Abstract
The molecular basis of pathogen clone emergence is relatively poorly understood. Acquisition of a bacteriophage encoding a previously unknown secreted phospholipase A2 (designated SlaA) has been implicated in the rapid emergence in the mid-1980s of a new hypervirulent clone of serotype M3 group A Streptococcus. Although several lines of circumstantial evidence suggest that SlaA is a virulence factor, this issue has not been addressed experimentally. We found that an isogenic ΔslaA mutant strain was significantly impaired in ability to adhere to and kill human epithelial cells compared with the wild-type parental strain. The mutant strain was less virulent for mice than the wild-type strain, and immunization with purified SlaA significantly protected mice from invasive disease. Importantly, the mutant strain was significantly attenuated for colonization in a monkey model of pharyngitis. We conclude that transductional acquisition of the ability of a GAS strain to produce SlaA enhanced the spread and virulence of the serotype M3 precursor strain. Hence, these studies identified a crucial molecular event underlying the evolution, rapid emergence, and widespread dissemination of unusually severe human infections caused by a distinct bacterial clone.
Keywords: bacteria, Group A Streptococcus, Streptococcus pyogenes
Although of tremendous concern to society and public health authorities, the molecular events, epidemiological processes, and host factors that contribute to rapid emergence of new pathogenic bacterial clones are poorly understood (1, 2). Information about these processes is needed to develop a predictive model of bacterial epidemics and new drugs, vaccines, and diagnostics. To better understand clone emergence and changes in disease frequency and severity, we have used genome-wide analysis methods to study group A Streptococcus (GAS), a model human pathogen (3–6). GAS causes infections ranging in severity from relatively mild pharyngitis and skin infections to life-ending invasive diseases such as septicemia, necrotizing fasciitis, and streptococcal toxic shock syndrome (7).
Serotype M3 strains have been of particular interest, because comprehensive population-based studies have shown that these organisms cause a disproportionate number of severe invasive disease infections, such as necrotizing fasciitis and death (8–11). Based on genome sequencing and molecular population genetic analysis of strains recovered over >60 years, we discovered that acquisition of a bacteriophage encoding a new secreted phospholipase A2 (PLA2) named SlaA created a new clone that now is responsible for the vast majority of human infections caused by serotype M3 strains in many countries (3, 4, 12). SlaA is secreted extracellularly and is related to a potent toxin (textilotoxin) made by the Australian common brown snake, Pseudonaja textilis (3, 13, 14).
Several lines of evidence suggest that SlaA is a GAS virulence factor. The slaA gene was not present in serotype M3 strains until the mid-1980s, a time frame that correlated with the increase in frequency and severity of serotype M3 invasive infections (3). Humans with GAS infections seroconvert to SlaA, indicating that this protein is made during infection (3). In addition, SlaA has enzymatic activity against several phospholipid head groups and acyl chains located at the sn-2 position (14). For example, SlaA cleaves and releases arachidonic acid, a potent mediator of the inflammatory cascade.
With the goal of directly testing the hypothesis that SlaA contributes to pathogenesis, we made a ΔslaA isogenic mutant strain from a wild-type serotype M3 strain and studied its role in GAS host–cell interaction and contribution to virulence in mouse and monkey models of infection.
Results
Exogenously Presented SlaA Is Not Cytotoxic to Cultured Human Epithelial Cells.
SlaA has PLA2 activity in vitro against several physiologically relevant substrates present in host cell membranes (14). Degradation of phospholipids by phospholipases can damage host membranes and decrease cell viability. To determine whether exogenous SlaA caused host-cell cytotoxicity, purified recombinant SlaA (rSlaA) was incubated with immortalized pharyngeal epithelial (D562) cells, and lactate dehydrogenase (LDH) released into the culture medium was measured as an indicator of cell damage. No significant increase in LDH activity was detected (data not shown). Similarly, rSlaA did not have a detrimental effect on host-cell morphology or membrane integrity, as assessed by transmission electron microscopy (Fig. 7, which is published as supporting information on the PNAS web site). These results indicated that exogenous rSlaA alone does not produce a substantive cytotoxic effect on human epithelial cells grown in vitro.
SlaA Increases GAS Attachment to and Killing of Human Epithelial Cells.
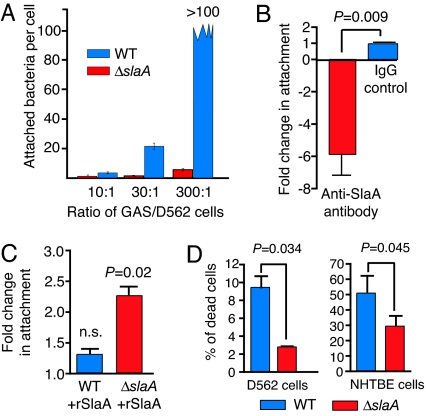
To determine whether SlaA contributes to pathogenesis, we made a ΔslaA isogenic mutant strain from a wild-type serotype M3 strain (Figs. 8 and 9, which are published as supporting information on the PNAS web site). We tested the hypothesis that SlaA production influenced GAS–host cell interaction by using D562 cells and normal human trancheobronchial epithelial (NHTBE) cells. In both cell types, significantly fewer ΔslaA GAS were associated with the host cells compared with the wild-type strain (Fig. 1). Importantly, decreased attachment could not be overcome by adding more mutant bacteria (Fig. 2A). The number of wild-type GAS attached to epithelial cells increased proportionally with an increased cfu/cell ratio, an effect not observed with the ΔslaA mutant strain. Depletion of SlaA by addition of anti-SlaA antibody significantly decreased GAS adherence to host cells (Fig. 2B), and addition of exogenous rSlaA increased the number of ΔslaA mutant GAS attached to host cells by >2-fold (Fig. 2C). Several reports indicate that GAS infection of epithelial cells results in apoptosis (15, 16). Thus, we hypothesized that the ΔslaA mutant strain would kill fewer host cells as a consequence of decreased adherence. We found that the ΔslaA mutant killed significantly fewer host epithelial cells compared with the wild-type strain (Fig. 2D).
Fig. 1.
Production of SlaA significantly enhances adherence of GAS to human epithelial cells. D562 and NHTBE cells were infected with wild-type or the ΔslaA mutant strain (moi = 100:1, 3 h), washed with PBS, fixed, and stained with crystal violet. Photomicrographs for NHTBE cells are not shown, but the results mirrored the D562 data. (A) Uninfected D562 cells. (B) D562 cells infected with wild-type strain MGAS315. (C) D562 cells infected with ΔslaA isogenic mutant strain. (Magnification, ×40.) (D) Quantitative differences in host-cell adherence between the wild-type and ΔslaA mutant strains by using D562 cells (Left) and NHTBE cells (Right). The number of cell-associated bacteria was determined by counting GAS attached to 50 randomly selected human cells in five different microscope fields. The results are expressed as the mean number of GAS per host cell. An enlarged version of Fig. 1 is presented as Fig. 10, which is published as supporting information on the PNAS web site.
Fig. 2.
Characterization of SlaA-dependent GAS–human cell interactions. (A) Adherence of the wild-type strain is dose-dependent, whereas adherence of the ΔslaA mutant strain is not. Experiments were performed with D562 cells as described but at the indicated mois. (B) Depletion of SlaA decreases GAS adherence to D562 cells. SlaA was titrated with 10 μg of anti-SlaA antibody during infection with wild-type MGAS315. Affinity-purified preimmune rabbit IgG at the same concentration was used as a control. (C) Addition of purified rSlaA to the infection assay increased adherence of the ΔslaA mutant strain. rSlaA (50 μg) was added simultaneously with the wild-type and ΔslaA mutant strains. n.s., not significant. (D) Production of SlaA significantly enhanced GAS killing of human epithelial cells. D562 or NHTBE epithelial cells were cocultured with wild-type or the ΔslaA isogenic mutant strain (moi = 100:1, 3 h) and stained with trypan blue. The number of stained cells in 50 randomly chosen fields was counted by light microscopy, and the number of positive cells was expressed as percent of total cells in the field. P values were determined by t test of data for mutant vs. wild-type. NHTBE are primary cells and are much more susceptible to killing by GAS than the immortalized D562 cells.
To determine whether SlaA or SlaA-producing bacteria entered the host cell, NHTBE cells were incubated with wild-type or ΔslaA mutant GAS, and cell lysates were analyzed by Western immunoblotting. Immunoreactive SlaA was found in the cell membrane and cytosolic fractions of cells incubated with wild-type GAS, indicating that SlaA gained access to the host cell cytoplasm (Fig. 3A). These results were confirmed with confocal microscopy (Fig. 3 B and C). Taken together, the data suggest that SlaA plays an important role in facilitating GAS adherence to cultured epithelial cells, and that entry of SlaA into host cells is required for cytotoxicity.
Fig. 3.
NHTBE cells were infected with wild-type GAS (lanes 1, 3, and 5) or the ΔslaA mutant strain (lanes 2, 4, and 6), fractionated, and analyzed by Western immunoblot with anti-SlaA antisera. Lanes 1 and 2, cell culture medium; lanes 3 and 4, NHTBE membrane fraction; lanes 5 and 6, NHTBE cytosolic fraction. (B) Confocal microscopy images of NHTBE cells 6 h after infection with wild-type GAS. Cell membranes were labeled with anti-CD44 antibody (red), and SlaA was visualized with Alexa 488-conjugated secondary antibody (green, green arrows). White arrowheads indicate the cell membrane. x-z and y-z crosssections through the cell layer (black arrowheads) are shown in Upper and Right, respectively. (C) SlaA enters epithelial cells. The dashed white line corresponds to the crosssection position. The cells were imaged in 0.5-μm sections from the apical to the basal surface (z-stack). A pixel intensity projection in the x-y orientation indicates the red peaks of the cell-surface CD44 staining (red arrowheads) and green SlaA intracellular peaks (green arrowheads). The red and green baselines represent background staining. (D) Intracellular transport of SlaA is streptolysin O-independent and cytochalasin D-dependent. Western immunoblot analysis of SlaA in cytoplasmic fractions of NHTBE cells after infection with a Δslo mutant strain (lane 2) or after infection with the same strain in the presence of cytochalasin D (lane 1).
To investigate the mode of SlaA entry into host cells, we tested the hypothesis that transmembrane pores generated by GAS streptolysin O (SLO) were involved. Pore formation by the GAS cytolytic toxin SLO has been postulated to be equivalent to type III secretion in Gram-negative bacteria (17). NHTBE cells were incubated with serotype M3 strain 950771Smslo−, which produces SlaA but not SLO, because of deletion of the slo structural gene (18). SlaA was present in the cytosolic fractions prepared from Δslo-infected host cells (Fig. 3D, lane 2), suggesting that SlaA does not require SLO to gain access to the host cell cytosol. In contrast, cytochalasin D blocked transport of SlaA into human epithelial cells (Fig. 3D, lane 1). These results suggest that SlaA enters host cells by an active transport process requiring intact cytoskeleton function.
SlaA Is a Virulence Factor.
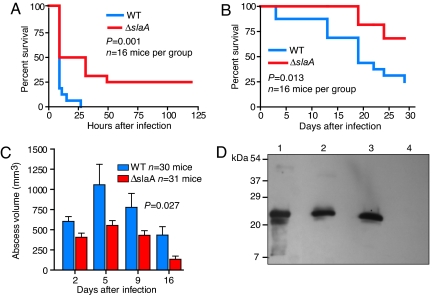
The observation that serotype M3 GAS strains expressing SlaA are overrepresented among invasive disease isolates (3, 4, 14, 19) suggests that SlaA contributes to GAS pathogenesis. In addition, patients with pharyngitis or invasive infections seroconvert to SlaA, indicating that this enzyme is secreted during infection (3). To determine whether SlaA plays a role in GAS invasive infection, we compared the ability of the wild-type and ΔslaA mutant strains to cause mouse near mortality after i.p. inoculation. Significantly fewer mice infected with the ΔslaA isogenic mutant strain reached near mortality compared with animals infected with the wild-type parental strain (Fig. 4A). One characteristic of serotype M3 human infections is unusually severe tissue destruction, as exemplified by necrotizing fasciitis (8, 10, 11). Important to note, the ΔslaA mutant strain also caused significantly less morbidity after s.c. inoculation compared with the wild-type strain (Fig. 4 B and C). Western immunoblot analysis of proteins extracted from skin lesions confirmed that SlaA was made by the wild-type strain at the site of infection (Fig. 4D).
Fig. 4.
SlaA contributes to GAS virulence in both septicemia and skin-lesion mouse models of infection. CD-1 Swiss male mice were inoculated i.p. with 2.5 × 107 cfu of wild-type MGAS315 or the ΔslaA isogenic mutant strain. Immunocompetent hairless mice (strain Clr:SKH1-hrBR) were s.c.-inoculated with 1 × 107 cfu. Kaplan–Meier survival curves were plotted to compare the difference in near mortality after i.p. (A) and s.c. (B) inoculation. (C) ΔslaA mutant strain induced significantly smaller lesions in the s.c. model of infection. Average lesion volumes were compared for the wild-type and ΔslaA mutant strains at various days postinfection. (D) Western immunoblot analysis showing production of SlaA at the lesion site. Mice were inoculated with wild-type or ΔslaA mutant strains, killed at 48 h postinfection, and tissue at the lesion site was excised. Proteins were extracted from the tissue and analyzed by Western immunoblot by using SlaA-specific polyclonal rabbit antibody. Lane 1, purified recombinant SlaA; lane 2, extract from wild-type-infected mice; lane 3, extract from ΔslaA-infected mice to which purified recombinant SlaA was added before extraction (extraction control); lane 4, extract from ΔslaA-infected mice.
Protection of Mice by Immunization with Recombinant SlaA.
Inasmuch as SlaA is produced during infection of humans and mice (ref. 3 and Fig. 4D) and contributes to virulence, it is possible that immunization with rSlaA would protect against GAS disease. Mice immunized with SlaA had significantly smaller abscesses compared with sham-immunized control animals (Fig. 5 A and B). In addition, mice with high levels of anti-SlaA antibody had significantly smaller skin lesions compared with animals with low anti-SlaA antibody levels (Fig. 5C). These results further support the idea that SlaA contributes to the enhanced virulence of serotype M3 strains containing the slaA gene.
Fig. 5.
Immunization of mice with SlaA abrogates GAS pathogenesis. (A) Immunocompetent hairless mice (strain Clr:SKH1-hrBR) were immunized with SlaA, boosted, and challenged with wild-type strain MGAS315. Lesion volumes were calculated, and averages were compared for unimmunized and SlaA-immunized animals at the indicated times postinfection. The difference in abscess volumes between immunized and sham-immunized control animals was statistically significant, as assessed by mixed-model repeated measures. (B) Representative lesions induced by wild-type GAS after s.c. inoculation of immunized mice. (Left) Sham-immunized mice. (Right) SlaA-immunized mice. (C) Increased α-SlaA antibody titers correlated with smaller lesion size upon GAS challenge. Serum was obtained from immunized mice 2 days before challenge with strain MGAS315. Antibody levels were determined by ELISA with purified rSlaA. Statistical significance was tested by the Pearson correlation test (Pearson r = −0.585; 95% confidence interval, −0.859 to −0.050).
SlaA Significantly Enhances Upper Respiratory Tract Infection.
Large epidemiological studies have shown that genotypes of GAS strains that are abundant causes of tonsillitis (“strep throat”) also are the dominant types causing invasive episodes in the same geographic area (20, 21). Recently, the cynomolgus macaque has become the gold standard for experimental GAS pharyngitis studies (22, 23). Thus, we tested the hypothesis that SlaA contributed to colonization and infection in this model. The mutant strain was strikingly attenuated for infection and persistence (Fig. 6A). Furthermore, animals infected with the wild-type strain seroconverted to SlaA (Fig. 6B), thus confirming that SlaA was expressed in vivo in the upper respiratory tract.
Fig. 6.
SlaA is required for GAS infection of cynomolgus macaques. Two groups of four monkeys were infected with wild-type (WT) strain MGAS315 or the ΔslaA isogenic mutant strain and monitored. (A) The tonsils of monkeys were swabbed at the indicated time points, and recovered bacteria were plated to determine cfus. (B) Monkey sera from the indicated time points were analyzed by ELISA for anti-SlaA antibody. PC, positive control.
Discussion
Although of great interest to many fields of biomedical research, the molecular genetic events contributing to the creation and very rapid emergence of new pathogenic bacterial clones are poorly understood (1, 2). GAS is an ideal model organism to study these processes, because population-based strain collections are available that are linked to detailed clinical information. In addition, the organism is known to exhibit precipitous changes in disease frequency and severity, and the genome sequences of 12 strains are available (3, 6, 12, 19, 24–28). The present study was motivated by several lines of circumstantial evidence that together implicated acquisition of a bacteriophage encoding SlaA as a key event underlying the recent and rapid emergence of a new unusually virulent serotype M3 clone (3, 4, 12). Our studies unambiguously demonstrate that SlaA is an important GAS virulence factor, thereby providing considerable support to the hypothesis that it contributed to clone emergence and the observed increased severity of serotype M3 infections.
Although we did not investigate the precise host substrate(s) for SlaA, the epithelial cell culture data suggest that the enzyme exerts its cytotoxic effects intracellularly. In this regard, SlaA has parallels with ExoU, a PLA2 virulence factor made by Pseudomonas aeruginosa (29–32), which is delivered directly to the host cell cytosol by the type III secretion machinery. As observed with SlaA, ExoU lacks cytotoxic effect when added exogenously to host cells grown in vitro; however, intracellular ExoU has a rapid and profound cytotoxic effect on cultured cells (29). The idea that SlaA acts from within host cells was further supported by data showing that expression of SlaA in yeast results in decreased host-cell viability (Fig. 11, which is published as supporting information on the PNAS web site). In addition, exhaustive attempts to clone the gene for genetic complementation of the mutant strain failed, consistent with the idea that SlaA is cytotoxic when expressed within cells (see Supporting Text, which is published as supporting information on the PNAS web site). It is a formal possibility that SlaA itself is not directly cytotoxic to human cells; rather, it may be that increased host-cell death occurs secondary to the enhanced adherence of GAS mediated by SlaA. However, the yeast expression data favor the idea that SlaA is directly cytotoxic, although both processes may be operative.
We also found that SlaA was secreted by GAS at the site of soft-tissue infection. Inactivation of the slaA gene resulted in significantly decreased virulence of the mutant strain in two mouse models of GAS invasive infection, and immunization with SlaA significantly decreased the amount of tissue pathology observed in mice after s.c. inoculation. These findings lead us to believe that SlaA enhances the pathogenic processes occurring during deep-tissue infection in the human, either directly or indirectly.
Thus, our data suggest a model in which SlaA acts in a multifaceted manner by enhancing host colonization and tissue destruction, which together would increase the number and severity of invasive infections caused by serotype M3 strains. SlaA increased the adherence of GAS to human epithelial cells, the first stage of host–pathogen cellular interaction. The ability of SlaA to enhance GAS adherence to epithelial cells would expand the population size and the possibility of transmission of these strains, thereby increasing the probability that SlaA-producing organisms would encounter a host susceptible to, or at risk for, invasive GAS infection. In this regard, we note that SlaA production is greatly increased when GAS interacts with human saliva or respiratory tract epithelial cells (13, 14, 33). Consistent with these findings, patients with GAS tonsillitis seroconvert to SlaA (3), which means that this enzyme is made during human upper respiratory tract infection. Together, these observations strongly suggest that SlaA is secreted very early in host–pathogen interaction, an optimal time to establish infection and drive clonal expansion.
Concluding Comment.
To summarize, many social, political, and economic factors contribute to the emergence and reemergence of infectious diseases (2). However, with the exception of the acquisition of genes conferring antimicrobial agent resistance, relatively little information is available about the molecular processes contributing to the very rapid emergence of distinct pathogenic bacterial clones. The sum of the evidence points to a model in which very recent acquisition of the gene encoding SlaA created a new clone of serotype M3 GAS with significantly enhanced epithelial cell colonization capacity and unusually high virulence traits.
Materials and Methods
Bacterial Strains, Culture Conditions, and DNA Manipulation.
The bacterial strains and plasmids used in this study are listed in Table 1, which is published as supporting information on the PNAS web site. GAS strains were grown in Todd–Hewitt broth (Difco, Sparks, MD) supplemented with 0.2% yeast extract (THY medium) at 37°C in 5% CO2/20% O2 atmosphere. THY medium or tryptose agar with 5% sheep blood (Becton Dickinson, Franklin Lakes, NJ) was used as solid medium. Cloning experiments were performed with Escherichia coli Nova Blue (Novagen, San Diego, CA). Ampicillin (100 μg/ml) and spectinomycin (150 μg/ml) were added when applicable.
Human Epithelial Cell Culture.
Immortalized Detroit 562 (D562) pharyngeal epithelial cells were purchased from the American Type Culture Collection (Manassas, VA; CCL-138). The cells were grown to 80–90% confluency in MEM (Invitrogen, Carlsbad, CA) supplemented with 10% FBS (BD Biosciences, San Jose, CA). NHTBE cells (Clonetics, San Diego, CA) were grown on collagen-coated glass cover slips in 24-well plates (Corning, Corning, NY) to 80–90% confluency in BEBM medium with supplements as recommended by the manufacturer (Clonetics) at 37°C with 5% CO2.
Construction of the ΔslaA Isogenic Mutant Strain.
Details are provided in Supporting Text. We exhaustively attempted to genetically complement the slaA deletion strain by introducing a wild-type copy of slaA into the mutant strain. Details are provided in Supporting Text.
Purification of SlaA.
rSlaA was purified to homogeneity from E. coli and tested for PLA2 activity (ref. 3 and Supporting Text).
In Vitro Infection, Attachment, and Cell Death Assays.
NHTBE cells were grown as previously described. Growth media were replaced the morning of infection. D562 epithelial cells were grown in MEM medium with serum for 48 h after seeding, and the media were replaced with serum-free MEM on the day of infection. Overnight GAS cultures were diluted into THY medium and grown to an OD600 ≈0.3. The bacteria were pelleted, washed with PBS, and suspended in PBS to an OD600 of ≈2.0. A 50-μl aliquot [resulting in an multiplicity of infection (moi) of 100:1] was added to each well of human cells and incubated for 3 h. Nonadherent GAS were removed by washing with PBS. For cytotoxicity assays, host cells were stained for 10 min with trypan blue (1:10 dilution in PBS), fixed in 2% paraformaldehyde (1 h), and the mean percentage of stained cells was determined. To determine the number of cell-associated bacteria, fixed cells were stained overnight with crystal violet, and the number of GAS attached to 50 randomly selected human cells in five different microscopic fields was determined. Lactate dehydrogenase release assays were performed per the manufacturer's instructions (CytoTox-ONE; Promega, Madison, WI).
Antibody-Inhibition Assay.
The attachment assay was performed as described above, except that 10 μg of affinity-purified rabbit anti-SlaA antibody was added to the D562 cells immediately before addition of bacteria. Affinity-purified preimmune rabbit IgG at the same concentration was used as a control.
Host-Cell Localization of SlaA.
For immunological localization of SlaA, NHTBE cells were infected for 2.5 h (moi = 100:1). Antibiotics (100 μg/ml clindamycin and 10 μg/ml penicillin) were then added for 30 min. Cytochalasin D (70 μg/ml) was added to selected samples during the infection. Epithelial cells were detached with trypsin-EDTA, collected by centrifugation, washed, and lysed by incubation with 1 mg/ml digitonin. Cell lysates were centrifuged at 20,800 × g for 5 min to yield a cytosolic and membrane fraction. The samples were analyzed by Western immunoblot with rabbit anti-SlaA antibody (1:10,000 dilution) followed by alkaline phosphatase-conjugated goat anti-rabbit antibody (1:5,000; Santa Cruz Biotechnology, Santa Cruz, CA).
For cell localization of SlaA using confocal microscopy, NHTBE cells were infected for 6 h (moi = 100:1). Infected NHTBE cells were then washed with PBS and fixed with 4% paraformaldehyde in PBS (pH 7.4), and host cell membranes were labeled with 5 μg/ml Alexa 568-conjugated anti-CD44 antibody (IM7.8.1; BD Biosciences). To visualize intracellular SlaA, CD44-labeled cells were washed with PBS, permeabilized with 0.1% Triton X-100 in PBS, and incubated with primary rabbit anti-SlaA antibodies followed by secondary staining with Alexa 488-conjugated donkey anti-rabbit IgG (Molecular Probes, Eugene, OR). Confocal microscopy images were collected by using a Zeiss (Oberkochen, Germany) LSM 510 Pascal confocal microscope equipped with an Argon/Krypton and two HeNe lasers (Zeiss) and images processed by using Zeiss software.
Mouse Infection Experiments.
GAS strains used for infection studies were grown in THY medium to mid-exponential phase (OD600 ≈0.5), harvested, washed twice with PBS, and used to infect either immunocompetent hairless mice (strain Crl:SKH1-hrBR; s.c. inoculation, administered dose 1.0 × 107 cfu) or CD-1 Swiss mice (i.p. inoculation, administered dose 2.5 × 107 cfu). Abscess length (L) and width (W) values were used to calculate abscess volume (V = 4/3π (L/2)2 × [W/2]) and area (A = π[L/2] × [W/2]) by using equations for a spherical ellipsoid (34).
Immunization of Mice with rSlaA.
Crl:SKH1-hrBR mice were vaccinated with purified rSlaA (50 μg) and TiterMax adjuvant (TiterMax USA, Norcross, GA). Control mice were injected with PBS and TiterMax. At 4 weeks postimmunization, mice were boosted with 25 μg of rSlaA. Two weeks later, they were challenged by s.c. injection as described above.
Serologic Analysis of SlaA.
In vitro production of SlaA was assessed by Western immunoblot analysis with specific rabbit polyclonal anti-SlaA antibody (3). ELISA on mouse sera (1:2,000 dilution) from SlaA-vaccinated animals was done by standard procedures with purified SlaA as antigen. ELISA on monkey sera (1:500 dilution) was done by standard procedures with purified SlaA as antigen.
Non-Human Primate Infection.
A non-human primate model of GAS pharyngitis was used (22, 23). Two groups of four anesthetized cynomolgus macaques were inoculated with a 1-ml suspension (8 × 107/ml) of wild-type and ΔslaA GAS strains into the nares of each animal. Throat swabs and peripheral blood samples were taken on days −7, 7, 15, 21, and 28. Throat swabs only were taken on days 0, 2, 4, 11, and 18.
Supplementary Material
Acknowledgments
We thank R. Ireland, I. Abdi, D. Dorward, R. Larson, J. Rosenblatt, K. Ponce, A. Raiford, and M. Strauss for technical assistance; T. Quitugua for generous hospitality; K. Stockbauer for editorial help; E. A. Graviss for help with statistical analysis; and R. M. Krause, D. Morens, and F. R. DeLeo for critical comments to improve the manuscript. This work was supported in part by Southwest National Primate Research Center Pilot Study Grant 05-3500-77.
Abbreviations
- GAS
Group A Streptococcus
- PLA2
phospholipase A2
- SlaA
streptococcal phospholipase A
- rSlaA
recombinant SlaA
- NHTBE
normal human tracheobronchial epithelial
- SLO
streptolysin O
- moi
multiplicity of infection.
Footnotes
The authors declare no conflict of interest.
References
- 1.Institute of Medicine. Emerging Infections: Microbial Threats to Health in the United States. Washington, DC: Natl Acad Press; 1992. [PubMed] [Google Scholar]
- 2.Morens DM, Folkers GK, Fauci AS. Nature. 2004;430:242–249. doi: 10.1038/nature02759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beres SB, Sylva GL, Barbian KD, Lei B, Hoff JS, Mammarella ND, Liu MY, Smoot JC, Porcella SF, Parkins LD, et al. Proc Natl Acad Sci USA. 2002;99:10078–10083. doi: 10.1073/pnas.152298499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beres SB, Sylva GL, Sturdevant DE, Granville CN, Liu M, Ricklefs SM, Whitney AR, Parkins LD, Hoe NP, Adams GJ, et al. Proc Natl Acad Sci USA. 2004;101:11833–11838. doi: 10.1073/pnas.0404163101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Musser JM, DeLeo FR. Am J Pathol. 2005;167:1461–1472. doi: 10.1016/S0002-9440(10)61232-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sumby P, Porcella SF, Madrigal AG, Barbian KD, Virtaneva K, Ricklefs SM, Sturdevant DE, Graham MR, Vuopio-Varkila J, Hoe NP, et al. J Infect Dis. 2005;192:771–782. doi: 10.1086/432514. [DOI] [PubMed] [Google Scholar]
- 7.Cunningham MW. Clin Microbiol Rev. 2000;13:470–511. doi: 10.1128/cmr.13.3.470-511.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davies HD, McGeer A, Schwartz B, Green K, Cann D, Simor AE, Low DE. N Engl J Med. 1996;335:547–554. doi: 10.1056/NEJM199608223350803. [DOI] [PubMed] [Google Scholar]
- 9.O'Brien KL, Beall B, Barrett NL, Cieslak PR, Reingold A, Farley MM, Danila R, Zell ER, Facklam R, Schwartz B, et al. Clin Infect Dis. 2002;35:268–276. doi: 10.1086/341409. [DOI] [PubMed] [Google Scholar]
- 10.Sharkawy A, Low DE, Saginur R, Gregson D, Schwartz B, Jessamine P, Green K, McGeer A. Clin Infect Dis. 2002;34:454–460. doi: 10.1086/338466. [DOI] [PubMed] [Google Scholar]
- 11.Li Z, Sakota V, Jackson D, Franklin AR, Beall B. J Infect Dis. 2003;188:1587–1592. doi: 10.1086/379050. [DOI] [PubMed] [Google Scholar]
- 12.Nakagawa I, Kurokawa K, Yamashita A, Nakata M, Tomiyasu Y, Okahashi N, Kawabata S, Yamazaki K, Shiba T, Yasunaga T, et al. Genome Res. 2003;13:1042–1055. doi: 10.1101/gr.1096703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Banks DJ, Lei B, Musser JM. Infect Immun. 2003;71:7079–7086. doi: 10.1128/IAI.71.12.7079-7086.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagiec MJ, Lei B, Parker SK, Vasil ML, Matsumoto M, Ireland RM, Beres SB, Hoe NP, Musser JM. J Biol Chem. 2004;279:45909–45918. doi: 10.1074/jbc.M405434200. [DOI] [PubMed] [Google Scholar]
- 15.Nakagawa I, Nakata M, Kawabata S, Hamada S. Cell Microbiol. 2001;3:395–405. doi: 10.1046/j.1462-5822.2001.00122.x. [DOI] [PubMed] [Google Scholar]
- 16.Tsai PJ, Lin YS, Kuo CF, Lei HY, Wu JJ. Infect Immun. 1999;67:4334–4339. doi: 10.1128/iai.67.9.4334-4339.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Madden JC, Ruiz N, Caparon M. Cell. 2001;104:143–152. doi: 10.1016/s0092-8674(01)00198-2. [DOI] [PubMed] [Google Scholar]
- 18.Bricker AL, Cywes C, Ashbaugh CD, Wessels MR. Mol Microbiol. 2002;44:257–269. doi: 10.1046/j.1365-2958.2002.02876.x. [DOI] [PubMed] [Google Scholar]
- 19.Banks DJ, Porcella SF, Barbian KD, Beres SB, Philips LE, Voyich JM, DeLeo FR, Martin JM, Somerville GA, Musser JM. J Infect Dis. 2004;190:727–738. doi: 10.1086/422697. [DOI] [PubMed] [Google Scholar]
- 20.Hoe NP, Vuopio-Varkila J, Vaara M, Grigsby D, De LD, Fu YX, Dou SJ, Pan X, Nakashima K, Musser JM. J Infect Dis. 2001;183:633–639. doi: 10.1086/318543. [DOI] [PubMed] [Google Scholar]
- 21.Muotiala A, Seppala H, Huovinen P, Vuopio-Varkila J. J Infect Dis. 1997;175:392–399. doi: 10.1093/infdis/175.2.392. [DOI] [PubMed] [Google Scholar]
- 22.Sumby P, Barbian KD, Gardner DJ, Whitney AR, Welty DM, Long RD, Bailey JR, Parnell MJ, Hoe NP, Adams GG, et al. Proc Natl Acad Sci USA. 2005;102:1679–1684. doi: 10.1073/pnas.0406641102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Virtaneva K, Porcella SF, Graham MR, Ireland RM, Johnson CA, Ricklefs SM, Babar I, Parkins LD, Romero RA, Corn GJ, et al. Proc Natl Acad Sci USA. 2005;102:9014–9019. doi: 10.1073/pnas.0503671102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferretti JJ, McShan WM, Ajdic D, Savic DJ, Savic G, Lyon K, Primeaux C, Sezate S, Suvorov AN, Kenton S, et al. Proc Natl Acad Sci USA. 2001;98:4658–4663. doi: 10.1073/pnas.071559398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Green NM, Zhang S, Porcella SF, Nagiec MJ, Barbian KD, Beres SB, LeFebvre RB, Musser JM. J Infect Dis. 2005;192:760–770. doi: 10.1086/430618. [DOI] [PubMed] [Google Scholar]
- 26.Holden MTG, Scott A, Cherevach I, Chillingworth T, Churcher C, Cronin A, Dowd L, Feltwell T, Hamlin N, Holroyd S, et al. J Bacteriol. 2006 doi: 10.1128/JB.01227-06. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smoot JC, Barbian KD, Van Gompel JJ, Smoot LM, Chaussee MS, Sylva GL, Sturdevant DE, Ricklefs SM, Porcella SF, Parkins LD, et al. Proc Natl Acad Sci USA. 2002;99:4668–4673. doi: 10.1073/pnas.062526099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beres SB, Richter EW, Nagiec MJ, Sumby P, Porcella SF, DeLeo FR, Musser JM. Proc Natl Acad Sci USA. 2006;103:7059–7064. doi: 10.1073/pnas.0510279103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Phillips RM, Six DA, Dennis EA, Ghosh P. J Biol Chem. 2003;278:41326–41332. doi: 10.1074/jbc.M302472200. [DOI] [PubMed] [Google Scholar]
- 30.Rabin SD, Hauser AR. Infect Immun. 2003;71:4144–4150. doi: 10.1128/IAI.71.7.4144-4150.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sato H, Frank DW. Mol Microbiol. 2004;53:1279–1290. doi: 10.1111/j.1365-2958.2004.04194.x. [DOI] [PubMed] [Google Scholar]
- 32.Sato H, Frank DW, Hillard CJ, Feix JB, Pankhaniya RR, Moriyama K, Finck-Barbancon V, Buchaklian A, Lei M, Long RM, et al. EMBO J. 2003;22:2959–2969. doi: 10.1093/emboj/cdg290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shelburne SA, 3rd, Granville C, Tokuyama M, Sitkiewicz I, Patel P, Musser JM. Infect Immun. 2005;73:4723–4731. doi: 10.1128/IAI.73.8.4723-4731.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lukomski S, Sreevatsan S, Amberg C, Reichardt W, Woischnik M, Podbielski A, Musser JM. J Clin Invest. 1997;99:2574–2580. doi: 10.1172/JCI119445. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.