI Prism 7700 sequence detector (Applied Biosystems, Foster City, CA). The PSA primers were 5′-GATGAAACAGGCTGTGCCG-3′ (forward) and 5′-CCTCACAGCTACCCACTGCA-3′ (reverse), and the probe was 5′-FAM-CAGGAACAAAAGCGTGATCTTGCTGGG-3′. The PLZF primers were 5′-GGAGGATGCCCTGGAGACA-3′ (forward) and 5′-CAGCAGACAGAAGACGGCC-3′ (reverse), and the probe was 5′-FAM-CAGGCAGA-CCCATACTGGCACTGACA-3′. The AR primers were 5′-GGAATTCCTGTGCATGAAA-3′ (forward) and 5′-CGAAGTTCATCAAAGAATT-3′ (reverse), and the probe was 5′-FAM-CTTCAGCATTATTCCAGTG-3′. The internal control used was 18S rRNA.
Affymetrix Microarrays.
LNCaP cells were split in RPMI medium 1640 with 5% CSS for 2 days, and 1 nM DHT was added for 2, 4, 6, 8, and 24 hr. Total RNA from duplicate samples was isolated with Trizol reagent and analyzed on an Affymetrix (Santa Clara, CA) GeneChip U133A array, as described (6).
Acknowledgments
We thank Drs. Azad Bonni, Li-Huei Tsai, Bradley M. Denker, and Lirim Shemshedini for plasmids; Victoria Petkova for real-time RT-PCR; Ediane L. Dutra for DNA sequencing; and Robert Borgesi for technique assistance. This work was supported by Department of Defense Grant PC040499 (to S.C.), the Dana–Farber/Harvard Cancer Center Prostate Cancer Specialized Program of Research Excellence (SPORE), and the Hershey Family Prostate Cancer Research Fund.
Abbreviations
- AR
androgen receptor
- PCa
prostate cancer
- ADT
androgen-deprivation therapy
- Cdk
cyclin-dependent kinase
- CSS
charcoal/dextran-stripped serum
- DHT
dihydrotestosterone
- PSA
prostate-specific antigen.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
References
- 1.Gelmann EP. Clin Oncol. 2002;20:3001–3015. doi: 10.1200/JCO.2002.10.018. [DOI] [PubMed] [Google Scholar]
- 2.van der Kwast TH, Schalken J, Ruizeveld de Winter JA, van Vroonhoven CC, Mulder E, Boersma W, Trapman J. Int J Cancer. 1991;48:189–193. doi: 10.1002/ijc.2910480206. [DOI] [PubMed] [Google Scholar]
- 3.Visakorpi T, Hyytinen E, Koivisto P, Tanner M, Keinanen R, Palmberg C, Palotie A, Tammela T, Isola J, Kallioniemi OP. Nat Genet. 1995;9:401–406. doi: 10.1038/ng0495-401. [DOI] [PubMed] [Google Scholar]
- 4.Taplin ME, Bubley GJ, Shuster TD, Frantz ME, Spooner AE, Ogata GK, Keer HN, Balk SP. N Engl J Med. 1995;332:1393–1398. doi: 10.1056/NEJM199505253322101. [DOI] [PubMed] [Google Scholar]
- 5.Holzbeierlein J, Lal P, LaTulippe E, Smith A, Satagopan J, Zhang L, Ryan C, Smith S, Scher H, Scardino P, et al. Am J Pathol. 2004;164:217–227. doi: 10.1016/S0002-9440(10)63112-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stanbrough M, Bubley GJ, Ross K, Golub TR, Rubin MA, Penning TM, Febbo PG, Balk SP. Cancer Res. 2006;66:2815–2825. doi: 10.1158/0008-5472.CAN-05-4000. [DOI] [PubMed] [Google Scholar]
- 7.Mohler JL, Gregory CW, Ford OH, III, Kim D, Weaver CM, Petrusz P, Wilson EM, French FS. Clin Cancer Res. 2004;10:440–448. doi: 10.1158/1078-0432.ccr-1146-03. [DOI] [PubMed] [Google Scholar]
- 8.Gregory CW, Hamil KG, Kim D, Hall SH, Pretlow TG, Mohler JL, French FS. Cancer Res. 1998;58:5718–5724. [PubMed] [Google Scholar]
- 9.Amler LC, Agus DB, LeDuc C, Sapinoso ML, Fox WD, Kern S, Lee D, Wang V, Leysens M, Higgins B, et al. Cancer Res. 2000;60:6134–6141. [PubMed] [Google Scholar]
- 10.Kuiper GG, Brinkmann AO. Biochemistry. 1995;34:1851–1857. doi: 10.1021/bi00006a005. [DOI] [PubMed] [Google Scholar]
- 11.Zhou ZX, Kemppainen JA, Wilson EM. Mol Endocrinol. 1995;9:605–615. doi: 10.1210/mend.9.5.7565807. [DOI] [PubMed] [Google Scholar]
- 12.Gioeli D, Ficarro SB, Kwiek JJ, Aaronson D, Hancock M, Catling AD, White FM, Christian RE, Settlage RE, Shabanowitz J, et al. J Biol Chem. 2002;277:29304–29314. doi: 10.1074/jbc.M204131200. [DOI] [PubMed] [Google Scholar]
- 13.Wong HY, Burghoorn JA, Van Leeuwen M, de Ruiter PE, Schippers E, Blok LJ, Li KW, Dekker HL, De Jong L, Trapman J, et al. Biochem J. 2004;383:267–276. doi: 10.1042/BJ20040683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gregory CW, Johnson RT, Jr, Mohler JL, French FS, Wilson EM. Cancer Res. 2001;61:2892–2898. [PubMed] [Google Scholar]
- 15.Mellinghoff IK, Vivanco I, Kwon A, Tran C, Wongvipat J, Sawyers CL. Cancer Cell. 2004;6:517–527. doi: 10.1016/j.ccr.2004.09.031. [DOI] [PubMed] [Google Scholar]
- 16.Bakin RE, Gioeli D, Sikes RA, Bissonette EA, Weber MJ. Cancer Res. 2003;63:1981–1989. [PubMed] [Google Scholar]
- 17.Bakin RE, Gioeli D, Bissonette EA, Weber MJ. Cancer Res. 2003;63:1975–1980. [PubMed] [Google Scholar]
- 18.Nazareth LV, Weigel NL. J Biol Chem. 1996;271:19900–19907. doi: 10.1074/jbc.271.33.19900. [DOI] [PubMed] [Google Scholar]
- 19.Sadar MD. J Biol Chem. 1999;274:7777–7783. doi: 10.1074/jbc.274.12.7777. [DOI] [PubMed] [Google Scholar]
- 20.Weber MJ, Gioeli D. J Cell Biochem. 2004;91:13–25. doi: 10.1002/jcb.10683. [DOI] [PubMed] [Google Scholar]
- 21.Craft N, Shostak Y, Carey M, Sawyers CL. Nat Med. 1999;5:280–285. doi: 10.1038/6495. [DOI] [PubMed] [Google Scholar]
- 22.Gregory CW, Fei X, Ponguta LA, He B, Bill HM, French FS, Wilson EM. J Biol Chem. 2004;279:7119–7130. doi: 10.1074/jbc.M307649200. [DOI] [PubMed] [Google Scholar]
- 23.Gregory CW, Whang YE, McCall W, Fei X, Liu Y, Ponguta LA, French FS, Wilson EM, Earp HS., III Clin Cancer Res. 2005;11:1704–1712. doi: 10.1158/1078-0432.CCR-04-1158. [DOI] [PubMed] [Google Scholar]
- 24.Black BE, Vitto MJ, Gioeli D, Spencer A, Afshar N, Conaway MR, Weber MJ, Paschal BM. Mol Endocrinol. 2004;18:834–850. doi: 10.1210/me.2003-0145. [DOI] [PubMed] [Google Scholar]
- 25.Veldscholte J, Berrevoets CA, Brinkmann AO, Grootegoed JA, Mulder E. Biochemistry. 1992;31:2393–2399. doi: 10.1021/bi00123a026. [DOI] [PubMed] [Google Scholar]
- 26.Fenton MA, Shuster TD, Fertig AM, Taplin ME, Kolvenbag G, Bubley GJ, Balk SP. Clin Cancer Res. 1997;3:1383–1388. [PubMed] [Google Scholar]
- 27.Kemppainen JA, Lane MV, Sar M, Wilson EM. J Biol Chem. 1992;267:968–974. [PubMed] [Google Scholar]
- 28.Shapiro GI. J Clin Oncol. 2006;24:1770–1783. doi: 10.1200/JCO.2005.03.7689. [DOI] [PubMed] [Google Scholar]
- 29.Porter LA, Donoghue DJ. Prog Cell Cycle Res. 2003;5:335–347. [PubMed] [Google Scholar]
- 30.Bach S, Knockaert M, Reinhardt J, Lozach O, Schmitt S, Baratte B, Koken M, Coburn SP, Tang L, Jiang T, et al. J Biol Chem. 2005;280:31208–31219. doi: 10.1074/jbc.M500806200. [DOI] [PubMed] [Google Scholar]
- 31.Meijer L, Borgne A, Mulner O, Chong JP, Blow JJ, Inagaki N, Inagaki M, Delcros JG, Moulinoux JP. Eur J Biochemd. 1997;243:527–536. doi: 10.1111/j.1432-1033.1997.t01-2-00527.x. [DOI] [PubMed] [Google Scholar]
- 32.Quarmby VE, Yarbrough WG, Lubahn DB, French FS, Wilson EM. Mol Endocrinol. 1990;4:22–28. doi: 10.1210/mend-4-1-22. [DOI] [PubMed] [Google Scholar]
- 33.Davies TG, Bentley J, Arris CE, Boyle FT, Curtin NJ, Endicott JA, Gibson AE, Golding BT, Griffin RJ, Hardcastle IR, et al. Nat Struct Biol. 2002;9:745–749. doi: 10.1038/nsb842. [DOI] [PubMed] [Google Scholar]
- 34.Ortega MA, Montoya ME, Zarranz B, Jaso A, Aldana I, Leclerc S, Meijer L, Monge A. Bioorg Med Chem. 2002;10:2177–2184. doi: 10.1016/s0968-0896(02)00069-x. [DOI] [PubMed] [Google Scholar]
- 35.Zhou ZX, Lane MV, Kemppainen JA, French FS, Wilson EM. Mol Endocrinol. 1995;9:208–218. doi: 10.1210/mend.9.2.7776971. [DOI] [PubMed] [Google Scholar]
- 36.Kang Z, Janne OA, Palvimo JJ. Mol Endocrinol. 2004;18:2633–2648. doi: 10.1210/me.2004-0245. [DOI] [PubMed] [Google Scholar]
- 37.Shang Y, Myers M, Brown M. Mol Cell. 2002;9:601–610. doi: 10.1016/s1097-2765(02)00471-9. [DOI] [PubMed] [Google Scholar]
- 38.Jiang F, Wang Z. Prostate. 2004;59:426–435. doi: 10.1002/pros.20000. [DOI] [PubMed] [Google Scholar]
- 39.Ngan ES, Hashimoto Y, Ma ZQ, Tsai MJ, Tsai SY. Oncogene. 2003;22:734–739. doi: 10.1038/sj.onc.1206121. [DOI] [PubMed] [Google Scholar]
- 40.Maddison LA, Huss WJ, Barrios RM, Greenberg NM. Prostate. 2004;58:335–344. doi: 10.1002/pros.10341. [DOI] [PubMed] [Google Scholar]
- 41.Ozen M, Ittmann M. Clin Cancer Res. 2005;11:4701–4706. doi: 10.1158/1078-0432.CCR-04-2551. [DOI] [PubMed] [Google Scholar]
- 42.Wu HC, Hsieh JT, Gleave ME, Brown NM, Pathak S, Chung LW. Int J Cancer. 1994;57:406–412. doi: 10.1002/ijc.2910570319. [DOI] [PubMed] [Google Scholar]
- 43.Kuiper GG, de Ruiter PE, Trapman J, Boersma WJ, Grootegoed JA, Brinkmann AO. Biochem J. 1993;291:95–101. doi: 10.1042/bj2910095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jenster G, de Ruiter PE, van der Korput HA, Kuiper GG, Trapman J, Brinkmann AO. Biochemistry. 1994;33:14064–14072. doi: 10.1021/bi00251a015. [DOI] [PubMed] [Google Scholar]
- 45.Chamberlain NL, Driver ED, Miesfeld RL. Nucleic Acids Res. 1994;22:3181–3186. doi: 10.1093/nar/22.15.3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee DK, Duan HO, Chang C. J Biol Chem. 2000;275:9308–9313. doi: 10.1074/jbc.275.13.9308. [DOI] [PubMed] [Google Scholar]
- 47.Lim JT, Mansukhani M, Weinstein IB. Proc Natl Acad Sci USA. 2005;102:5156–5161. doi: 10.1073/pnas.0501203102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Narayanan R, Adigun AA, Edwards DP, Weigel NL. Mol Cell Biol. 2005;25:264–277. doi: 10.1128/MCB.25.1.264-277.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McEwan IJ, Gustafsson J. Proc Natl Acad Sci USA. 1997;94:8485–8490. doi: 10.1073/pnas.94.16.8485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen CD, Welsbie DS, Tran C, Baek SH, Chen R, Vessella R, Rosenfeld MG, Sawyers CL. Nat Med. 2004;10:33–39. doi: 10.1038/nm972. [DOI] [PubMed] [Google Scholar]
- 51.Vassilev LT, Tovar C, Chen S, Knezevic D, Zhao X, Sun H, Heimbrook DC, Chen L. Proc Natl Acad Sci USA. 2006;103:10660–10665. doi: 10.1073/pnas.0600447103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen SY, Wulf G, Zhou XZ, Rubin MA, Lu KP, Balk SP. Mol Cell Biol. 2006;26:929–939. doi: 10.1128/MCB.26.3.929-939.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]