Abstract
Estrogen receptor α (ERα) functions as both a transcription factor and a mediator of rapid estrogen signaling. Recent studies have shown a role for ERα-interacting membranous and cytosolic proteins in ERα action, but our understanding of the role of the microtubule network in the modulation of ERα signaling remains unclear. Here we found that endogenous ERα associates with microtubules through the microtubule-binding protein hematopoietic PBX-interaction protein (HPIP). Biochemical and RNA-interference studies demonstrated that HPIP influences ERα-dependent rapid estrogen signaling by acting as a scaffold protein and recruits Src kinase and the p85 subunit of phosphatidylinositol 3-kinase to a complex with ERα, which in turn stimulates AKT and MAPK. We also found that ERα interacts with β-tubulin through HPIP. Destabilization of microtubules activated ERα signaling, whereas stabilization of microtubules repressed ERα transcriptional activity in a HPIP-dependent manner. These findings revealed a role for HPIP–microtubule complex in regulating 17β-estradiol–ERα responses in mammalian cells and discovered an inherent role of microtubules in the action of nuclear receptor.
Keywords: 17β-estradiol, estrogen receptor, hematopoietic PBX-interaction protein
Estrogen regulates a plethora of functionally divergent physiological processes including development, homeostasis, and reproduction (1). The diversity of estrogen action results in part from the ability of estrogen receptors (ERs) to act both as transcription factors that regulate gene expression (i.e., genomic effects) and as signaling proteins that rapidly recruit and activate kinase-dependent signaling pathways (rapid effects). There is growing evidence that a subpopulation of the conventional nuclear steroid receptor localized in the vicinity of the cell membrane mediates many of the rapid signaling actions of steroid hormones; however, membrane receptors unrelated to conventional steroid receptors have also been implicated (2, 3). Several studies support the concept that estrogen can activate multiple cytosolic signaling pathways through direct interactions of conventional estrogen receptor (ERα or ERβ) with various cytoplasmic and membranous proteins, including kinases and adaptor proteins, by forming different multiprotein complexes (2, 4). In addition, sequestration of ER by MTA1s (metastasis-associated antigen 1 short form) also triggers estrogen rapid signaling. So it appears that relative subcellular distribution of ERs plays a critical role in estrogen signaling. Besides these mechanistic studies, recent reports have suggested that extranuclear estrogen signaling is directly implicated in cell migration through actin cytoskeleton remodeling (5).
Microtubules are structural components of the cytoskeleton required for cell motility that regulate a variety of signaling pathways, including the inducible nitric oxide synthase, NF-κB, ERK, JNK, Wnt, and Hedgehog signaling pathways (6). The functional role of microtubules in signal transduction has been further elucidated by recent findings of interactions between microtubules and various classes of regulatory proteins, including p53 (7), Smads (8), and p120 catenin (9), by different mechanisms. This evidence suggests the existence of crosstalk between microtubules and signaling cascades. In this context, the role of the microtubule network in estrogen signaling has not been studied, except that the overexpressed activation function 1 (AF1) domain of ERα has been shown to bind to α- and β-tubulins in MCF-7 breast carcinoma cells (10). Here we investigated the possibility of a potential interaction between ERα and microtubules, which may in turn regulate ERα-mediated signaling through a scaffold mechanism. Using the yeast two-hybrid system, we have identified an ERα-interacting protein called hematopoietic PBX-interacting protein (HPIP; also known as PBX-interacting protein). HPIP is a transcriptional repressor of PBX1 (11) and has been shown to be associated with microtubules through a leucine-rich domain (12). Our data suggest that HPIP mediates binding of ERα with tubulins. In addition, HPIP recruits p85 subunit and Src kinases to 17β-estradiol (E2)–ERα complex, which eventually leads to the activation of AKT and MAPK pathways in response to short-term treatment with estrogen. In addition, destabilization of microtubules activates ERα transcriptional activity, whereas stabilization of microtubules represses ERα transactivation in an HPIP-dependent manner. These findings document a mechanism in which HPIP tethers ERα to microtubules and allows them to influence ERα signaling.
Results
ERα Associates with the Microtubule-Binding Protein HPIP.
A yeast two-hybrid system screening of a mammary gland cDNA expression library with the full-length ERα (amino acids 1–595) as bait resulted in the isolation of several positive clones, one of which matched with full-length HPIP (GenBank accession no. NM_020524). The specificity of interaction between ERα and HPIP in yeast was confirmed by using cotransformation followed by the yeast cell survival assay in nutrient selection medium. The pGBK-ERα- and pBAD-HPIP-transformed yeast colonies grew in medium lacking adenosine, histidine, tryptophan, and leucine, whereas the cells cotransformed with the control pGBK vector and pBAD-HPIP did not grow, suggesting a basic interaction between ERα and HPIP in yeast (Fig. 1A). In the presence of estrogen, pGBK-ERα- and pBAD-HPIP-transformed yeast colonies grew better, indicating that estrogen triggers the interaction between ERα and HPIP. Further, immunoprecipitation assays using either transient transfection of HPIP and ERα in HeLa cells (Fig. 1 B and C) or in HPIP-overexpressing MCF-7 cells (Fig. 1 D and E) demonstrated that HPIP interacts with ERα in mammalian cells. There was detectable basal interaction between ERα and HPIP, and such interaction was further induced by short-term exposure of cells to estrogen. Notably, HPIP appeared to migrate as if it had a mass of ≈100 kDa instead of ≈80 kDa (expected size) in both transient and stable transfection of HPIP in these cells. This larger size may have been the result of posttranslational modification of HPIP, because its amino acid sequence suggests the presence of three potential glycosylation sites (Fig. 7B, which is published as supporting information on the PNAS web site). Consistent with immunoprecipitation results, confocal analysis also suggested a strong cytoplasmic colocalization of transiently transfected HPIP with ERα in HeLa cells in the presence of estrogen (Fig. 8A, which is published as supporting information on the PNAS web site).
Fig. 1.
ERα interacts with HPIP. (A) A yeast survival assay shows the growth of yeast cells on nutrient selection medium, either in −LT or −AHLT, transformed with ERα and HPIP plasmids. −LT, lack of leucine and tryptophan; −AHLT, lack of adenosine, histidine, leucine, and tryptophan. (B and C) Interaction between T7-ERα and Flag-HPIP in HeLa cells treated with E2 (10 nM) for 5 min. IP, immunoprecipitation. (D and E) Interaction between HPIP and ERα in T7-HPIP-overexpressing MCF-7 cells treated with E2 (10 nM) for 5 min. (F and G) GST pull-down assays show the minimal interacting regions between HPIP and ERα. AF1, activation function 1 domain; DBD, DNA-binding domain; H, hinge region; AF2, activation function 2 domain. (H) Interaction of wild-type HPIP (WT-HPIP) but not mutant HPIP (MT-HPIP) with ERα in MCF-7 cells.
Further interaction studies with deletion mutants of ERα and HPIP identified the region of ERα that mediates HPIP binding as amino acids 410–731 in the C-terminal AF2 domain and hinge region of ERα (Fig. 1 F and G and Fig. 8 B and C). Because HPIP contains an LASLL sequence (LXXLL motif or nuclear receptor-interacting motif) within amino acids 615–619, we replaced the leucines with alanines. Coimmunoprecipitation analysis revealed that the LASLL motif is indeed involved in the association between HPIP and ERα because mutant HPIP did not interact efficiently with ERα (Fig. 1H). All together, these results suggest that ERα interacts with HPIP in mammalian cells.
HPIP Modulates ERα-Mediated Estrogen Rapid Signaling.
Because HPIP is expressed in a variety of breast cancer cell lines (Fig. 7A), we sought to characterize the physiological consequences of the interaction between ERα and HPIP. Analysis of motif scan (http://scansite.mit.edu) of HPIP amino acid sequence revealed that HPIP carries a number of PXXP motifs located in both the N and C termini (Fig. 7 B and C). In general, PXXP motifs mediate interaction with the Src homology-3 domains present in multiple signal-transducing molecules (13). This information led us to hypothesize that HPIP induces cell survival through the PXXP motifs. Indeed, overexpression of HPIP in MCF-7 cells (Fig. 9A, which is published as supporting information on the PNAS web site) increased phosphorylation of AKT at Ser-473 (activated AKT) and correspondingly increased the phosphorylation of its substrate GSK3-β at Ser-9 and also phosphorylation of MAPK by at least 3- to 4-fold over control pcDNA-expressing MCF-7 cells (Fig. 9B). Conversely, depletion of HPIP by HPIP-specific siRNA in MCF-7 cells decreased the phosphorylation of AKT and GSK3-β, demonstrating that HPIP is required for AKT activation (Fig. 9C).
On the basis of the above findings that the HPIP associates with ERα in the cytoplasm and overexpression of HPIP promotes AKT and MAPK signaling, we hypothesized that HPIP participates in estrogen-mediated rapid signaling. Previous studies have shown that short-term estrogen treatment triggers the activation of the Src/phosphatidylinositol 3-kinase (PI3K)/AKT pathway in MCF-7 cells (14). Indeed, we found a significant enhancement of AKT and MAPK activation in the HPIP-overexpressing MCF-7 cells relative to the control pcDNA-overexpressing cells in response to short-term (15 min) estrogen treatment (Fig. 2A). This activation of AKT and MAPK could be inhibited by ICI182,780, an antiestrogen, suggesting that the HPIP-mediated estrogen-induced rapid cytoplasmic estrogen signaling is ERα-dependent (Fig. 2A). Consistent with this result, there is a weak activation or loss of activation of MAPK and AKT in cells transfected with LASLL mutant of HPIP compared with the cells transfected with wild type T7-HPIP suggesting that existence of functional involvement of LASLL motif in the interaction between HPIP and ERα (Fig. 2B). Furthermore, knock-down of HPIP by HPIP siRNA in MCF-7 cells compromised the ability of estrogen to stimulate MAPK and AKT, suggesting that HPIP is required in mediating estrogen-induced ERα rapid signaling (Fig. 2C).
Fig. 2.
HPIP is required for E2-induced ERα-mediated activation of AKT and MAPK. (A) Effect of ICI182,780 on the activation of AKT and MAPK in either pcDNA- or WT-HPIP-transfected MCF-7 cells upon E2 treatment for 15 min. Numbers beneath each lane indicate fold increase in the activation of either AKT or MAPK relative to control. (B) Activation of AKT and MAPK in MCF-7 cells transfected with pcDNA, WT-HPIP, or MT-HPIP in response to E2. (C) Effect of HPIP-specific siRNA upon E2 (10 nM, 15 min) effect on signaling proteins. Con siRNA, control siRNA.
HPIP Recruits Src Kinase and p85 Subunit of PI3K to E2–ERα Complex.
Because HPIP contains potential SH3 domain-interacting motifs, such as PXXP motifs, and because HPIP activates AKT and MAPK in response to estrogen (Fig. 2A), we next explored the possibility that HPIP could recruit PI3K and Src to E2–ERα complex. Consistent with this idea, estrogen treatment promoted the coprecipitation of the p85 subunit of PI3K, Src kinase and ERα with HPIP; however, treatment with ICI182,780 did not result in the dissociation of either p85 or Src from HPIP (Fig. 3A). This observation suggests that HPIP interacts with p85 and Src independent of ERα, but because estrogen treatment promotes this association, implying the ERα recruitment through HPIP enables a ternary complex with p85/Src. Further depletion of HPIP in MCF-7 cells by using HPIP-specific siRNA prevented the formation of ERα-p85-Src complex supporting the notion that HPIP acts as scaffold or an adaptor to form such a complex (Fig. 3B). These results also suggest that the activation of AKT and MAPK requires such complex formation in response to estrogen. Accordingly, either treatment of pcDNA or HPIP overexpressing MCF-7 cells with the Src kinase inhibitor PP2 (Fig. 3C) or the PI3K inhibitor wortmannin (Fig. 3D) inhibited the HPIP-mediated activation of AKT and MAPK. Together these results suggest that HPIP acts as an anchor to form a complex with ERα, p85, and Src that activates AKT and MAPK pathways.
Fig. 3.
HPIP recruits Src and p85 to E2–ERα complex in response to short-term exposure to E2. (A) Effect of E2 (10 nM, 5 min) on the interaction among HPIP, Src, p85 of PI3K, and ERα in HPIP-expressing MCF-7 cells. Cells were treated with ICI182,780 (10−8 M) for 3 h before E2 treatment. DL, direct lysate. (B) Lysates of MCF-7 cells transfected with either control or HPIP siRNA treated with E2 for 5 min were immunoprecipitated with anti-ERα antibody and blotted with the indicated antibodies. (C and D) Effect of either Src kinase inhibitor PP2 (C) or PI3K inhibitor wortmannin (D) on the activation of AKT and MAPK in MCF-7 cells overexpressing either pcDNA or WT HPIP in response to E2.
HPIP Tethers ERα to Microtubules.
Because HPIP is known to associate with microtubules (12), ERα has been shown to interact with α- and β-tubulins (10), and HPIP interacts with ERα (this study), we reasoned that ERα could associate with microtubules through HPIP. We found that T7-tagged HPIP predominantly localizes in the cytoplasm and colocalizes with β-tubulin (Fig. 4A). Immunoprecipitation combined with confocal analysis showed the association of HPIP with β-tubulin and ERα in HPIP-expressing MCF-7 cells (Fig. 4B and Fig. 10A, which is published as supporting information on the PNAS web site). Further short-term exposure to estrogen enhances ERα interaction with HPIP–microtubule complex (Fig. 4B, lane 4). In general, ERα distribution varies from cell to cell. In MCF-7 cells, ERα is primarily localized in the nucleus and a relatively small proportion is in the cytoplasm (Fig. 10A), whereas ERα is predominantly localized in the cytoplasm in HepG2 cells and strongly associates with microtubules (Fig. 10B Upper). We next reasoned that microtubules and HPIP might regulate ERα distribution in these cells. Consistent with this idea, immunoprecipitation analysis showed that treatment of HepG2 cells with nocodazole, which disrupts microtubules (Fig. 4C, lanes 5 and 6), or depletion of HPIP by HPIP siRNA (Fig. 4D, lane 5 and 6) resulted in the loss of ERα and β-tubulin interaction but not in the cells either untreated or treated with Taxol (paclitaxel), which stabilizes microtubules (Fig. 4C, lanes 3 and 4, 7 and 8). More evidently, fractionation and confocal analysis confirmed that treatment of HepG2 cells with either nocodazole or depletion of HPIP by HPIP siRNA resulted in the substantial amount of nuclear localization of ERα (Figs. 4E and 10B), whereas untreated or Taxol-treated cells promoted ERα accumulation in the cytoplasm (Figs. 4E and 10B). Together, these findings suggested that HPIP tethers ERα to microtubules.
Fig. 4.
HPIP tethers ERα to microtubules. (A) Colocalization of T7-HPIP with β-tubulin in MCF-7 cells. (Upper) T7-HPIP (red, labeled with rhodamine), β-tubulin (green, labeled with FITC), and Topro-3-stained DNA (blue). Indicated with white boxes are blow-ups that are shown in Lower. (B) Interaction of HPIP with β-tubulin and ERα. (C) Interaction of ERα with β-tubulin in HepG2 cells treated with E2 (10 nM, 10 min) and/or nocodazole (Noc: 4 μM, 1 h) or Taxol (1 μM, 1 h). (D) HepG2 cells transfected with control or HPIP siRNA and treated with E2 (10 nM, 15 min) were immunoprecipitated with β-tubulin antibody and Western blotted. (E) Nuclear translocation of ERα in either HPIP siRNA-transfected or nocodazole- (4 μM, 4 h) treated HepG2 cells. (F and G) Lysates from MCF-7 cells expressing pcDNA or HPIP treated with colchicine (col; 0.1 μM) (F) or Taxol (0.1 μM) (G) for 1 h before exposure to E2 (10 nM, 15 min) were blotted with the indicated antibodies.
Consistent with coimmunoprecipitation data (Fig. 4D, lane 4), where p85 is also precipitated with β-tubulin along with ERα in response to estrogen treatment, treatment of either pcDNA- or HPIP-overexpressing MCF-7 cells with the microtubule-destabilizing agent colchicine abolished the ability of estrogen to activate AKT (Fig. 4F), whereas the cells treated with Taxol exhibited AKT activation (Fig. 4G). These findings suggest a role for microtubule integrity in the modulation of ERα-dependent rapid estrogen signaling, presumably as a result of HPIP–microtubule interaction.
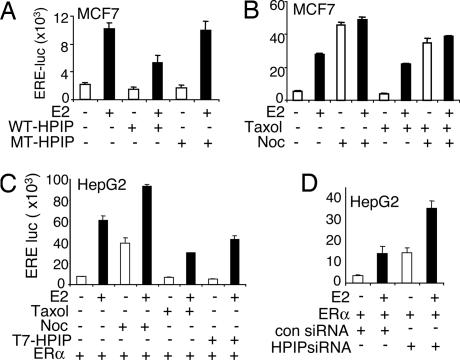
Depolymerization of Microtubules Induces ERα Transcriptional Activity.
Because HPIP is predominantly a cytoplasmic protein that interacts with both ERα and microtubules, we next investigated the role of HPIP on ERα transcriptional activity by using in vitro ERE-luc reporter assay. Indeed, ERα transactivation was inhibited in MCF-7 cells transfected with wild-type HPIP, but not in cells transfected with mutant HPIP or empty vector, suggesting HPIP acts as a negative regulator of ERα transcriptional activity (Fig. 5A). Next, we reasoned that if HPIP acts as a negative regulator of estrogen receptor nuclear signaling, then disruption of HPIP association with microtubules might augment the transcriptional activity of ERα. Various cell lines, including MCF-7, ZR75, HepG2, and HeLa, were transfected with an ERE-luc reporter construct and treated with either nocodazole or Taxol in the presence or absence of E2. We found a remarkable stimulation of ERα transcriptional activity by nocodazole in these cell lines (Fig. 5 B and C and Fig. 11 A and B, which is published as supporting information on the PNAS web site), whereas treatment with Taxol substantially reduced both basal and estrogen-induced luciferase activity. However E2 treatment had no further effect on ERE-luc activity in nocodazole-treated MCF-7 and ZR75 cells (Figs. 5B and 11A), whereas there was a modest increase in ERE-luc activity in nocodazole-treated HepG2 cells, which contains cytoplasmic ERα (Fig. 5C). Treatment of MCF-7 cells with nocodazole for 18 h in the presence or absence of estrogen followed by Taxol for an additional 1 h reduced the ability of nocodazole to stimulate ERE-luc activity (Fig. 5B, lanes 7 and 8), indicating the importance of microtubule integrity in ERα transcriptional activity. Notably, microtubule-affecting drugs had no effect on ERE-luc activity in ERα-negative cell lines such as HeLa (Fig. 11B), suggesting the specificity and dependence of drug-induced up-regulation of ERE-luc activity by ERα.
Fig. 5.
HPIP and microtubules negatively regulate ERα transcriptional activity. (A) Effect of T7-HPIP on ERE-luc in MCF-7 cells. (B and C) Effect of nocodazole (Noc; 4 μM, 18 h) or Taxol (1 μM, 18 h) on ERE-luc activity in MCF-7 cells (B) or in HepG2 cells (C). MCF7 cells were treated with Taxol for an additional 1 h in the presence of nocodazole and/or E2 (lanes 7 and 8). (D) Effect of control or HPIP siRNA on ERE-luc activity in HepG2 cells.
To generalize these findings, we expanded these studies to another microtubule-depolymerizing drug, colchicine. We found a significant stimulation of ERE-luc activity in MCF-7 cells similar to that seen in cells treated with nocodazole (Fig. 11C). In agreement with these results, knockdown of HPIP also enhanced the ERE-luc activity by 3- to 4-fold in HepG2 cells (Fig. 5D). To further substantiate these findings, we carried out a chromatin immunoprecipitation (CHIP) assay in HepG2 cells. HepG2 cells were either transfected with control siRNA or HPIP siRNA or treated with nocodazole or Taxol in the presence or absence of estrogen. Because ERα is predominantly localized in cytoplasm of HepG2 cells, we did not observe the recruitment of ERα onto pS2 promoter, an estrogen-inducible gene, whereas either nocodazole or depletion of HPIP promoted ERα recruitment onto pS2 promoter but not with Taxol (Fig. 11D). Together, these findings suggest that HPIP negatively regulates ERα transcriptional activity by tethering it to microtubules and that microtubule-destabilizing agents enhance the ERα transcriptional activity.
HPIP–ERα Interaction Promotes Breast Cancer Cell Motility and Tumorigenesis.
Rapid estrogen signaling has been implicated in cell migration (5). To assess whether HPIP-regulated rapid estrogen signaling influences this physiological process, we examined the migration of MCF-7 cells stably expressing HPIP and pcDNA by Boyden-chamber and wound-healing assays. HPIP-overexpressing cells showed enhanced motility under basal conditions; however, estrogen stimulation enhanced it further (Fig. 6A). Interestingly, treatment with either the Src kinase inhibitor PP2 or the PI3K inhibitor wortmannin resulted in a significant reduction in cell motility in MCF-7 cells stably expressing either HPIP or pcDNA. The antiestrogen ICI182,780 (Tocris, Ellisville, MO) also reduced the cell migration mediated by HPIP (Fig. 6A). Similar results were obtained when the above studies were repeated with a wound-healing assay. In this assay, HPIP-overexpressing MCF-7 cells showed significantly greater migration than the control cells in response to estrogen (Fig. 6B and Fig. 12A, which is published as supporting information on the PNAS web site). Although the HPIP-overexpressing MCF-7 cells showed elevated basal migration, they continued to respond to estrogen treatment by promoting wound closure. Overall, these results suggest a role for HPIP- and ERα-dependent formation of multiprotein complex with Src/PI3K in cytoplasmic ERα signaling and in estrogen-induced cell migration.
Fig. 6.
HPIP promotes breast cancer cell motility and tumorigenesis. (A and B) Uncoated Boyden-chamber assay (A) cell-migration assays (B) were performed with HPIP- and pcDNA-MCF-7 cells treated with E2 (10 nM, 2 days). n = 3. Con, untreated control; ICI, ICI182,780; WRT, wortmannin. (C) Anchorage-independent growth potential of pcDNA or T7-HPIP MCF-7 cells. (D) Induction of tumors in nude mice by MCF-7 cells expressing either pcDNA or HPIP at 8 weeks. (E) A simple model representing the regulation of cytoplasmic and nuclear functions of ERα by HPIP and microtubule (MT) complex.
To further strengthen the physiological implications of these findings, we next examined the ability of MCF-7 cells stably expressing HPIP and pcDNA to grow in an anchorage-independent manner. HPIP clones formed more colonies than did the control pcDNA clones under both basal and estrogen-stimulated conditions, in a manner sensitive to ICI182,780 (Fig. 6C). Consistent with these results, HPIP clones showed tumorigenic potential in nude mice (Fig. 6D) and activated Src, AKT, and MAPK in tumors (Fig. 12B). Further, histopathology diagnosis confirmed these tumors as invasive adenocarcinoma type. Together these findings suggest a close relationship between HPIP–ERα interaction and enhanced AKT and MAPK activation and tumorigenesis.
Discussion
Building on the previous finding that HPIP interacts with microtubules (12), we discovered that ERα associates with microtubules through HPIP. Consistent with a role for microtubules in ERα signaling, disruption of microtubules by nocodazole or colchicine markedly increased ERα transcriptional activity, whereas microtubule stabilization by Taxol inhibited ERα transcriptional activity. A previous report showed that the AF1 domain of ERα interacts with α- and β-tubulins in MCF-7 cells, suggesting a role for microtubules in rapid estrogen signaling (10). HPIP interacts with ERα through the LXXLL motif and with Src kinase and the p85 subunit of PI3K, possibly through its PXXP motifs, which aids the formation of a signaling complex and eventually the activation of AKT and MAPK in response to rapid estrogen signaling. It has been shown that p85 subunit of PI3K in concert with Src kinase is involved in estrogen-induced AKT activation by directly interacting with ERα (14). However, our studies suggest that HPIP is required to act as an anchor for such complex formation. Also, because such a multimeric protein complex might have limited diffusion in the cytoplasm, these observations raise the possibility that ERα interaction with microtubules through HPIP may be required to facilitate rapid ERα signaling.
Because HPIP contains both nuclear localization signals (NLS) and nuclear export signals (NES) (12) and ERα interacts with HPIP (this study), signals affecting microtubule–HPIP–ERα interaction may also influence HPIP and ERα nuclear localization and, hence, modify ERα transcriptional activity. In this context, it is notable that estrogen has been shown to inhibit the assembly of microtubules (15). It remains possible that partial inhibition of microtubule assembly by estrogen and/or phosphorylation of ERα or HPIP could act as an intermediate step in the signaling pathway leading to subcellular redistribution of HPIP and ERα. Microtubules have been shown to modulate the activity of transcription factors Smad and NF-κB by cytoplasmic sequestration (8, 16). The subcellular localization of ERα, however, varies in different cell types; it is predominantly nuclear in MCF-7 and ZR75 cells, whereas it is cytoplasmic in HepG2 cells. An earlier study of human breast cancer cell lines suggested the presence of three distinct cell phenotypes, distinguished by their arrangement of cytoplasmic microtubules: cells with an extensive array of microtubules, cells with diffuse microtubules only near the cell margins, and cells with only diffuse microtubules and no apparent arrays (17). The relationship of such microtubule arrangements with estrogen action remains unknown. In general, microtubules can affect signal transduction by (i) sequestration and release or (ii) microtubule delivery or (iii) microtubule scaffolding of signaling molecules. We found that HPIP forms a complex with Src, the p85 subunit of PI3K, and ERα to activate a major signaling cascade, which suggest a microtubule scaffolding of signaling molecules as a potential mechanism. Although the precise mechanism by which microtubule depolymerization leads to ERα activation remains to be elucidated, our model suggests that ERα is tethered to microtubules through HPIP, and ERα is activated in response to depolymerization of microtubules (Fig. 6E).
Because cytoskeletal changes are likely to be induced by cell–substrate and cell–cell interactions, it is also possible that such dynamic cellular changes provide a signal(s) that mediates the participation of microtubules in estrogen signaling. It will be of interest to learn the nature of the physiological signals that influence the dynamic status of microtubules and thereby regulate ERα signaling.
As with many cancer therapeutic agents, acquired resistance remains a significant problem when using microtubule-affecting agents to treat malignancies. In this context, because HPIP overexpression enhances estrogen sensitivity and AKT activation in breast cancer cells upon exposure to Taxol (Fig. 4G), it is possible that these events may participate in the development of resistance to microtubule–targeting agents. In summary, we have identified a mechanism wherein the microtubule-binding protein HPIP participates in cytoplasmic and nuclear signaling of ERα. The data presented here suggest that the interaction of HPIP with both ERα and microtubules regulates ER functionality in mammalian cells.
Materials and Methods
Plasmids and Yeast Two-Hybrid Screening.
The full-length ERα (1,788 bp) was cloned into the Gal4-binding domain vector pGBK (Clontech, Palo Alto, CA) and used to screen a mammary gland cDNA library fused to the Gal4 activation domain according to the supplier's instructions. The full-length T7-tagged HPIP was subcloned into pcDNA 3.1A mammalian expression vector (Invitrogen, Carlsbad, CA).
Generation of MCF-7 Cells Stably Expressing HPIP or pcDNA.
MCF-7 cells grown in 60-mm culture dishes were transfected with 5 μg of either pcDNA 3.1A or T7-HPIP by using the FuGENE 6 transfection reagent (Roche Applied Science, Indianapolis, IN) and following the supplier's protocol.
Biochemical Methods.
Cell extractions, immunoblotting, immunoprecipitation, and cell fractionation were performed as described in refs. 10 and 18.
siRNA Transfection and RT-PCR Analysis.
Transfection of HPIP-specific and control nonspecific siRNA (Dharmacon, Lafayette, CO) were performed as described in ref. 18. RT-PCR was done with an RT-PCR kit (Promega, Madison, WI) using HPIP-specific primers: 5′-GAAGGCTGAGCACTGGAAAC-3′ (forward) and 5′-CCTTAGTCCCTTCCCTCCAC-3′ (reverse).
Confocal Microscopy and Immunohistochemistry.
Confocal imaging and immunostaining of tissue sections were performed as described in ref. 18.
Reporter Gene Assays.
Cells grown in the culture medium with 5% DCC (dextran-coated charcoal) were cotransfected with 200 ng of ERE-Luc reporter and β-gal plasmids along with either 0.5 μg of empty vector or 0.5 μg of T7-HPIP by using the FuGENE-6 reagent according to the supplier's protocol.
Chromatin Immunoprecipitation (CHIP) Analysis.
Chromatin immunoprecipitation was done as previously described (19) but with ERα antibody and pS2-promoter-specific primers.
Biological Assays.
Soft-agar colony-growth assays and tumorigenesis studies in nude mice were done as previously described (18) by using MCF-7 cells stably expressing either pcDNA or HPIP. Boyden-chamber and wound-healing assays were performed as previously described (5).
Supplementary Material
Acknowledgments
We thank R. K. Humphries for Flag-HPIP. The study was supported by National Institutes of Health Grants CA98823 and CA109379 (to R.K.).
Abbreviations
- E2
17β-estradiol
- ER
estrogen receptor
- HPIP
hematopoietic PBX-interacting protein
- IP
immunoprecipitation
- MT-HPIP
mutant HPIP
- PI3K
phosphatidylinositol 3-kinase.
Footnotes
The authors declare no conflict of interest.
References
- 1.Edwards DP. Annu Rev Physiol. 2005;67:335–376. doi: 10.1146/annurev.physiol.67.040403.120151. [DOI] [PubMed] [Google Scholar]
- 2.Manavathi B, Kumar R. J Cell Physiol. 2006;207:594–604. doi: 10.1002/jcp.20551. [DOI] [PubMed] [Google Scholar]
- 3.Pedram A, Razandi M, Levin ER. Mol Endocrinol. 2006;20:1996–2009. doi: 10.1210/me.2005-0525. [DOI] [PubMed] [Google Scholar]
- 4.Cheskis BJ. J Cell Biochem. 2004;93:20–27. doi: 10.1002/jcb.20180. [DOI] [PubMed] [Google Scholar]
- 5.Acconcia F, Barnes CJ, Kumar R. Endocrinology. 2006;147:1203–1212. doi: 10.1210/en.2005-1293. [DOI] [PubMed] [Google Scholar]
- 6.Gundersen GG, Cook TA. Curr Opin Cell Biol. 1999;11:81–94. doi: 10.1016/s0955-0674(99)80010-6. [DOI] [PubMed] [Google Scholar]
- 7.Giannakakou P, Sackett DL, Ward Y, Webster KR, Blagosklonny MV, Fojo T. Nat Cell Biol. 2000;2:709–717. doi: 10.1038/35036335. [DOI] [PubMed] [Google Scholar]
- 8.Dong C, Li Z, Alvarez R, Jr, Feng XH, Goldschmidt-Clermont PJ. Mol Cell. 2000;5:27–34. doi: 10.1016/s1097-2765(00)80400-1. [DOI] [PubMed] [Google Scholar]
- 9.Roczniak-Ferguson A, Reynolds AB. J Cell Sci. 2003;116:4201–4212. doi: 10.1242/jcs.00724. [DOI] [PubMed] [Google Scholar]
- 10.Azuma K, Horie K, Inoue S, Ouchi Y, Sakai R. FEBS Lett. 2004;577:339–344. doi: 10.1016/j.febslet.2004.10.025. [DOI] [PubMed] [Google Scholar]
- 11.Abramovich C, Shen WF, Pineault N, Imren S, Montpetit B, Largman C, Humphries RK. J Biol Chem. 2000;275:26172–26177. doi: 10.1074/jbc.M001323200. [DOI] [PubMed] [Google Scholar]
- 12.Abramovich C, Chavez EA, Lansdorp PM, Humphries RK. Oncogene. 2002;21:6766–6771. doi: 10.1038/sj.onc.1205784. [DOI] [PubMed] [Google Scholar]
- 13.Musacchio A, Gibson T, Lehto VP, Saraste M. FEBS Lett. 1992;307:55–61. doi: 10.1016/0014-5793(92)80901-r. [DOI] [PubMed] [Google Scholar]
- 14.Castoria G, Migliaccio A, Bilancio A, Di Domenico M, de Falco A, Lombardi M, Fiorentino R, Varricchio L, Barone MV, Auricchio F. EMBO J. 2001;20:6050–6059. doi: 10.1093/emboj/20.21.6050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kipp JL, Ramirez VD. Neuroendocrinology. 2003;77:258–272. doi: 10.1159/000070281. [DOI] [PubMed] [Google Scholar]
- 16.Rosette C, Karin M. J Cell Biol. 1995;128:1111–1119. doi: 10.1083/jcb.128.6.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brinkley BR, Beall PT, Wible LJ, Mace ML, Turner DS, Cailleau RM. Cancer Res. 1980;40:3118–3129. [PubMed] [Google Scholar]
- 18.Vadlamudi RK, Manavathi B, Balasenthil S, Nair SS, Yang Z, Sahin AA, Kumar R. Cancer Res. 2005;65:7724–7732. doi: 10.1158/0008-5472.CAN-05-0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mazumdar A, Wang RA, Mishra SK, Adam L, Bagheri-Yarmand R, Mandal M, Vadlamudi RK, Kumar R. Nat Cell Biol. 2001;3:30–37. doi: 10.1038/35050532. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.