Abstract
Administration of IL-1β results in a profound and long-lasting hypoglycemia. Here, we show that this effect can be elicited by endogenous IL-1 and is related to not only the capacity of the cytokine to increase glucose uptake in peripheral tissues but also to mechanisms integrated in the brain. We show that (i) blockade of IL-1 receptors in the brain partially counteracted IL-1-induced hypoglycemia; (ii) peripheral administration or induction of IL-1 production resulted in IL-1β gene expression in the hypothalamus of normal and insulin-resistant, leptin receptor-deficient, diabetic db/db mice; (iii) IL-1-treated normal and db/db mice challenged with glucose did not return to their initial glucose levels but remained hypoglycemic for several hours. This effect was largely antagonized by blockade of IL-1 receptors in the brain; and (iv) when animals with an advanced Type II diabetes were treated with IL-1 and challenged with glucose, they died in hypoglycemia. However, when IL-1 receptors in the brains of these diabetic mice were blocked, they survived, and glucose blood levels approached those that these mice had before IL-1 administration. The prolonged hypoglycemic effect of IL-1 is insulin-independent and develops against increased levels of glucocorticoids, catecholamines, and glucagon. These findings, together with the present demonstration that this effect is integrated in the brain and is paralleled by IL-1β expression in the hypothalamus, indicate that this cytokine can reset glucose homeostasis at central levels. Such reset, along with the peripheral actions of the cytokine, would favor glucose uptake by immune cells during inflammatory/immune processes.
Keywords: CNS, IL-1 receptor antagonist, glucoregulation, type II diabetes, immune cell metabolism
There is evidence that immune-derived cytokines can mediate metabolic alterations during the course of infective, inflammatory, autoimmune, and neoplastic processes (1–4), either by acting locally or by interacting with different endocrine mechanisms (2, 3, 5). Under physiological conditions, glucose is the principal and most readily available source of energy. When IL-1, a prototypic proinflammatory cytokine, is administered to mice, a profound and long-lasting hypoglycemia is observed. The hypoglycemic effect of IL-1 is not mediated by insulin, because it is clearly observed in insulin-resistant diabetic mice and rats; is not caused by glucose loss; and is independent from the anorexic effect of the cytokine (6–12). IL-1-induced hypoglycemia might be explained by the capacity of this cytokine to stimulate glucose uptake in vitro in a variety of tissues such as adipose cells (13), fibroblasts (14), articular chondrocytes (15), keratinocytes (16), intestinal macrophages (17), peritoneal mesothelial cells (18), and glial cells (19). Studies in vivo also show that overproduction of IL-1 results in increased 2-deoxyglucose uptake in all tissues tested, including lymphoid organs and the brain (20). Several of the mentioned in vitro studies on the effect of IL-1 on glucose transport have been performed by using human cells, and injection of very low doses of recombinant IL-1β to patients with cancer induces a transient hypoglycemia (21). Administration of low doses of LPS to healthy volunteers increases glucose utilization (22) and decreases glucose levels (23). Because IL-1 is among the proinflammatory cytokines whose production is induced by LPS, these results indicate that IL-1 may also induce hypoglycemia in humans. IL-1β-stimulated glucose transport is PKC- and p38 MAP-activation-dependent and is accompanied by increased expression and membrane incorporation of different glucose transporters (24). The type of transporter affected depends on the tissue, for example, GLUT3 in the liver and brain and GLUT1 in chondrocytes (24). Furthermore, IL-1 inhibits gluconeogenesis by inhibiting the activity of phosphoenolpyruvate carboxykinase and also mediates a reduction in hepatic glycogen content (12, 20).
Besides these peripheral actions, there are indications that IL-1 can affect glucose homeostasis by acting at brain levels. Indeed, IL-1-mediated hypoglycemia develops against the enhanced output of counterregulatory hormones such as glucocorticoids, catecholamine, and glucagon (6, 9) and is very long lasting. Such a prolonged hypoglycemia is surprising, because the regulation of glucose levels in blood is one of the homeostatic mechanisms under the most tight feedback control at the level of the CNS. Studies have shown that injection of picogram amounts of IL-1 into the lateral brain ventricle induces hypoglycemia (10, 12). There is also evidence for brain IL-1 in the CNS, particularly in the hypothalamus, where it is present in neurons, and the quantity is increased by LPS administration (25, 26). These facts, together with the complex pattern of endocrine alterations caused by the cytokine, led us to hypothesize that IL-1, besides stimulating glucose transport and oxidation in the periphery, can also change the set point of CNS-mediated glucoregulation.
Here, we show that endogenous levels of IL-1 can induce hypoglycemia and trigger the expression of its own gene in the hypothalamus of normal and insulin-resistant mice. Furthermore, we provide evidence that IL-1 affects the set point for glucose homeostasis by acting at central levels.
Results
Endogenous IL-1 Mediates Hypoglycemia.
We have administered a low dose (0.5 μg per mouse) of LPS together with the IL-1 receptor antagonist (IL-1ra) to study whether endogenous IL-1 contributes to LPS-induced hypoglycemia. Fig. 1A shows that i.p. administration of IL-1ra (300 μg) inhibits the hypoglycemic effect of LPS.
Fig. 1.
IL-1 induces hypoglycemia. (A) Immediately after a blood sample was obtained (time 0), C57BL/6J male mice received LPS (0.5 μg per mouse) or saline and, 30 min later, IL-1ra (100 μg or 300 μg) or the corresponding vehicle. All substances were injected i.p. Additional blood samples were collected at the times indicated and used for glucose determinations. Each point in the curves represents the mean ± SEM from determinations performed in four (in groups with saline) or seven (in groups with LPS) mice per group. ∗, P < 0.005 vs. saline + vehicle and vs. saline + IL-1ra; ‡, P < 0.015 vs. LPS + vehicle; #, P < 0.015 vs. saline + IL-1ra. (B) Immediately after a blood sample was obtained (time 0), mice received either the vehicle alone, IL-1 (0.1 μg per mouse), or regular insulin (0.25 units) injected i.p. Additional blood samples were collected at the times indicated. Each point in the curves represents the mean ± SEM from determinations performed in 5–10 mice per group. ∗, P < 0.01 vs. vehicle; §, P < 0.005 vs. insulin. The shaded area in each panel indicates the range of the mean ± SEM of time 0 from all groups together.
IL-1 Induces a More Prolonged Hypoglycemia Than Regular Insulin.
We have compared the hypoglycemic effect of IL-1 with that of a dose of regular insulin that decreases glucose levels in a comparable magnitude. Fig. 1B shows the time kinetics of the effect of a single i.p. injection of 0.1 μg of IL-1 and 0.25 units (11.4 μg) of regular insulin on the glucose blood levels of normal mice. It is clear that insulin-injected mice returned to euglycemic values within 5 h, whereas the glucose levels of the mice that received IL-1 were still below the normal basal range 24 h later.
IL-1 in the Brain Contributes to Peripherally Induced IL-1 Hypoglycemia.
To explore the possibility that IL-1 induces hypoglycemia by acting in the brain, IL-1 was injected i.p. and IL-1ra (50 μg) intracerebroventricularly (i.c.v.). When injected i.p., even 100 μg of IL-1ra are not enough to significantly affect IL-1-induced hypoglycemia (see Fig. 1A). As shown in Fig. 2, blockade of IL-1 effects in the brain significantly attenuated the hypoglycemia induced by peripheral administration of IL-1.
Fig. 2.
Blockade of IL-1 receptors in the brain interferes with IL-1-induced hypoglycemia. IImmediately after a blood sample was obtained (time 0), IL-1ra (50 μg in 5 μl of vehicle) or the vehicle alone was injected i.c.v., and IL-1 (0.1 μg per mouse) or the vehicle was injected i.p. Additional blood samples were collected at the times indicated and used for glucose determinations. Each point in the curves represents the mean ± SEM from determinations performed in three (in groups with control i.p) and six to eight (in groups with IL-1 i.p.) mice per group. ∗, P < 0.001 vs. control + vehicle and vs. control + IL-1ra; §, P < 0.005 vs. all other groups. The shaded area indicates the range of the mean ± SEM of time 0 from all groups together.
IL-1 Induces the Expression of Its Own Gene in the Hypothalamus of Normal and Diabetic Mice.
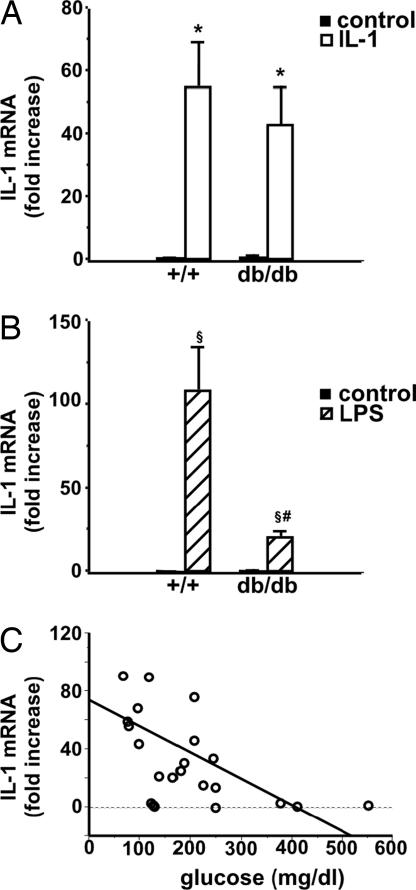
We then tested the possibility that IL-1 injected peripherally could result in the induction of its own gene in the hypothalamus and compared this effect with that of LPS. IL-1 induced IL-1 gene expression in the hypothalamus of normal and insulin-resistant, diabetic mice (Fig. 3A). However, whereas IL-1-induced IL-1 gene expression in the brain was comparable in both types of mice, the effect of LPS was less marked in diabetic mice (Fig. 3B). Interestingly, a correlation between IL-1 gene expression in the hypothalamus and glucose blood levels is established in both normal and db/db mice when values of control-, IL-1-, and LPS-injected mice are plotted together (Fig. 3C).
Fig. 3.
IL-1 and LPS induce IL-1 gene expression in the hypothalamus of normal and diabetic mice. Male C57BL/Ks +/+ and db/db mice (8 weeks of age) received control vehicle, IL-1 (0.1 μg per mouse) (A), or LPS (0.5 μg per mouse) (B) injected i.p. Animals were killed 2 h later, blood was collected, and the hypothalamus was dissected and immediately frozen until used for the determination of IL-1 gene expression as described in Materials and Methods. Each bar represents the mean ± SEM from determinations performed in three to five mice per group. (A) ∗, P < 0.05 vs. corresponding control. (B) §, P < 0.02 vs. corresponding control; #, P < 0.01 vs. LPS-injected +/+ mice. (C) The correlation between IL-1 gene expression in the hypothalamus and glucose blood levels. The data shown in A and B are plotted together with the corresponding glucose concentration determined in the blood of each individual mouse (r2 = 0.023, P < 0.02).
IL-1-Treated Mice Remain Hypoglycemic After a Glucose Load.
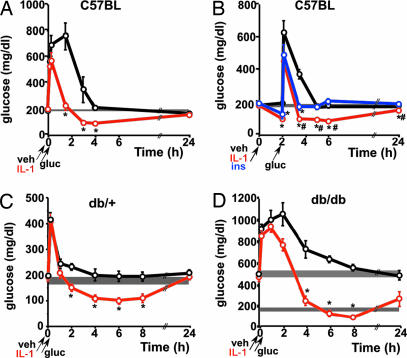
We have tested whether IL-1-induced hypoglycemia persists even after exogenous administration of glucose. Fig. 4A shows the results obtained when a glucose load was administered together with IL-1. After a transient increase in glucose blood levels, IL-1-treated mice were hypoglycemic for at least 4 h (Fig. 4A). Fig. 4B shows the results of a similar experiment when the glucose load was given 2 h after IL-1 administration, i.e., when glucose levels were ≈50% reduced. We also included in these studies a group of mice injected with a dose of regular insulin that induced a hypoglycemia comparable with that of IL-1. The results clearly show that mice that received IL-1 returned to the reduced levels of glucose they had before the glucose load and remained at these levels during several hours. In contrast, insulin-injected mice approached basal euglycemic levels rapidly.
Fig. 4.
Effect of IL-1 on glucose (gluc) tolerance in normal and db/db mice. (A) After a blood sample was obtained, 8-week-old C57BL/6J male mice received vehicle (veh) or IL-1 (0.1 μg per mouse) injected i.p., immediately followed by glucose [2 mg/g of body weight (g b.w.)], also administered i.p. Additional blood samples were obtained at the times indicated. Each point in the curves represents the mean ± SEM from glucose determinations performed in four mice per group. ∗, P < 0.05 vs. vehicle. (B) The same procedure as described in A was followed, but the glucose load was administered 2 h after the injection of vehicle, IL-1, or regular insulin (ins). Each point in the curves represents the mean ± SEM from glucose determinations performed in five mice per group. ∗, P < 0.01 vs. vehicle; #, < P < 0.05 vs. insulin. (C) After obtaining a blood sample, C57BL/Ks db/+ mice (mean body weight, 22.4 ± 0.3 g) received either vehicle or IL-1 (0.25 μg per mouse) injected i.p., immediately followed by glucose (2 mg/g b.w.) also administered i.p. Additional blood samples were obtained at the times indicated. Each point in the curves represents the mean ± SEM from glucose determinations performed in five mice per group. ∗, P < 0.005 vs. vehicle. (D) The same procedure as described in C was followed but using C57BL/Ks db/db mice and a dose of 0.5 μg of IL-1 per mouse, because the body weight of these mice (48.3 ± 0.9 g) is approximately twice that of db/+ mice. Each point in the curves represents the mean ± SEM from glucose determinations performed in four or five mice per group. ∗, P < 0.005 vs. vehicle. The shaded area in each panel indicates the range of the mean ± SEM of time 0 from all groups together. The lower shaded area in D corresponding to the glucose range of normoglycemic db/+ mice (C) has been included for comparison.
IL-1-Treated Diabetic Mice Have Reduced Glucose Levels Even After a Glucose Load.
We have previously reported that IL-1 can reduce glucose levels in db/db mice. On this basis, we studied the effect of IL-1 when db/+ and db/db mice were subjected to a glucose load. Because, as we and others have shown, these mice are resistant to insulin, a group injected with this hormone was not included in these studies. The results obtained in the heterozygous, normoglycemic db/+ mice are comparable with those obtained in C57BL/6J mice: After a transient hyperglycemia due to the glucose load, IL-1-injected db/+ mice remained hypoglycemic for at least 8 h (Fig. 4C).
The basal levels of glucose in db/db mice are more than twice those of normal mice, and the effect of IL-1 during a glucose-tolerance test in these mice was quantitatively even more pronounced than in normal littermates (Fig. 4D). After a transient further increase in glucose levels due to its exogenous administration, diabetic mice treated with IL-1 did not return to their previous hyperglycemic values, but glucose levels were within the basal range of normal animals during a remarkably prolonged time. Glucose levels of IL-1-injected db/db mice were still significantly lower than those of vehicle-injected db/db mice 24 h after the beginning of the experiment.
Occupancy of IL-1 Receptors in the Brain Is Necessary to Maintain IL-1-Induced Hypoglycemia After a Glucose Load.
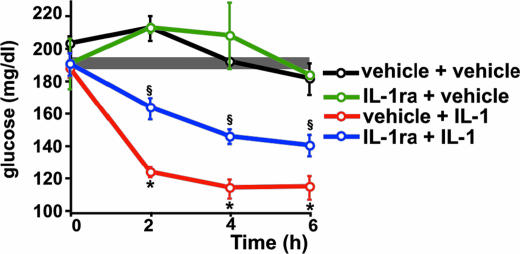
We have investigated whether IL-1 affects the set point of glucoregulation at central levels. IL-1 and glucose were injected i.p. and IL-1ra was administered i.c.v. to block the receptors for the cytokine in the brain. Whereas IL-1-injected mice that received only the vehicle i.c.v. remained hypoglycemic for at least 8 h after the glucose load, those animals that received IL-1ra i.c.v. returned to euglycemic values 2 h after glucose administration (Fig. 5).
Fig. 5.
Brain IL-1 maintains IL-1-induced-hypoglycemia even after a glucose load in normal mice. After a blood sample was obtained (time 0), C57BL/6J mice received either vehicle or IL-1 (0.1 μg per mouse) injected i.p. and either vehicle or IL-1ra (50 μg in 5 μl of vehicle) injected i.c.v. Immediately after injection, a glucose load (2 mg/g b.w.) was administered i.p. Additional blood samples were collected at the times indicated and used for glucose determinations. Each point in the curves represents the mean ± SEM from determinations performed in three to six mice per group. ∗, P < 0.001 vs. control + saline and control + IL-1ra; §, P < 0.05 vs. IL-1 + IL-1ra. The shaded area indicates the range of the mean ± SEM of time 0 from all groups together.
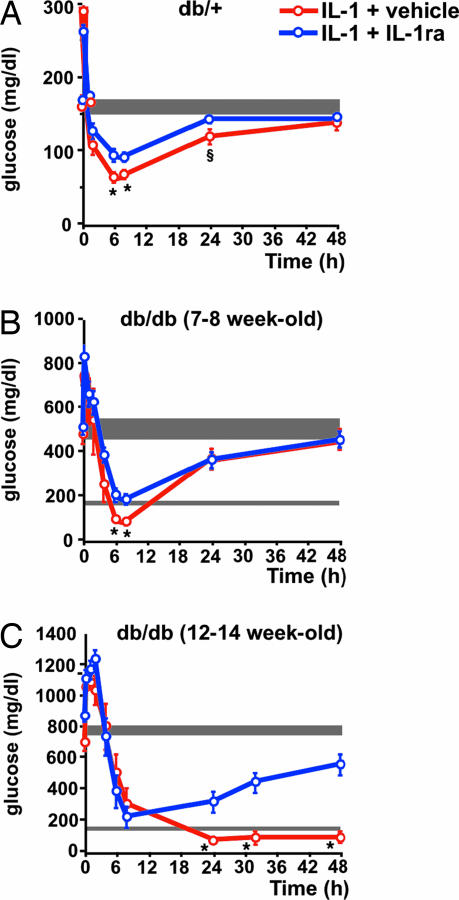
IL-1 Resets Glucose Homeostasis at Brain Levels in db/db Mice.
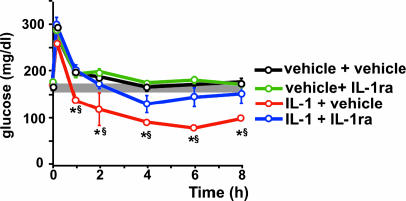
A study comparable with that in normal C57BL/6J mice was performed in db/+ and db/db mice. Blockade of IL-1 receptors in the brain of db/+ mice significantly reduced the hypoglycemia that followed a glucose load in IL-1-injected mice (Fig. 6A). However, this effect was less marked than in C57BL/6J mice. The basal glucose level of 7- to 8-week-old db/db mice was between 450 and 600 mg/dl When these mice received IL-1 and glucose i.p. and the vehicle i.c.v., a remarkable decrease (≈5-fold) in blood glucose levels was observed for at least 8 h after the glucose load. This reduction was significantly less pronounced in diabetic mice treated in the same way but that received IL-1ra i.c.v. (Fig. 6B). The same type of experiment was done at a more advanced stage of the disease, as reflected by glucose concentrations in blood between 700 and 900 mg/dl when 12- to 14-week-old db/db mice were used (Fig. 6C). In these mice, a strong reduction in glucose levels was noticed during the first 8 h after the glucose load, independently of whether they received vehicle or IL-1ra injected i.c.v. However, a remarkable difference was detected between these two groups 24 h later. The average concentration of blood glucose in diabetic mice treated with IL-1 that received vehicle injected i.c.v. was ≈100 mg/dl, and this value was maintained for at least 2 days. Four of six mice in this group died between 4 and 5 days after initiation of the treatment, most likely because of a relative hypoglycemia, because 24 h before death, their glucose concentrations were between 25 and 75 mg/dl. In contrast, glucose levels in the blood of IL-1-treated mice that received IL-1ra i.c.v. started to increase 24 h after the glucose load and approached their previous basal levels 2 days later. No mortality was observed in this group of mice.
Fig. 6.
Brain IL-1 maintains IL-1-induced-hypoglycemia after a glucose load in db/db mice. (A) After a blood sample was obtained (time 0), C57BL/Ks db/+ mice received IL-1 (0.1 μg per mouse) injected i.p. and either vehicle or IL-1ra (50 μg in 5 μl of vehicle) injected i.c.v. Immediately after injection, a glucose load (2 mg/g b.w.) was administered i.p. Additional blood samples were collected at the times indicated in the curves and used for glucose determinations. Each point in the curves represents the mean ± SEM from determinations performed in six mice per group. ∗, P < 0.025 vs. IL-1 + IL-1ra-injected mice; §, P < 0.025 vs. time 0. (B) The same procedure and doses of IL-1, IL-1ra, and glucose as in A but using 7- to 8-week-old C57BL/Ks db/db mice. Each point in the curves represents the mean ± SEM from determinations performed in six or seven mice per group. ∗, P < 0.025 vs. IL-1 + IL-1ra-injected mice. (C) The same procedure and doses of IL-1, IL-1ra, and glucose as in A but using 12- to 14-week-old C57BL/Ks db/db mice. Each point in the curves represents the mean ± SEM from determinations performed in six mice per group. ∗, P < 0.01 vs. IL-1 + IL-1ra-injected mice. The shaded area in each panel indicates the range of the mean ± SEM of time 0 from all groups together.
Discussion
The studies reported here provide evidence that the reduction of blood glucose levels induced by IL-1 in mice is not only based on increased glucose uptake and utilization in peripheral tissues but also determined by glucoregulatory mechanisms in the brain. These studies support the view that IL-1 resets glucose homeostasis at the level of the CNS. We have first established that endogenously produced IL-1 decreases glucose levels, results that are in line with those obtained in IL-1α/IL-1β knock out mice (27). We also show that, even after a glucose load, hypoglycemia lasted longer in IL-1-treated mice than in those that received regular insulin. Furthermore, when glucose was injected 2 h after IL-1, i.e., when glycemia was rather low, hypoglycemic values were quickly regained and were maintained for several hours. These results agree with those obtained in IL-1ra knockout mice, showing that, under prolonged fasting, these animals display an accelerated response to a glucose load (28). In a further step, we show that blockade of IL-1 receptors in the brain partially interfered with the hypoglycemic effect of IL-1 and that IL-1 transcripts in the hypothalamus were increased after peripheral administration of the cytokine or its inducer LPS. Such increases in IL-1 gene expression were negatively correlated with blood glucose levels. Taken together with studies showing that i.c.v. injection of picogram amounts of IL-1 induces hypoglycemia (10, 12), we concluded that this cytokine can alter glucose homeostasis by acting at central levels. The results discussed above suggested that IL-1 could change the set point of glucoregulation, which, as for other essential variables such as thermo- and osmoregulation, is orchestrated at central levels. To approach this issue directly, we blocked IL-1 receptors in the brain and studied the time needed by IL-1-treated normal and insulin-resistant db/db mice to return to their basal glucose levels after a glucose load. As mentioned, IL-1-treated mice challenged with a glucose load needed several hours to recover from hypoglycemia. This prolonged delay occurs despite increased levels of counterregulatory hormones such as glucocorticoids, glucagon, and catecholamines (6, 9). We show here that blockade of IL-1 receptors in the brain significantly diminished the capacity of peripherally injected IL-1 to induce hypoglycemia. Also after a glucose load, normal mice, in which IL-1 receptors in the brain were blocked, approached euglycemia more quickly than untreated mice. Comparable studies were done in db/db mice. These animals, which are commonly used as a model of Type II diabetes, do not express functional leptin receptors and are obese and insulin-resistant. We performed glucose-tolerance tests at different stages of the disease in db/db mice that received either vehicle or IL-1ra i.c.v., followed by a single i.p. IL-1 injection. After the short hyperglycemic episode due to the glucose load, the maintenance of reduced glucose levels induced by IL-1 was partially interfered with in young diabetic db/db mice in which IL-1 receptors in the brain were blocked. The effect was comparable with that observed in control db/+ mice. A different pattern was observed in older db/db mice with a glycemia of ≈700–900 mg/dl. After a further increase in glucose levels caused by the glucose load, the glycemia in IL-1-injected mice dropped to 25% of their basal values within 8 h. Later on, blood glucose concentration in mice that received IL-1ra in the brain was significantly higher than those of the db/db animals that received the vehicle alone and approached the basal hyperglycemic levels. In contrast, db/db mice that received IL-1 and the glucose load, and in which IL-1 could exert effects in the brain, remained hypoglycemic for at least 2 days, when they started to die without recovering from hypoglycemia. It appears that these mice did not tolerate a relatively low glucose level for a long time, because most of them died 4–5 days after the beginning of the treatment. Thus, the effect of IL-1 in the brain of insulin-resistant diabetic mice appears to be related to their initial glycemia and to be potentiated by exogenous administration of glucose.
It is surprising that animals injected with a low, subpyrogenic dose of IL-1 that have ≈50% reduction in glucose blood levels during several hours do not show neurological or other symptoms that characterize hypoglycemia. The finding that peripheral administration of IL-1 results in increased IL-1 mRNA expression in the brain, as shown here in normal and db/db mice, considered together with previous reports showing that IL-1 stimulates glucose uptake by brain cells (19) and that 2-deoxy-glucose incorporation in the brain is increased during a prolonged increase in IL-1 blood levels (20), may provide a plausible explanation for this fact. Thus, it is conceivable that an increased central production of IL-1 contributes to protect neural cells by compensating the reduced glucose availability during IL-1-induced hypoglycemia. On the other hand, it should also be considered that an uncontrolled glucose uptake by the brain might be detrimental because of increased metabolism involving oxidative stress and ROS-induced neuronal damage, as it seems to occur during diabetic encephalopathy.
Control and regulatory systems operate efficiently to keep essential physiologic parameters within a programmed set point. However, a set point that is optimal under healthy conditions may need to be adjusted at other levels during diseases, in particular those in which the activity of the immune system is increased. The regulation of glucose levels is at the core of homeostasis, because glucose is the main source of energy for most cells. Essential immune processes such as endocytosis, phagocytosis, increased cell turnover, clonal expansion, production of numerous mediators, and generation of effector cells and molecules are very expensive in terms of energy (2, 3, 5, 29). During acute physical stress, energy based on glucose supply needs to be deviated, for example, to muscles for an efficient “fight or flight” response. In contrast, IL-1, TNF-α, or endotoxin administration decreases the rate of 2-deoxyglucose uptake by muscle tissue (30). Indeed, glucose supply needs to be deviated toward immune cells for an adequate immune response. Our data support the hypothesis that IL-1 produced locally in inflamed/infected tissue would stimulate glucose uptake and utilization by immune cells even during hypoglycemic conditions. Thus, IL-1 would play a relevant role in orchestrating the necessary central and peripheral adjustments of glucose homeostasis during acute immune processes.
The complexity of these adjustments is attested to by the fact that proinflammatory cytokines induce an increased output of catecholamines, glucocorticoids, and glucagon, which increase glucose mobilization, and there is evidence that the effects of IL-1 are clearly deepened when these mechanisms cannot operate (6). However, host responses mediated by counterregulatory hormones can only partially neutralize the hypoglycemic effect of IL-1. Insulin itself can also mediate a counterregulatory effect, because a clear reduction in the levels of this hormone is observed after administration of IL-1 to normal and fa/fa rats (11) and to adrenalectomized animals (6). Thus, IL-1 triggers a reorganization of the main neuroendocrine mechanisms that control glucose homeostasis, an effect that is further indicated by the prolonged reduction of glucose blood levels that follows a glucose load in IL-1-treated animals. Considering that glucoregulation is orchestrated at central levels, the evidence obtained by blocking IL-1 receptors in the brain supports the hypothesis that IL-1 changes the set point of glucose homeostasis. One possible mechanism by which brain-borne IL-1 could contribute to maintain glucose levels within this reduced set point is by down-regulating counterregulatory neuroendocrine mechanisms. However, the molecular bases underlying the effect of IL-1 in the brain still need to be established.
Materials and Methods
Animals.
C57BL/6J and C57BL/Ks db/+ breeding pairs were obtained from Harlan Winkelmann (Borchen, Germany), and further bred at our animal facilities. C57BL/Ks +/+, db/+, and db/db mice were obtained by crossing C57BL/Ks db/+ pairs. Animals were caged individually for 1 week before experimentation and were kept isolated throughout. They were permanently housed in temperature-, humidity- and light- (12-h cycles) controlled rooms and fed ad libitum. The studies were approved by the Institutional Review Board.
Reagents.
Purified recombinant human IL-1β and recombinant human IL-1ra were kindly provided by A. Shaw (Glaxo Institute for Molecular Biology, Geneva, Switzerland) and J. Relton (Upjohn, Boulder, CO). Lipopolysaccharide from Escherichia coli 0111:B4 was purchased from Difco (Detroit, MI). Regular insulin (Velosulin) was obtained from Nordisk (Gentofte, Denmark) and D (+) glucose from Merck (Darmstadt, Germany). TRIzol Reagent and MMLV reverse transcriptase were obtained from Invitrogen Life Technologies (Karlsruhe, Germany); DNaseI from Epicentre Biotechnologies (Madison, WI); Buffer Y+/Tango from MBI Fermentas (St. Leon Rot, Germany); RNeasy Mini Spin Columns from Qiagen (Hilden, Germany); oligo primers from Amersham Biosciences (Freiburg, Germany); AmpliTaqGold nucleotides and Uracil-DNA-Glycosylase from PE Applied Biosystems (Darmstadt, Germany); and Rox dye from TIB MOLBIOL (Berlin, Germany).
IL-1, LPS, and IL-1ra Administration.
IL-1 and IL-1ra were diluted to the desired concentration with endotoxin-free medium consisting of 0.15 M NaCl and 0.01% human serum albumin. LPS was dissolved in 0.15 M NaCl. Substances were injected at the concentrations indicated in the figures in a volume of 200 μl or 5 μl when administered i.p. or i.c.v., respectively.
Glucose-Tolerance Test.
Glucose (2 mg/g b.w.) was injected i.p. Blood samples (50 μl) were obtained in <1 min from the tip of the tail under light ether narcosis at the times indicated in the figures.
Glucose Determinations.
Glucose concentrations in plasma were determined by an enzymatic (hexokinase) method (kit 115A; Sigma, St. Louis, MO).
Determination of IL-1 Gene Expression by RT-PCR.
Hypothalami were frozen on dry ice immediately after collection and stored at −80°C until use. RNA extraction was performed by using TRIzol Reagent according to a standard protocol (31). The RNA was treated with 2 units of DNaseI in 10× Buffer Y+/Tango, followed by purification using RNeasy Mini Spin Columns according to the manufacturer's instruction and eluted in 30 μl of RNase-free water. Reverse transcription (RT) was performed from 1 μg of total RNA by using 40 units of SuperScript II reverse transcriptase and 0.1 μg of oligop(dT)12–18 primer in a volume of 20 μl. RT was performed at 42°C for 60 min and 70°C for 15 min. cDNA was diluted 1:3 before use. PCR was performed on an ABI PRISM 7700 Sequence Detection System (Applied Biosystems, Foster City, CA) in a final volume of 25 μl containing 12.5 μl of modified 2× PCR buffer (Applied Biosystems), a 200 nM solution of each primer, a 100 nM solution of the corresponding probe, and 4 μl of cDNA. All PCRs were performed by using the following conditions: initial 50°C for 2 min and 95°C for 10 min, followed by 40 cycles at 95°C for 15 s and 60°C for 1 min.
Relative Quantitation of PCR Products.
Primer and probes were chosen to bind in different exons or to span exon junctions to prevent amplification of genomic DNA. The forward primers (sequence 5′–3′) used were CAA CCA ACA AGT GAT ATT CTC CAT G (amplicon length 152 bp) for IL-1β (32) and CAA CGG GAA GCC CAT CAC CAT (amplicon length 66 bp) for glyceraldehyde-3-phosphate-dehydrogenase (GAPDH). The fluorogenic internal probes used were CTG TGT AAT GAA AGA CGG CAC ACC CAC C for IL-1β and TTC CAG GAG CGA GAC CCC ACT AAC for GAPDH. The reverse primer used were GAT CCA CAC TCT CCA GCT GCA for IL-1β and CCG GCC TCA CCC CAT TTG A for GAPDH. The comparative ΔΔCT method was used to calculate relative gene expression data, because we had determined in preliminary experiments that the amplification efficiencies of the target gene (IL-1β) and the reference gene (GAPDH) are comparable. Thus, the IL-1β mRNA level was normalized to the GAPDH mRNA level in each sample. The value of control animals was arbitrarily set at 1.0.
Statistical Analysis.
Results are expressed as mean ± SEM. Data were analyzed by using one-way ANOVA, followed by Fisher's test for multiple comparisons. Correlations were analyzed by using the StatView 5.1 program, version 2000. Differences were considered significant when P values were <0.05.
Acknowledgments
This work was supported by German Research Council (Deutsche Forschungsgemeinschaft) Grant RE 1451/2-2.
Abbreviations
- g b.w.
grams of body weight
- IL-1ra
IL-1 receptor antagonist
- i.c.v.
intracerebroventricular(ly).
Footnotes
References
- 1.Riedemann NC, Guo RF, Ward PA. J Clin Invest. 2003;112:460–467. doi: 10.1172/JCI19523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Powanda MC, Beisel WR. J Nutr. 2003;133:S322–S327. doi: 10.1093/jn/133.1.322S. [DOI] [PubMed] [Google Scholar]
- 3.Bachmann MF, Kopf M. Curr Opin Immunol. 2002;14:413–419. doi: 10.1016/s0952-7915(02)00363-1. [DOI] [PubMed] [Google Scholar]
- 4.Straub RH, Besedovsky HO. Faseb J. 2003;17:2176–2183. doi: 10.1096/fj.03-0433hyp. [DOI] [PubMed] [Google Scholar]
- 5.Calder PC. Proc Nutr Soc; 1995. pp. 65–82. [DOI] [PubMed] [Google Scholar]
- 6.del Rey A, Besedovsky H. Am J Physiol. 1987;253:R794–R798. doi: 10.1152/ajpregu.1987.253.5.R794. [DOI] [PubMed] [Google Scholar]
- 7.Besedovsky H, del Rey A. J Neurosci Res. 1987;18:172–178. doi: 10.1002/jnr.490180124. [DOI] [PubMed] [Google Scholar]
- 8.del Rey A, Besedovsky H. Proc Natl Acad Sci USA. 1989;86:5943–5947. doi: 10.1073/pnas.86.15.5943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berkenbosch F, de Goeij DE, Rey AD, Besedovsky HO. Neuroendocrinology. 1989;50:570–576. doi: 10.1159/000125283. [DOI] [PubMed] [Google Scholar]
- 10.del Rey A, Besedovsky HO. Eur J Clin Invest. 1992;22(Suppl 1):10–15. [PubMed] [Google Scholar]
- 11.del Rey A, Monge-Arditi G, Klusman I, Besedovsky HO. Exp Clin Endocrinol Diabetes. 1996;104:317–326. doi: 10.1055/s-0029-1211461. [DOI] [PubMed] [Google Scholar]
- 12.del Rey A, Monge-Arditi G, Besedovsky HO. Ann NY Acad Sci. 1998;840:153–161. doi: 10.1111/j.1749-6632.1998.tb09559.x. [DOI] [PubMed] [Google Scholar]
- 13.Garcia-Welsh A, Schneiderman JS, Baly DL. FEBS Lett. 1990;269:421–424. doi: 10.1016/0014-5793(90)81207-5. [DOI] [PubMed] [Google Scholar]
- 14.Bird TA, Davies A, Baldwin SA, Saklatvala J. J Biol Chem. 1990;265:13578–13583. [PubMed] [Google Scholar]
- 15.Shikhman AR, Brinson DC, Valbracht J, Lotz MK. J Immunol. 2001;167:7001–7008. doi: 10.4049/jimmunol.167.12.7001. [DOI] [PubMed] [Google Scholar]
- 16.Gould GW, Cuenda A, Thomson FJ, Cohen P. Biochem J. 1995;311(Pt 3):735–738. doi: 10.1042/bj3110735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fukuzumi M, Shinomiya H, Shimizu Y, Ohishi K, Utsumi S. Infect Immun. 1996;64:108–112. doi: 10.1128/iai.64.1.108-112.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fischereder M, Schroppel B, Wiese P, Fink M, Banas B, Schmidbauer S, Schlondorff D. J Nephrol. 2003;16:103–109. [PubMed] [Google Scholar]
- 19.Vega C, Pellerin L, Dantzer R, Magistretti PJ. Glia. 2002;39:10–18. doi: 10.1002/glia.10080. [DOI] [PubMed] [Google Scholar]
- 20.Metzger S, Nusair S, Planer D, Barash V, Pappo O, Shilyansky J, Chajek-Shaul T. Endocrinology. 2004;145:5150–5156. doi: 10.1210/en.2004-0323. [DOI] [PubMed] [Google Scholar]
- 21.Crown J, Jakubowski A, Kemeny N, Gordon M, Gasparetto C, Wong G, Sheridan C, Toner G, Meisenberg B, Botet J, et al. Blood. 1991;78:1420–1427. [PubMed] [Google Scholar]
- 22.Agwunobi AO, Reid C, Maycock P, Little RA, Carlson GL. J Clin Endocrinol Metab. 2000;85:3770–3778. doi: 10.1210/jcem.85.10.6914. [DOI] [PubMed] [Google Scholar]
- 23.Bloesch D, Keller U, Spinas GA, Kury D, Girard J, Stauffacher W. J Clin Endocrinol Metab. 1993;77:1156–1163. doi: 10.1210/jcem.77.5.8077306. [DOI] [PubMed] [Google Scholar]
- 24.Shikhman AR, Brinson DC, Lotz MK. Am J Physiol Endocrinol Metab. 2004;286:E980–E985. doi: 10.1152/ajpendo.00243.2003. [DOI] [PubMed] [Google Scholar]
- 25.Rettori V, Dees WL, Hiney JK, Lyson K, McCann SM. Neuroimmunomodulation. 1994;1:251–258. doi: 10.1159/000097173. [DOI] [PubMed] [Google Scholar]
- 26.Wong ML, Bongiorno PB, Rettori V, McCann SM, Licinio J. Proc Natl Acad Sci USA. 1997;94:227–232. doi: 10.1073/pnas.94.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oguri S, Motegi K, Iwakura Y, Endo Y. Clin Diagn Lab Immunol. 2002;9:1307–1312. doi: 10.1128/CDLI.9.6.1307-1312.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsuki T, Horai R, Sudo K, Iwakura Y. J Exp Med. 2003;198:877–888. doi: 10.1084/jem.20030299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fox CJ, Hammerman PS, Thompson CB. Nat Rev Immunol. 2005;5:844–852. doi: 10.1038/nri1710. [DOI] [PubMed] [Google Scholar]
- 30.Ling PR, Bistrian BR, Mendez B, Istfan NW. Metabolism. 1994;43:279–284. doi: 10.1016/0026-0495(94)90093-0. [DOI] [PubMed] [Google Scholar]
- 31.Chomczynski P, Sacchi N. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 32.Overbergh L, Valckx D, Waer M, Mathieu C. Cytokine. 1999;11:305–312. doi: 10.1006/cyto.1998.0426. [DOI] [PubMed] [Google Scholar]