Abstract
Adrenomedullin (AM) belongs to the calcitonin gene-related peptide (CGRP) family and is a well known potent vasodilator. We show here that AM is a powerful pain-inducing neuropeptide. AM-like immunoreactivity is widely distributed in both CGRP-containing and lectin IB4-binding nociceptors in dorsal root ganglion and axon terminals in the superficial dorsal horn of the rat spinal cord. Specific binding sites for the radioligand, [125I]AM13–52 as well as immunoreactivity for receptor markers such as the calcitonin receptor-like receptor and three receptor-activity-modifying proteins are localized in the superficial dorsal horn, demonstrating the existence of AM/CGRP receptors in this region. Intrathecal injection of rat AM1–50, dose- and time-dependently, induced long-lasting heat hyperalgesia and increased the phosphorylation of Akt and GSK3β in the dorsal horn. Pre- and posttreatments with the AM receptor antagonist AM22–52 and PI3 kinase inhibitors (LY294002 and Wortmannin) significantly blocked or reversed AM-induced heat hyperalgesia. Pre- and posttreatments with AM22–52 and Wortmannin also significantly blocked or reversed intraplantar capsaicin-induced heat hyperalgesia. Taken together, our results demonstrate that AM acts as a pain-inducing peptide in the dorsal horn. By activating specific receptors (likely AM2) and the PI3K/Akt/GSK3β signaling pathway, AM could play a significant role in long-lasting heat hypersensitivity and inflammatory heat hyperalgesia.
Keywords: dorsal root ganglion, neuropeptide, nociception, nociceptor, signal transduction
Adrenomedullin (AM) is a member of the calcitonin gene-related peptide (CGRP) superfamily and shares similarities in structure and function with CGRP (1). AM was initially isolated from human pheochromocytoma and is derived from preproAM, a product of the AM gene localized on human chromosome 11 (2). AM has a wide range of biological effects including vasodilatation, cell proliferation, and pro- or anti-inflammatory properties (3). AM exerts its effects by acting on either CGRP1 or specific AM (AM1 and AM2) receptors. These receptors are pharmacologically unique but share a seven-transmembrane domain, the calcitonin receptor-like receptor (CLR) (1). To be functional, CLR requires the receptor-activity-modifying proteins (RAMPs) 1–3 to act as chaperone proteins and to confer pharmacological specificity (4) as well as the receptor component protein (RCP) to facilitate coupling to intracellular pathways (5). The CGRP1 receptor is generated by the association of CLR with RAMP1, whereas AM1 and AM2 receptors are produced by the association of CLR with RAMP 2 and 3, respectively (1, 6).
CGRP is enriched in small-to-medium neurons in dorsal root ganglia (DRG) (7, 8) and its role in pain (9), migraine (10), and neurogenic inflammation (2) are well established. Small-sized DRG neurons, which give rise to C and Aδ axons and can be activated by noxious mechanical, thermal, or chemical stimuli, are known as nociceptors. These nociceptors have been classified in two categories (11). The first is usually known as “peptidergic nociceptors” because they express CGRP, substance P and trkA, thus being responsive to nerve growth factor (NGF). Projections of this type of nociceptors terminate mostly in lamina I (LI) and outer lamina II (LIIo) of the dorsal horn. The second is frequently referred to as “nonpeptidergic nociceptors” and binds the isolectin B4 (IB4) and expresses receptors for glia-derived neurotrophic factor (GDNF). Afferent axons of this type of nociceptor primarily terminate in inner lamina II (LIIi).
Recently, AM mRNA and protein were shown to be expressed in DRG, and some AM-IR neurons also contained CGRP (12). This pattern of expression led us to hypothesize that AM could play a role in nociception. To test this hypothesis, our first objective was to clearly establish the presence of AM in nociceptors in DRG. The second objective was to demonstrate the presence of AM receptors in neurons in the superficial dorsal horn. The third objective was to establish whether intrathecal (i.t.) injection of AM induces nociceptive responses and whether spinal AM plays a role in peripheral inflammation. Finally, we explored the mechanisms underlying AM-induced pain response and found that the phosphatidylinositol 3-kinase (PI3K)/Akt/GSK3β signaling pathway is particularly involved. Our data demonstrate that AM should be considered as a pain neuropeptide.
Results
AM-IR Is Present in Nociceptors in DRG Neurons.
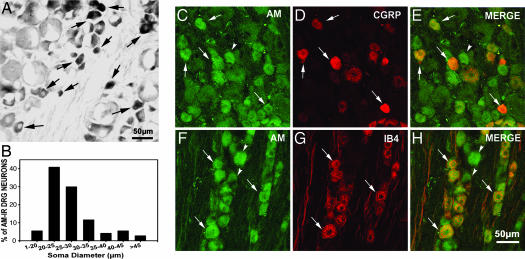
In lumbar DRG of naïve rats, AM-IR was frequently localized in small-to-medium neurons (Fig. 1A, arrows) and only occasionally in large neurons. Quantitative analysis revealed that ≈30% of DRG neurons were AM-containing (Fig. 6A, which is published as supporting information on the PNAS web site) and that 85% of AM-IR neurons were within the range of small-to-medium size (Fig. 1B). AM-IR neurons with similar profiles were also observed in trigeminal ganglia and DRG at other spinal levels (data not shown). Incubation of DRG sections with AM antiserum preabsorbed with rAM1–50 (Fig.6D) or with omission of AM antiserum (Fig. 6E) resulted in lack of staining. The presence of AM in DRG tissue was further confirmed by using ELISA (Fig. 6B). A significant amount of AM was present in DRG tissue (1,808 ± 316 pg), heart (1,981 ± 250 pg), and adrenal gland (878 ± 62 pg) per gram of wet tissue, although they were below detection level in kidney and liver (<10 pg per gram of wet tissue).
Fig. 1.
AM-IR neurons in two types of nociceptors in DRG. (A) AM-IR is localized in small and medium neurons (arrows). (B) Size–frequency histogram reveals that 85% of AM-IR neurons were distributed within the range of small (<25 μm in soma diameter) to medium (25–35 μm in soma diameter) neurons. AM-IR (C, arrows) and CGRP-IR (D, arrows) were coexpressed in some neurons (E, yellow, arrows). AM-IR (F, arrows) was colocalized in numerous IB4-binding neurons (G, arrows) when merged (H, arrows). Some AM-IR neurons did not coexpress CGRP (E, arrowhead) or bind IB4 (H, arrowheads).
We expected to find that AM-IR was predominantly coexpressed in CGRP-containing neurons in DRG, because it belongs to the CGRP superfamily (13). However, double-immunostaining studies showed that only ≈33% of AM-IR neurons (Fig. 1C, arrows) were CGRP-IR (Fig. 1 D and E, arrows). Interestingly, 58% of AM-IR neurons (Fig. 1F, arrows) bound IB4 (Fig. 1 G and H, arrows) and as much as 56% of IB4-binding neurons contained AM-IR. CGRP (Fig. 6F, arrowheads) and IB4 (Fig. 6G, arrows) are basically distributed in two distinct populations of DRG neurons (Fig. 6H), with only 9% of CGRP-IR neurons binding IB4.
We examined the distribution of AM-IR axon terminals in the spinal dorsal horn and its relationship to those of CGRP- and IB4-positive axonal terminals. AM-IR axonal terminals were predominantly enriched in LI, LIIo, and LIIi (Fig. 7 A and D, which is published as supporting information on the PNAS web site). CGRP-IR axons were mostly seen in LI and LIIo (Fig. 7B), whereas IB4-binding axons were particularly enriched in LIIi (Fig. 7E). Thus, the distribution of AM-IR axon terminals overlaps those of both CGRP and IB4 (Fig. 7 C and F). The coexistence of AM and CGRP (Fig. 7G, arrows) or AM and IB4 (Fig. 7H, arrows) in the same axon terminals was also noted. Few AM-IR cell profiles were observed in the dorsal horn at the light microscopic level.
At the electron microscopic level, AM-IR axonal boutons were abundantly distributed in LI and LII (Fig. 8 A–D, which is published as supporting information on the PNAS web site) and established synapses with postsynaptic elements such as somatic dendrites (Fig. 8A, arrowhead), small dendritic branches (Fig. 8 B and D, arrowheads) and dendritic spines (Fig. 8C, arrowhead). AM-IR axonal boutons were occasionally observed to be postsynaptic to other axonal boutons (data not shown). AM-IR was associated more frequently with round clear synaptic vesicles than with large dense core vesicles (Fig. 8 A, C, and D, arrows).
CLR, RAMPs, and AM Receptor-Binding Sites Are Present in the Superficial Dorsal Horn.
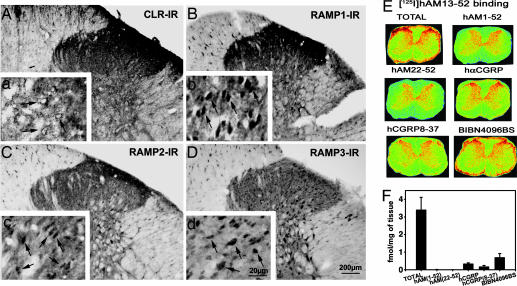
The presence and localization of CLR and RAMP 1, 2, and 3 in the lumbar spinal cord were examined. CLR-, RAMP1-, and RAMP2-IR axons and cells were predominantly localized in LI and LII (Fig. 2A–C). Only RAMP3-IR cells were observed throughout all laminae (Fig. 2D). CLR-IR was mostly localized in the plasma membrane of labeled cells (Fig. 2a, arrows), whereas RAMP 1-, 2-, and 3-IR were mostly cytoplasmic (Fig. 2 b–d, arrows). Incubation of spinal cord sections with CLR and RAMP1–3 antisera that were preabsorbed with their respective blocking peptides resulted in lack of staining (Fig. 9, which is published as supporting information on the PNAS web site).
Fig. 2.
CGRP1 and AM receptors (AM1 and AM2) in the dorsal horn of rat lumbar spinal cord. (A) CLR-IR axons and cells were observed throughout all laminae but mainly in LI and LII. CLR-IR was localized in plasma membrane of cells (arrows). RAMP1-IR (B) and RAMP2-IR (C) axons and cells are localized throughout all laminae but predominantly in LI-LII. RAMP1-IR and RAMP2-IR were distributed in the cytoplasm of cells (b and c, arrows). Only RAMP3-IR was localized in the cells in all laminae (D) and its IR is cytoplasmic (arrows). (E) Total [125I]hAM13-52-binding sites were predominantly localized in LI-LII, which was competed by saturation concentrations of both hAM1-52 and hAM22-52 and, to a somewhat lesser extent, by hαCGRP, hCGRP8-37 or BIBN4096BS. (F) The quantification of the total [125I]hAM13-52-binding sites and levels remaining in the presence of 1 μM hAM1-52, hAM22-52, hαCGRP, hCGRP8-37, or BIBN4096BS.
Receptor autoradiography revealed that specific binding sites for [125I] hAM13–52 are predominantly localized in LI and II in the dorsal horn of the lumbar spinal cord (Fig. 2E). Specific [125I]hAM13-52-binding was almost completely inhibited in the presence of saturating concentrations of hAM1-52 (agonist) and hAM22-52 (antagonist) (Fig. 2 E and F). CGRP, CGRP8-37, and BIBN4096BS were less potent competitors against specific [125I]AM13-52 binding as expected for AM receptors (Fig. 2 E and F).
i.t. Injection of AM Induced a Dose- and Time-Dependent Nociceptive Response.
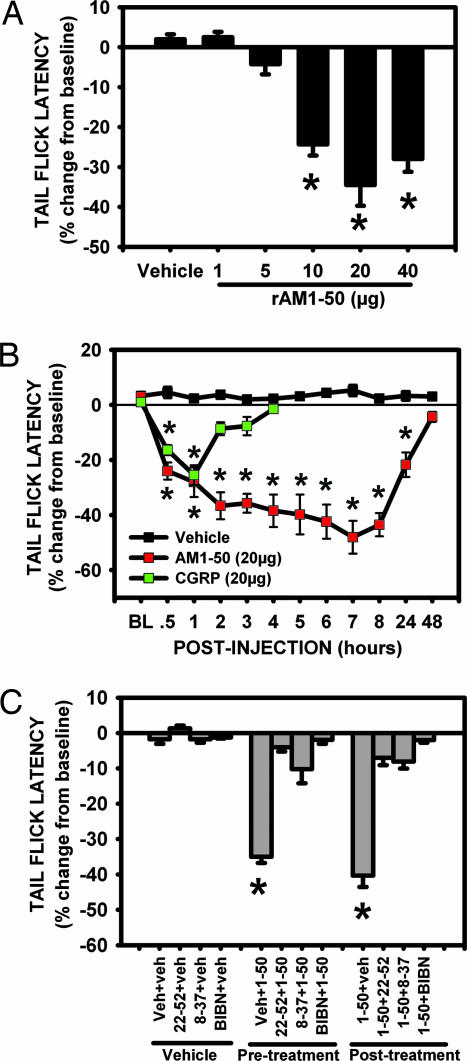
i.t. rat (r)AM1-50 injected, at the doses of 10, 20, and 40 μg, induced significant, long-lasting reduction in tail-flick latency (TFL) (Fig. 3A). Doses of 1 and 5 μg were ineffective, whereas 20 μg exerted the maximum effect. The effect of rAM1-50 (20 μg) was observed as early as 30 min after injection (Fig. 3B). The reduction in TFL was progressive and reached a maximum by 7 h. TFL returned to preinjection level by 48 h (Fig. 3B), demonstrating the long-lasting effect of rAM1-50. By using von Frey filaments to stimulate the hindpaws of injected rats, i.t. AM at all doses and time points did not cause signs of mechanical allodynia (data not shown). As reported (14), i.t. injection of rat αCGRP (20 μg) induced a significant reduction in TFL but only at 30 min and 1 h after injection (Fig. 3B).
Fig. 3.
i.t. injection of rAM1-50 dose- and time-dependently induced heat hyperalgesia. (A) i.t. rAM1-50 (10, 20, and 40 μg) significantly reduced TFL compared with control (∗, P < 0.01) at 2 h after injection. The dose of 20 μg exerted the maximum effect, whereas 1 and 5 μg were ineffective. (B) i.t. rAM1-50 (20 μg) significantly reduced TFL between 30 min and 24 h, with a peak effect observed 7 h after injection (∗, P < 0.001). By 48 h after injection, AM-reduced TFL returned to control level. i.t. αCGRP (20 μg) significantly reduced TFL at 30 and 60 min after injection (∗, P < 0.01). (C) i.t. rAM1-50 (20 μg) significantly reduced TFL at 2 h after injection (∗, P < 0.01). This effect was blocked or reversed by i.t.AM22-52, CGRP8-37, or BIBN4096BS 1 h before and after i.t. rAM1-50. i.t. vehicle, AM22-52, CGRP8-37, and BIBN4096BS failed to significantly affect TFL on their own. Mean ± SEM, n = 5–10 per group.
Preemptive i.t. injection of the AM receptor antagonist, AM22-52 (20 μg), significantly blocked AM-reduced TFL from 2 (Fig. 3C) to 6 (data not shown) h after injection. Pretreatment with the CGRP receptor antagonists CGRP8-37 (20 μg) and BIBN4096BS (0.1 μg) also significantly blocked AM-reduced TFL between 2 (Fig. 3C) and 6 (data not shown) h. Interestingly, after treatment with AM22-52 (20 μg), CGRP8-37 (20 μg) or BIBN4096BS (0.1 μg) significantly reversed AM-reduced TFL (Fig. 3C). i.t. injection of vehicle, AM22-52, CGRP8-37, or BIBN4096BS did not significantly alter TFL by themselves (Fig. 3C).
Intrathecal AM-Induced Pain Response Is Mediated Through the Activation of the PI3K–Akt–GSK3β Pathway.
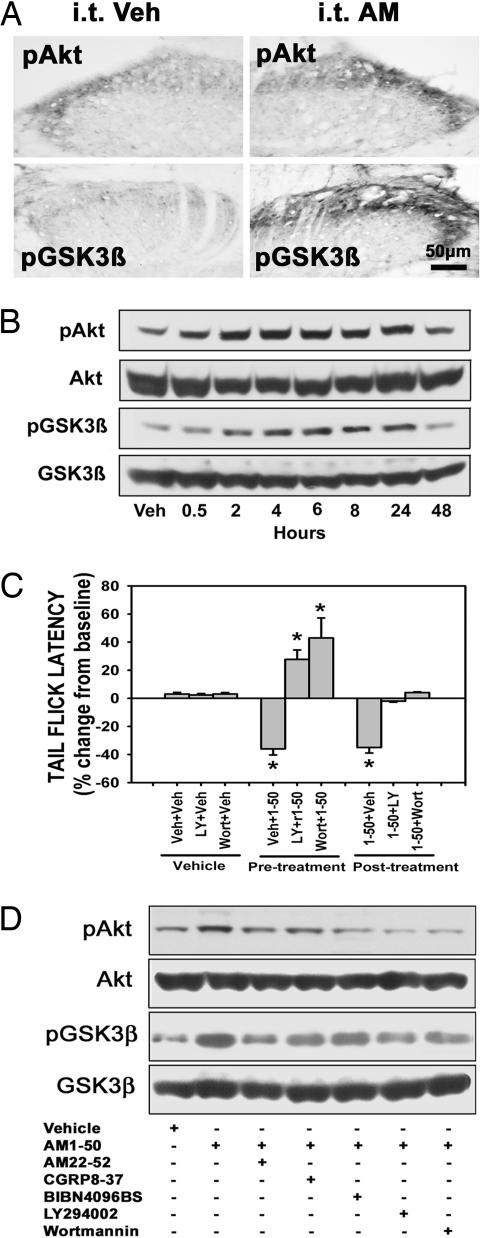
AM-induced vasodilatation and myocardial protection is mediated through the activation of the phosphatidylinositol 3-kinase (PI3K)/Akt-dependent pathway (15, 16). The phosphorylation of Akt in DRG is involved in the maintenance of inflammatory pain caused by intraplantar injection of capsaicin (17). Accordingly, we investigated whether i.t. rAM1-50 could induce the phosphorylation of downstream kinases such as Akt and GSK3β in the dorsal horn neurons and whether PI3 kinase inhibitors (LY294002 and Wortmannin) were able to block i.t. rAM1-50-reduced TFL. After i.t. injection of 20 μg rAM1-50, the levels of pAkt- and pGSK3β-IR were significantly increased in the dorsal horn neurons and primary afferent axons compared with vehicle treatment (Fig. 4A; and see Fig. 10A, which is published as supporting information on the PNAS web site). The level of pAkt was gradually increased from 30 min to 48 h, with the peak increase seen at 4–6 h after injection (Figs. 4B and 10B). Increased phosphorylation of GSK3β was seen at 2 h after injection, reaching a peak level at 8 h and returning to control value by 48 h after injection (Figs. 4B and Fig. 10B).
Fig. 4.
i.t. AM-induced heat hyperalgesia is mediated by the activation of the PI3K/Akt/GSK3β pathway. (A) i.t. rAM1–50 (20 μg) increased pAkt-IR and pGSK3β-IR in L5 spinal dorsal horn at 2 h after injection. (B) Western blot analysis shows that pAkt and pGSK3β expression were remarkably increased from 2 to 24 h after AM injection and reached peak levels at 2 and 8 h, respectively. Veh, vehicle. (C) i.t. rAM1–50 significantly reduced TFL at 2 h after injection (∗, P < 0.001), but this effect was blocked or reversed by pre- or posttreatment with AM22–52 (20 μg), LY294002 (10 μg) and wortmannin (0.1 μg). Veh, vehicle. Moreover, pretreatment with both LY294002 and Wortmannin also significantly increased TFL compared with control (∗, P < 0.01). Mean ± SEM, n = 8 in each group. (D) Western blot analysis shows that pretreatment with AM22–52, CGRP8–37, BIBN4096BS, LY294002, or Wortmannin attenuated AM-induced increase in the levels of pAkt and pGSK3β in the lumbar dorsal horn.
Preemptive i.t. injection of either LY294002 (10 μg) or Wortmannin (0.2 μg) significantly blocked rAM1-50-reduced TFL at 2 h after AM injection (Fig. 4C). The blocking effects persisted up to 6 h after i.t. rAM1-50 injection (data not shown). Interestingly, both inhibitors not only blocked AM-reduced TFL, but also significantly increased TFL (Fig. 4C), suggesting an exaggerated antihyperalgesic response (antinociception). Moreover, i.t. injection of both inhibitors 1 h after i.t. rAM1-50 significantly reversed AM-reduced TFL (Fig. 4C). Western blots revealed that pretreatment with AM22-52 (20 μg), CGRP8-37 (20 μg), BIBN9046BS (0.1 μg), LY294002 (10 μg), and Wortmannin (0.2 μg) suppressed rAM1-50 (20 μg) -induced phosphorylation of Akt and GSK3β (Fig. 4D).
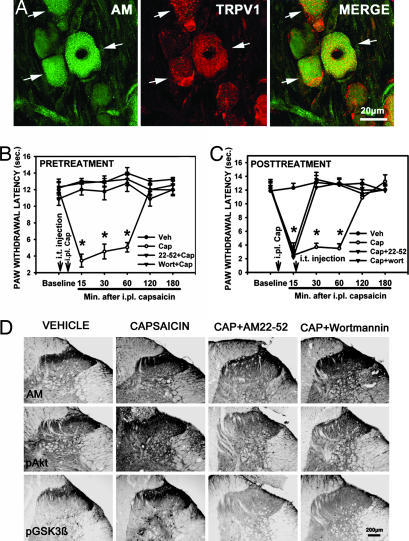
AM Released from the Dorsal Horn Is Involved in Intraplantar Capsaicin-Induced Inflammatory Pain.
The transient receptor potential vanilloid receptor 1 (TRPV1) is a ligand-gated nonselective cation channel activated by capsaicin, heat, and proton (18). In lumbar DRG, ≈62% (AM-IR cells, 33 ± 3; TRPV1-IR cells, 31 ± 3; AM+TRPV1-IR cells, 21 ± 3 cells per field, n = 10) of AM-IR neurons coexpressed TRPV1 (Fig. 5A). We tested whether AM released from nociceptors could be involved in capsaicin-induced heat hyperalgesia. Intraplantar injection of capsaicin (30 μg) significantly reduced paw-withdrawal latency (PWL) 15, 30, and 60 min after injection (Fig. 5 B and C). i.t. injection of AM22-52 (20 μg) and Wortmannin (0.1 μg) 1 h before and 15 min after capsaicin injection significantly prevented (Fig. 5B) or reversed (Fig. 5C) capsaicin-reduced PWL. One hour after capsaicin injection, AM-, pAkt-, and pGSK3β-IR were significantly increased in the ipsilateral dorsal horn compared with vehicle (Fig. 5D; and see Fig. 11 A–C, which is published as supporting information on the PNAS web site). i.t. injection of AM22-52 (20 μg) at 15 min after capsaicin significantly suppressed increase in AM-IR, pAkt-IR and pGSK3β-IR induced by capsaicin, whereas i.t. Wortmannin (0.1 μg) did not alter increases in AM-IR but strongly inhibited the phosphorylation of Akt and GSK3β (Figs. 5D and 11 A–C).
Fig. 5.
AM and PI3K/Akt/GSK3β is involved in intraplantar capsaicin-induced heat hyperalgesia. (A) AM-IR (green, arrows) coexpressed TRPV1-IR (red, arrows) in the same neurons (yellow, arrows). Intraplantar injection of capsaicin (30 μg) significantly reduced PWL at 15, 30, and 60 min after injection compared with control (B and C, ∗, P < 0.001). i.t. AM22-52 (20 μg) and Wortmannin (0.1 μg) 1 h before (B) and 15 min after (C) capsaicin injection prevented or reversed capsaicin-reduced PWL. Mean ± SEM, n = 7 or 8 per group. (D) One hour after capsaicin injection, AM-, pAkt-, and pGSK3β-IR were dramatically increased in the ipsilateral dorsal horn. i.t. AM22-52 (20 μg) at 15 min after capsaicin injection significantly attenuated capsaicin-increased AM-, pAkt-, and pGSK3β-IR. i.t. Wortmannin (0.2 μg) did not affect capsaicin-increased AM-IR in the dorsal horn, but reduced capsaicin-increased pAkt and pGSK3β-IR levels. Cap, capsaicin; i.pl., intraplantar.
Discussion
Anatomical Evidence That AM Is a Pain Neuropeptide.
In this study, we observed that the majority of AM-IR DRG neurons were of small-to-medium size, with one-third coexpressing CGRP, whereas two-thirds were IB4-positive, demonstrating that AM is widely distributed in both NGF- and GDNF-responsive nociceptors (11). AM should hence be considered as a more universal nociceptive transmitter or modulator than CGRP. Naturally, immunohistochemical studies reveal only the localization of a given protein and depend on the full specificity of the antibodies used. It would be interesting to perform in situ hybridization using highly selective riboprobes to establish the expression of AM mRNA in these two type of nociceptors. Interestingly, it has recently been reported that AM mRNA is expressed in DRG tissues (12). In accordance with findings that capsaicin treatment eliminated AM-IR in perivascular nerves (12), we observed that the majority of AM-IR neurons coexpressed TRPV1. AM has been recognized as an inflammatory mediator (3, 19). Circulating AM is dramatically increased by multiple cellular sources during local and systemic inflammation such as sepsis (3). AM is believed to stimulate the production of cytokines from immune cells and to increase blood flow and vascular permeability in inflamed tissues. The presence of AM in DRG neurons suggests these neurons as an important source of AM released in target tissues.
We have shown here that CLR and RAMP 1-, 2- and 3-IR neurons are enriched in the superficial dorsal horn. RCP-IR neurons are also present in this area (20). Thus, the various components required to generate functional CGRP1, AM1, and AM2 (1) receptors are expressed in dorsal horn neurons. Moreover, specific [125I]AM13-52-binding sites are predominantly localized in the area, with AM1-52 and AM22-52 potently competing for these sites, whereas CGRP8-37 and BIBN4096BS were less effective. This ligand-selectivity profile reveals that specific [125I]AM13-52-binding sites mostly represent AM receptors (1, 21). AM could activate both pre- and postsynaptic receptors in the spinal cord because CLR and all RAMPs are expressed in DRG neurons (22). As shown for CGRP (23), AM may act on presynaptic autoreceptors to regulate DRG functions.
Functional Evidence That AM Is a Pain Neuropeptide.
We observed that i.t. rAM1-50 induced a long-lasting heat hyperalgesia in rats. Both pre- and posttreatments with AM22-52, CGRP8-37, or BIBN4096BS significantly blocked or reversed AM-induced heat hyperalgesia. This pharmacological profile is similar to that of the AM2 receptor subtype, although a role for CGRP1 and/or AM1 receptors cannot be excluded at this time. i.t. CGRP was shown earlier to induce only a transient heat hyperalgesia (14) in contrast to the long-lasting hyperalgesia shown here by i.t. AM1-50. These distinct response profiles could be related to the following observations. First, AM is extensively distributed in both CGRP-containing and IB4-binding nociceptors and highly colocalized with TRPV1. Second, AM acts on AM1, AM2, and CGRP1 receptors, whereas CGRP binds mostly to CGRP1 receptors (1, 2). Finally, AM was also shown to be a more potent vasorelaxing peptide than CGRP (24). However, it has yet to be fully established whether AM-induced pain response is mediated by a direct activation of AM receptors located on nociceptive neurons in the dorsal horn or via an indirect mechanism (as suggested for CGRP), AM potentially inducing the release of other pain-stimulating substances such as substance P or glutamate. Additional experiments will be required to clarify this point.
The PI3K/Akt/GSK3β Signaling Pathway Is Involved in AM-Induced Heat Hyperalgesia.
i.t. rAM1-50 significantly increased the phosphorylation of Akt and GSK3β in dorsal horn neurons and axons, an effect suppressed by pretreatments with an AM receptor antagonist and PI3K inhibitors. Before and after treatment with the AM antagonist (AM22-52), and PI3K inhibitors also blocked or reversed rAM1-50-induced heat hyperalgesia that was temporally associated with increased phosphorylation of Akt and GSK3β in the dorsal horn. Taken together, these data suggest that AM-induced heat hyperalgesia is mediated, at least in part, through the activation of the PI3K/Akt/GSK3β signaling pathway.
Intraplantar injection of capsaicin was shown to induce heat hyperalgesia and increase Akt phosphorylation in DRG neurons (17). In this study, intraplantar capsaicin not only induced heat hyperalgesia but also increased the levels of AM-, pAkt-, and pGSK3β-IR in neurons and axons of the dorsal horn. Moreover, the capsaicin receptor TRPV1 has been reported to colocalize with both CGRP and IB4 in DRG neurons (25). We found here that the majority of AM-IR neurons expressed TRPV1. Capsaicin could thus act on TRPV1 to induce the release of AM from both CGRP-containing and IB4-binding nociceptors. Moreover, before and after treatment with either AM22-52 or Wortmannin completely blocked or reversed capsaicin-induced heat hyperalgesia and the associated increase in pAkt- and GSK3β-IR in the dorsal horn. These data suggest that AM released from primary nociceptive afferents is involved in capsaicin-induced heat hyperalgesia through the activation of the PI3K/Akt/GSK3β signaling pathway. Because both AM22-52 and Wortmannin reversed capsaicin-induced pAkt and pGSK3β to levels that were significantly lower than controls, their endogenous basal level of activity is likely to be affected as well. We also observed that AM22-52 attenuated capsaicin-induced increase in AM levels in the dorsal horn. AM22-52 may thus act on “autoreceptors” to regulate the release of AM from nociceptive afferents, as suggested for CGRP (20). The increase in AM-IR induced by capsaicin may result from a direct effect on the synthesis and release of AM and/or from an indirect action through the stimulation of the production of other inflammatory mediators, which, in turn, act on their receptors on nociceptors to increase AM synthesis and release.
In conclusion, this study provides morphological and functional data showing that AM is a potent pain-related neuropeptide. Its enrichment in both NGF- and GDNF-responsive nociceptors suggests its key role in nociceptive processes, particularly in heat hyperalgesia. Interestingly, the PI3K/Akt/GSK3β signaling pathway is likely involved in AM-induced heat hyperalgesia. AM should thus be considered as a pain-related neuropeptide.
Materials and Methods
Animals.
Sprague–Dawley rats (male, 250–350 g; Charles River Breeding Laboratories, St-Constant, QC, Canada) were used in this study. All surgery and animal care procedures were according to protocols and guidelines approved by the McGill University Animal Care Committee and the Canadian Council for Animal Care.
The sources of all chemicals and reagents are described in detail in supporting information which is published on PNAS website.
Immunocytochemistry.
All immunostaining procedures are described in greater detail in Supporting Materials and Methods, which is published as supporting information on the PNAS web site. Briefly, DRG sections (10 μm) or L4–L5 spinal cord sections (40 μm) were incubated in the following antisera: purified rabbit polyclonal anti-rat AM antiserum, goat polyclonal anti-CLR, rabbit anti-RAMP 1, 2, and 3, rabbit anti-pAkt and -pGSK3β. Subsequently, sections were processed by using biotinylated IgG and Elite Vectastain ABC kit.
Double immunostaining of AM/CGRP, AM/IB4, or AM/TRPV1 are described in detail in Supporting Materials and Methods. DRG or spinal cord sections were incubated in a mixture of rabbit polyclonal anti-AM (1:100)/guinea pig polyclonal anti-CGRP (1:500) or anti-TRPV1 (1:100) antisera; or only in rabbit polyclonal anti-AM (1:100) overnight. Sections were next incubated in biotinylated goat anti-guinea pig (1:200) or biotinylated IB4 (1:500). Finally, sections were incubated in goat-anti rabbit IgG conjugated with Alexa Fluor 488 to label AM-IR and streptavidin conjugated with Alexa Fluor 568 to label CGRP-IR, IB4 binding or TRPV1-IR.
The methods used for the quantification of AM-IR in DRG neurons and AM-IR, pAkt-IR and pGSK3β-IR in the dorsal horn are described in detail in Supporting Materials and Methods.
125I-Labeled Human AM13-52 Receptor-Binding Autoradiography.
Sprague–Dawley rats (n = 4) were decapitated and L4–L6 spinal cord segments were removed and cut on a cryostat. Spinal cord sections (20 μm) were incubated with 35 pM [125I]hAM13–52. Human(h)AM1–52 and hAM22–52 were used to determine levels of specific binding whereas hαCGRP, hαCGRP8–37 and BIBN4096BS were used as related competitors (1 μM for all). Radioligand-incubated sections were exposed to films for 6 days and developed films were analyzed by using image analysis software and specific binding was calculated by subtracting nonspecific binding seen in presence of 1 μM hAM1-52 or hAM22-52 from total binding.
Intrathecal Catheter Indwelling and Drug Treatments.
The detailed description of i.t. catheter indwelling is provided in Supporting Materials and Methods. All drugs were injected through the exteriorized portion of the catheter in a 10-μl volume, followed by a flush with 10 μl of artificial cerebrospinal fluid (aCSF). The following drugs were i.t. injected: rAM1-50 (1, 5, 10, 20, and 40 μg), rαCGRP (20 μg), hAM22-52 (20 and 40 μg), hαCGRP8-37 (20 μg), BIBN4096BS (0.1 μg), and the PI3K inhibitors LY294002 (10 μg) and Wortmannin (0.2 μg). All drugs were dissolved in DMSO and diluted into their final concentration in aCSF. Vehicle was prepared as 20% DMSO dissolved in aCSF. Antagonists and inhibitors were injected 45–60 min before or 60 min after rAM1-50 injection.
Intraplantar Injection of Capsaicin and Drug Treatments.
Animals were anesthetized by inhalation of 4% isoflurane. Capsaicin (30 μg) dissolved in 10 μl of normal saline containing 20% ethanol and 7% Tween-80 was injected into the central plantar region of a hindpaw by using a 30-gauge syringe. Vehicle consisted of the mixture of solvents. AM22-52 (20 μg) and Wortmannin (0.2 μg) were i.t. injected either 45 min before or 15 min after capsaicin injection.
Nociceptive Behavioral Testing.
TFL was measured by using a Tail Flick Analgesia Meter (Model 33A; IITC Life Science, Woodland Hills, CA). A radiant-heat beam was focused on the middle portion of the tail until the tail was quickly flicked away. Baseline of the TFL was adjusted to the range of 6–8 s. Cut-off time was set at 10 s. All values obtained after i.t. injection were normalized as the percentage of changes from preinjection baselines: percentage change = [(TFL after injection − TFL preinjection)/TFL preinjection × 100%]. A one-way ANOVA with post hoc Dunnett's multiple-comparison method was used. TFL from vehicle-injected rats served as control. Significance level was set at P < 0.05.
PWL of both hindpaws in response to thermal stimulation were measured by using a radiant-heat beam (model 336 Plantar Analgesia Meter; IITC Life Science). The cut-off time was set at 20 sec. The mean from each group was determined and compared statistically by using one-way repeated-measures ANOVA with post hoc Dunnett's multiple-comparison method. The significance level was set at P < 0.05.
Western Blot Analysis.
The dorsal half of L4–L6 spinal cord segment was sonicated in modified RIPA buffer. The lysates were centrifuged, and the protein concentration in the supernatant was determined. Samples with equal amounts (20 μg per 20 μl per lane) of protein were loaded and then separated by 4–20% polyacrylamide gel electrophoresis, and the resolved proteins were transferred to Hybond-C nitrocellulose membranes. Membranes were then incubated in rabbit anti-pAkt (Ser-473) and anti-pGSK3β (Ser-9) (1:1,000), probed with a HRP conjugated goat anti-rabbit IgG and finally detected by using chemiluminescence. The membranes were stripped and reprobed with anti-Akt and Anti-GSK3β antisera (1:1,000) again. The loading and blotting of equal amounts of protein were also verified by reprobing the membrane with anti β-actin antiserum (data not shown).
Supplementary Material
Acknowledgments
This study was supported by a grant from Canadian Institutes of Health Research (to R.Q.).
Abbreviations
- AM
adrenomedullin
- aCSF
artificial cerebrospinal fluid
- CGRP
calcitonin gene-related peptide
- CLR
calcitonin receptor-like receptor
- DRG
dorsal root ganglion
- GSK3β
glucose synthase kinase 3β
- IR
immunoreactivity or immunoreactive
- i.t
intrathecal
- PWL
paw-withdrawal latency
- TFL
tail-flick latency.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission
References
- 1.Poyner DR, Sexton PM, Marshall I, Smith DM, Quirion R, Born W, Muff R, Fischer JA, Foord SM. Pharmacol Rev. 2002;54:233–246. doi: 10.1124/pr.54.2.233. [DOI] [PubMed] [Google Scholar]
- 2.Brain SD, Grant AD. Physiol Rev. 2004;84:903–934. doi: 10.1152/physrev.00037.2003. [DOI] [PubMed] [Google Scholar]
- 3.Bunton DC, Petrie MC, Hillier C, Johnston F, McMurray JJ. Pharmacol Ther. 2004;103:179–201. doi: 10.1016/j.pharmthera.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 4.McLatchie LM, Fraser NJ, Main MJ, Wise A, Brown J, Thompson N, Solari R, Lee MG, Foord SM. Nature. 1998;393:333–339. doi: 10.1038/30666. [DOI] [PubMed] [Google Scholar]
- 5.Luebke AE, Dahl GP, Roos BA, Dickerson IM. Proc Natl Acad Sci USA. 1996;93:3455–3460. doi: 10.1073/pnas.93.8.3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Born W, Fischer JA, Muff R. Recept Channels. 2002;8:201–209. [PubMed] [Google Scholar]
- 7.Gibson SJ, Polak JM, Bloom SR, Sabate IM, Mulderry PM, Ghatei MA, McGregor GP, Morrison JF, Kelly JS, Evans RM, et al. J Neurosci. 1984;4:3101–3111. doi: 10.1523/JNEUROSCI.04-12-03101.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hokfelt T, Arvidsson U, Ceccatelli S, Cortes R, Cullheim S, Dagerlind A, Johnson H, Orazzo C, Piehl F, Pieribone V, et al. Ann NY Acad Sci. 1992;657:119–134. doi: 10.1111/j.1749-6632.1992.tb22762.x. [DOI] [PubMed] [Google Scholar]
- 9.Mogil JS, Miermeister F, Seifert F, Strasburg K, Zimmermann K, Reinold H, Austin JS, Bernardini N, Chesler EJ, Hofmann HA, et al. Proc Natl Acad Sci USA. 2005;102:12938–12943. doi: 10.1073/pnas.0503264102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edvinsson L. CNS Drugs. 2001;15:745–753. doi: 10.2165/00023210-200115100-00001. [DOI] [PubMed] [Google Scholar]
- 11.Snider WD, Mcmahon SB. Neuron. 1998;20:629–632. doi: 10.1016/s0896-6273(00)81003-x. [DOI] [PubMed] [Google Scholar]
- 12.Hobara N, Nakamura A, Ohtsuka A, Narasaki M, Shibata K, Gomoita Y, Kawasaki H. Peptides. 2004;25:589–599. doi: 10.1016/j.peptides.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 13.van Rossum D, Hanisch UK, Quirion R. Neurosci Biobehav Rev. 1997;21:649–678. doi: 10.1016/s0149-7634(96)00023-1. [DOI] [PubMed] [Google Scholar]
- 14.Cridland RA, Henry JL. Neuropeptides. 1988;11:23–32. doi: 10.1016/0143-4179(88)90024-8. [DOI] [PubMed] [Google Scholar]
- 15.Nishimatsu H, Suzuki E, Nagata D, Moriyama N, Satonaka H, Walsh K, Sata M, Kangawa K, Matsuo H, Goto A, et al. Circ Res. 2001;89:63–70. doi: 10.1161/hh1301.092498. [DOI] [PubMed] [Google Scholar]
- 16.Okumura H, Nagaya N, Itoh T, Okano I, Hino J, Mori K, Tsukamoto Y, Ishibashi-Ueda H, Miwa S, Tambara K, et al. Circulation. 2004;109:242–248. doi: 10.1161/01.CIR.0000109214.30211.7C. [DOI] [PubMed] [Google Scholar]
- 17.Zhuang ZY, Xu H, Clapham DE, Ji RR. J Neurosci. 2004;24:8300–8309. doi: 10.1523/JNEUROSCI.2893-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 19.Hamid SA, Baxter GF. Pharmacol Ther. 2005;105:95–112. doi: 10.1016/j.pharmthera.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 20.Ma W, Chabot JG, Powell KJ, Jhamandas K, Dickerson IM, Quirion R. Neuroscience. 2003;120:677–694. doi: 10.1016/s0306-4522(03)00159-3. [DOI] [PubMed] [Google Scholar]
- 21.Juaneda C, Dumont Y, Quirion R. Trends Pharmacol Sci. 2000;21:432–438. doi: 10.1016/s0165-6147(00)01555-8. [DOI] [PubMed] [Google Scholar]
- 22.Cottrell GS, Roosterman D, Marvizon JC, Song B, Wick E, Pikios S, Wong H, Berthelier C, Tang Y, Sternini C, et al. J Comp Neurol. 2005;490:239–255. doi: 10.1002/cne.20669. [DOI] [PubMed] [Google Scholar]
- 23.Powell KJ, Quirion R, Jhamandas K. Eur J Neurosci. 2003;18:1572–1583. doi: 10.1046/j.1460-9568.2003.02887.x. [DOI] [PubMed] [Google Scholar]
- 24.Lainchbury JG, Cooper GJ, Coy DH, Jiang NY, Lewis LK, Yandle TG, Richards AM, Nicholls MG. Clin Sci (London) 1997;92:467–472. doi: 10.1042/cs0920467. [DOI] [PubMed] [Google Scholar]
- 25.Guo A, Vulchanova L, Wang J, Li X, Elde R. Eur J Neurosci. 1999;11:946–958. doi: 10.1046/j.1460-9568.1999.00503.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.